中国科学院微生物研究所、中国微生物学会主办
文章信息
- 王俊欢, 李先军, 吴巍, 樊双虎, 贾阳, 王嘉翼, 闫艳春
- Wang Junhuan, Li Xianjun, Wu Wei, Fan Shuanghu, Jia Yang, Wang Jiayi, Yan Yanchun
- 混合菌群YC-BJ1对有机磷阻燃剂的降解及16S rRNA基因多样性分析
- Characterization and 16S rRNA gene-based metagenomic analysis of the organophosphorous flame retardants degrading consortium YC-BJ1
- 生物工程学报, 2019, 35(11): 2050-2060
- Chinese Journal of Biotechnology, 2019, 35(11): 2050-2060
- 10.13345/j.cjb.190195
-
文章历史
- Received: May 14, 2019
- Accepted: July 17, 2019
有机磷酸酯(Organophosphorus esters, OPEs)是一类典型的有机磷阻燃剂(Organophosphorus flame retardants, OPFRs)。作为阻燃剂和增塑剂,OPEs广泛应用于多种产品中,包括塑料、纺织品、家具、电子产品、车辆以及建筑用品等。常用的OPFRs分为三类:烷基磷酸酯,如磷酸三(丁氧基乙基)酯(Tris(2-butoxyethyl) phosphate, TBEP)和磷酸三辛酯(Tris(2-ethylhexyl) phosphate, TEHP);含氯磷酸酯,常用的有磷酸三(2-氯丙基)酯(Tris(1-chloro-2-propyl) phosphate, TCPP)、磷酸三(2-氯乙基)酯(Tris(2-chloroethyl) phosphate, TCEP)和磷酸三(1, 3-二氯异丙基)酯(Tris(1, 3-dichloroisopropyl) phosphate, TDCPP);以及芳基磷酸酯,如磷酸三苯酯(Triphenyl phosphate, TPhP)和磷酸三甲苯酯(Tricresyl phosphate, TCrP)[1-3]。因其持久性和危害性,2009年起,多溴联苯醚(Polybrominated diphenyl ethers, PBDEs)已禁止使用,作为替代物的OPFRs的使用量持续增长[4]。
OPFRs作为添加剂,并没有与产品基质通过化学键连接,在生产、运输以及使用的过程中有大量的OPFRs释放到环境中[5-6]。因此,OPFRs在不同的环境介质中都有分布,比如室内环境[7-8]、水环境如饮用水[9]、地表水[10]以及江河湖海[11-13]、大气[14-15]、土壤和沉积物[16-17]以及生物体[18]。甚至在极地和远洋环境中,都有OPFRs检出,说明OPFRs具有远程运输能力并在全球范围内普遍存在[19-20]。
在所有的OPFRs中,含氯的TCPP和TDCPP疑似有致癌性[6],磷酸三丁酯(Tri-n-butyl phosphate, TnBP)、TBEP和TCEP则对斑马鱼幼虫具有神经毒性[21]。人类细胞系和斑马鱼的实验表明,一些OPFRs (TDCPP、TPhP、TCrP)具有内分泌干扰潜能并能够影响性荷尔蒙平衡[22]。一些毒理学实验表明OPFRs对斑马鱼胚胎具有心脏毒性[23]、发育毒性[24]以及生殖毒性[25]。500 μg/L TPhP的长期暴露对大型蚤具有发育以及生殖毒性[26]。有报道指出TDCPP暴露会损害人类角膜[27]。因此,环境中无处不在的OPFRs对人类健康是一大威胁。
已报道的OPFRs去除方法有光催化[28]、高级氧化工艺[29]、动物分解[30]和微生物降解[31]等多种。其中,生物修复具有极大的优势,高效、成本低、环境友好。目前,已报道的OPFRs降解菌不多,且多为混合菌群[31-37],单菌只有几种,玫瑰杆菌属Roseobacter[38]、鞘氨醇单胞菌Sphingomonas和鞘脂菌属Sphingobium[39-40]以及短短芽孢杆菌Brevibacillus brevis[41],表 1列举了部分已报道的混合菌群以及纯菌,包括来源、可降解底物以及降解能力。表 1列举的7篇混合菌群降解OPFRs的报道中,只有3篇提到了具体的降解率,其中最高的为分离自美国污水处理厂的活性淤泥,28 h内能够将20 mg/L的TPhP降解99.0%±24.3%[37];表 1列举了已报道的全部5株OPFRs降解菌,其中活性最高的是一株Sphingomonas sp.,能够在63 h内降解超过90%的TnBP (100 mg/L),并且是已报道的唯一一株能够利用OPFRs作为唯一碳源生长的菌株[40];分离自日本的Sphingomonas sp. TCM1和Sphingobium sp. TDK1能够降解TDCPP和TCEP,作为唯一磷源,培养6 h能够100%降解TCEP (5.7 mg/L)和TDCPP (8.6 mg/L)[39]。目前已报道的OPFRs降解微生物不仅活性较低,亦缺乏对其降解能力的综合评估,尤其是对环境变化的耐受能力。
| Year | Source & microorganisms | Substrates & degradation efficiency | Reference |
| 1975 | A mixed bacterial population isolated from mud sample of Shell Lake, Canada | TCrP, TPhP, trixylenyl phosphate (TXP) | [31] |
| 1979 | Naturally occurring mixed-microbial populations present in activated sludge and river water | TPhP, TCrP, TXP, TEHP, cresyl diphenyl phosphate (CDP), tert-butylphenyl diphenyl phosphate (t-BPDP), isodecyl diphenyl phosphate (IDDP), 2-ethylhexyl diphenyl phosphate (EHDP) | [32] |
| 1984 | Mixed sediment microflora collected from Missouri, US | Isopropylphenyl diphenyl phosphate (IPDP) | [33] |
| 1986 | Microcosms containing sediment and water from five different ecosystems in US | Over 37% of t-BPDP (5–55mg/L) was mineralized after 8 weeks | [34] |
| 2002 | Leachate from a sea-based solid waste disposal site, osaka North Port, Japan | Aryl-phosphates (TCrP, TPhP), alkyl-phosphates (TnBP), chloro-alkylphosphates (TCEP, TDCPP) | [35] |
| 2008 | Mixed bacterial cultures 67E and 45D enriched from soil and sediment samples, Niigata, Japan | Completely degraded 20 μmol/L TCEP and TDCPP within 6 h | [36] |
| 2014 | Activated sludge from either the Amsterdam West or the Amstelveen sewage treatment plants, US | Aromatic organophosphorus flame retardants TPhP (20 mg/L, 99.0%±24.3% within 28 h), resorcinol bis diphenyl phosphate (PBDPP or RDP) and bisphenol- A bis diphenyl phosphate (BPABDPP or BDP) | [37] |
| 2004 | Roseobacter YS-57, isolated from the leachate of a seabased waste disposal site, Osaka North Port, Japan | TCrP, TPhP (0.5 mg/L, over 99% within 3 days) | [38] |
| 2010 | Sphingomonas sp. TCM1 and Sphingobium sp. TDK1, isolated from soil and sediment samples, Niigata, Japan | TCEP and TDCPP (20 µmol/L, 100%, as the sole phosphorus source within 6 h) | [39] |
| 2018 | Brevibacillus brevis, isolated from an e-waste dismantling area in Guiyu, Guangdong Province, China | TPhP (the highest degradation efficiency of 3 μmol/L TPhP by B. brevis reached 92.1% at pH 7 and 30 ℃) | [41] |
| 2019 | Sphingomonas sp., isolated from the tailing sand of Hechi City, Guangxi Province, China | TnBP could be utilized as the sole carbon source (375 μmol/L, over 90%, 63 h) | [40] |
因此,本研究的主要目的是富集能够高效降解OPFRs的微生物,并关注可能影响降解效率的环境因子。从北京某垃圾场渗滤液中富集到一个混合菌群YC-BJ1,能够高效降解OPFRs,并对它的降解特性、底物谱以及物种组成进行了分析。
1 材料与方法 1.1 材料 1.1.1 药品和试剂所有化学品均购自商业来源。表 2列出了本研究中涉及的OPFRs。标准品TnBP (99.25%)、TBEP (93.0%)、TEHP(98.74%)、TCEP (98.73%)、TDCPP (95.44%)、TCPP (99.65%)、TCrP (98.6%)和TPhP (98%)购自Dr. Ehrenstorfer GmbH公司(德国,奥格斯堡),溶于甲醇或者乙腈(色谱纯),配制成浓度为2×104 mg/L的母液。
| Type | IUPAC name | Abbreviation | CAS |
| Alkyl-phosphates | Tri-n-butyl phosphate | TnBP | 126-73-8 |
| Tris(2-ethylhexyl) phosphate | TEHP | 78-42-2 | |
| Tris(2-butoxyethyl) phosphate | TBEP | 78-51-3 | |
| Chloro-alkyl-phosphates | Tris(2-chloroethyl) phosphate | TCEP | 115-96-8 |
| Tris(1, 3-dichloroisopropyl) phosphate | TDCPP | 13674-87-8 | |
| Tris(1-chloro-2-propyl) phosphate | TCPP | 13674-84-5 | |
| Aryl-phosphates | Tricresyl phosphate | TCrP | 1330-78-5 |
| Triphenyl phosphate | TPhP | 115-86-6 |
富集用培养基TEM配方:1.0 L无菌水中添加2.0 g (NH4)2SO4、1.5 g Na2HPO4·12H2O、1.5 g K2HPO4、0.2 g MgSO4·7H2O、0.01 g CaCl2和100 µL TES储存液。TES储存液包含FeSO4·7H2O (5 g/L)、ZnSO4·7H2O (2.2 g/L)、CuSO4·5H2O (0.3 g/L)、MnSO4·2H2O (14.3 g/L)、CoSO4·7H2O (1.2 g/L)、Na2MoO4·2H2O (0.2 g/L)和Na2WO4·2H2O (2.3 g/L)。用NaOH或者HCl (2 mol/L)调节培养基pH为7.2±0.2,或者其他需要的pH。所有培养基在121 ℃下高压灭菌20 min。降解实验中,在TEM培养基中添加OPFRs作为唯一碳源。
1.2 方法 1.2.1 细胞生长检测使用紫外-可见分光光度计(美国,马萨诸塞州,赛默科技)检测600 nm下的光密度(OD600),以此衡量细胞生长。
1.2.2 高效液相色谱使用高效液相色谱仪(美国,加州,安捷伦)检测TPhP浓度,具体配置如下:C18柱(Agilent Eclipse XDB, 5 µm, 4.6 mm×150 mm),二极管阵列检测器,205 nm,进样量10 µL,流动相(乙腈和水9︰1),流速1 mL/min,柱温25 ℃。因为TPhP的溶解度极低,所以在水相中加入等体积的乙腈,混匀,0.22 µm膜(上海,安谱)过滤后使用液相检测。
1.2.3 气相色谱表 2列举的其余OPFRs则使用气相色谱仪检测,日本岛津GC-2010系统,配置如下,HP-5毛细管柱(内径0.25 mm,长度30 m,膜厚0.25 µm),火焰离子检测器(300 ℃),进样量5 µL,载体N2,流速1.51 mL/min,柱温以10 ℃/min的速度从160 ℃逐渐增加到280 ℃,并在280 ℃保持4 min,检测器的载气为H2 (40 mL/min)和空气(400 mL/min)。水相中的OPFRs首先需要用等体积正己烷萃取到有机相,然后使用气相检测。
1.2.4 OPFRs降解菌的富集从北京某垃圾处理厂采集渗透液,4 ℃储存。在100 mL的锥形瓶中,加入30 mL的TEM培养基,添加20 mg/L的TPhP和5 mL水样,30 ℃、180 r/min培养7 d,取1 mL该培养液,转接到新鲜的TEM培养基中并添加40 mg/L的TPhP,相同条件下继续培养7 d。如此重复5次,至TPhP的浓度增加至100 mg/L。为尽可能减少母液中溶剂的干扰,富集过程中交替使用溶于甲醇和乙腈的母液。经过35 d的富集和驯化,得到了一瓶培养液,根据它的来源命名YC-BJ1,储存在4 ℃以备使用。在后续的降解实验中,该富集培养液作为种子液,接种比例为1%。
将富集培养液1%接种到含有100 mg/L OPFRs的10 mL TEM培养基中,30 ℃、180 r/min振荡培养4 d。每个处理组设3个重复,对照不接菌,并根据1.2.2和1.2.3描述的方法检测底物浓度。因为色谱信号与OPFRs的浓度具有线性关系(数据未列出),所以OPFRs的降解率根据对照和实验组之间的色谱信号比率计算。
1.2.5 TPhP降解酶的细胞定位检测上清液和细胞提取液的降解能力。取1 mL培养5 d的富集培养液,12 000 r/min离心5 min,使用0.22 µm的膜过滤上清并于4 ℃保存,菌体重悬浮在1 mL新鲜的TEM培养基中,超声破碎细胞至液体澄清,12 000 r/min离心5 min,此上清即为细胞提取液。在上清和细胞提取液中加入20 mg/L TPhP,30 ℃、180 r/min振荡培养12 h,对照为添加等量底物的新鲜TEM培养基。每组设3个重复。按1.2.2所述方法检测底物浓度。
1.2.6 环境因子对TPhP降解的影响为评估环境耐受性,检测不同环境因子对TPhP (100 mg/L)的降解影响,包括温度(15 ℃、20 ℃、25 ℃、30 ℃、35 ℃、40 ℃)、pH (4、5、6、7、8、9、10、11、12)和盐度(0%、2%、4%、6%、8% g/mL NaCl添加)。培养4 d,每组设3个重复。按1.2.2所述方法检测底物浓度。
1.2.7 高通量测序分析富集培养液菌落结构使用TaKaRa总基因组提取试剂盒(中国,大连,宝日医生物技术)提取混合菌群总DNA,共3个重复,分开测序。扩增16S rRNA基因的可变保守区域(V3+V4),构建双端小片段文库,通过Illumina HiSeq 2500高通量测序平台测序。通过拼接(UCHIME v4.2)、过滤(Trimmomatic v0.33)和嵌合体去除(UCHIME v4.2),从原始下机双端序列中获得最终的有效序列。使用QIIME[42] (version 1.8.0)中的UCLUST聚类有效序列,以97%的相似性获得运算分类单位(Operational taxonomic units, OTUs)。基于Silva (细菌分类数据库)对OTUs进行分类和注释。使用Mothur (version v.1.30)软件进行alpha多样性分析。
1.3 核酸序列编号将混合菌群YC-BJ1的16S rRNA基因多样性分析数据上传到NCBI数据库,序列号为SRS4436212。
2 结果与分析 2.1 菌群富集及其对TPhP的降解本研究从北京某垃圾处理厂渗透液中富集到一个混合菌群,根据环境样品来源命名为YC-BJ1。降解研究发现,混合菌群YC-BJ1能够高效降解TPhP (100 mg/L),培养4 d的降解率高达99.55%,1 d内就有超过一半的TPhP被降解(图 1)。

|
| 图 1 混合菌群YC-BJ1对TPhP (100 mg/L)的降解 Fig. 1 Biodegradation curve of 100 mg/L TPhP by bacterial consortium YC-BJ1 and cell growth curve. Each data point represents the mean of three replicates. |
| |
能够降解TPhP的混合菌群已有报道。一个富集自加拿大的混合群落能够利用TPhP作为唯一碳源生长,但没有提到具体的降解率[31]。一个自然形成的混合微生物群落能够在7 d内降解浓度高达91 mg/L的TPhP,降解率为93%±11%[32]。有报道称活性淤泥能够在28 h内将TPhP (20 mg/L)完全矿化并释放CO2,降解率高于60%[37]。与已报道的微生物群落相比,本研究中的混合菌群YC-BJ1显然具有更高的TPhP降解活性,只需要4 d就能够实现对100 mg/L TPhP的降解,降解率高于99%。
2.2 混合菌中的TPhP降解酶定位HPLC检测底物TPhP的减少,以此比较上清和细胞提取液的酯酶活性。与细胞提取液相比,上清对TPhP几乎没有降解(表 3),表明混合菌群YC-BJ1中的TPhP水解酶并没有释放到细胞外。以往的研究发现,TPhP水解酶既有胞外酶,又有胞内酶。Roseobacter sp. YS-57中的TPhP水解酶为胞外酶[38],而富集自加拿大的TPhP降解菌群中的水解酶则为胞内酶[31]。除了简单的定位,关于TPhP水解酶需要更深入的研究,包括纯化、催化动力学分析以及机理研究。
| Hydrolases source | Degradation rate (%) a |
| Supernatant | 0.27±0.28 |
| Cell extract | 97.22±2.62 |
| a: each value represents average (n=3) ± STEDV. | |
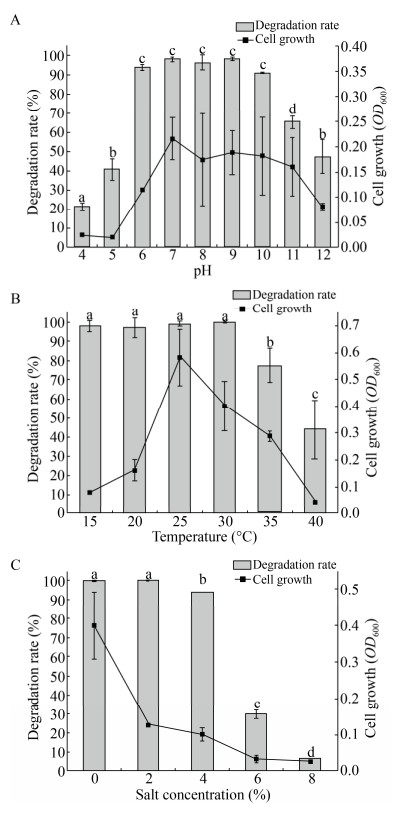
为了评估混合菌群YC-BJ1的环境适应性,按前所述,在不同的条件下进行了一系列的降解实验,包括pH (4、5、6、7、8、9、10、11、12)、温度(15 ℃、20 ℃、25 ℃、30 ℃、35 ℃、40 ℃)和盐度(0%、2%、4%、6%、8% g/mL NaCl添加)。结合菌株生长以及降解数据,发现混合菌群YC-BJ1降解TPhP的最适条件为pH 7、25 ℃,无盐添加。并且混合菌群YC-BJ1表现出了优越的环境适应性,能够在较宽的环境范围内保持较高的TPhP降解活性,pH (5.0–12.0)、温度(15–40 ℃)和盐度(0%– 4%) (图 2)。在上述环境范围内,混合菌群YC-BJ1对TPhP的降解率均维持在40%以上,表现出极强的环境耐受能力,说明其具有广泛的应用潜能。
|
| 图 2 环境因子对混合菌群YC-BJ1降解TPhP的影响 Fig. 2 Effect of environmental factors on degradation of TPhP by bacterial consortium YC-BJ1. (A) Effect of pH (4, 5, 6, 7, 8, 9, 10, 11, 12). (B) Effect of temperature (15 ℃, 20 ℃, 25 ℃, 30 ℃, 35 ℃, 40 ℃). (C) Effect of salinity (0%, 2%, 4%, 6%, 8% g/mL NaCl addition). The initial concentration of TPhP is 100 mg/L. Each data point represents the mean of three replicates. The different letters represent significant differences at P≤0.05 (One-way ANOVA). |
| |
除了降解率的直接比较,我们还对图 2的降解数据作了两个维度的显著性分析:1)与未接种的对照作比较,在pH 4–12、温度(15–40 ℃)和盐度(0%–6%)的广泛条件下,培养4 d后,混合菌群YC-BJ1对TPhP有显著降解(One-way ANOVA, P≤0.05);2)对同一环境因子下的数据进行组内比对,发现该实验条件下,在pH 6–10区间内(图 2A,“c”标注),15–30 ℃温度区间内(图 2B,“a”标注),0%–2%的盐度范围内(图 2C,“a”标注),混合菌群YC-BJ1对TPhP的降解没有显著差异。显著性分析表明,混合菌群YC-BJ1不仅能够在广泛的环境条件下降解TPhP,更能够保持较高的降解效率。
pH、温度以及盐度对微生物降解具有极强的影响力[43],但是目前还没有环境因子影响群落对TPhP降解的报道。最近发表的一篇文章分析了一个富集自海水的混合菌群SWO的环境适应性,该群落能够在pH 4.0–9.0、温度25–37 ℃和盐度0%–10%范围内降解多环芳烃[44]。与混合菌群SWO相比,除了盐度,混合菌群YC-BJ1对温度和pH都有更宽的耐受范围,考虑到SWO富集自海水,而YC-BJ1则富集自垃圾场渗滤液,对盐度的适应能力弱于菌群SWO。
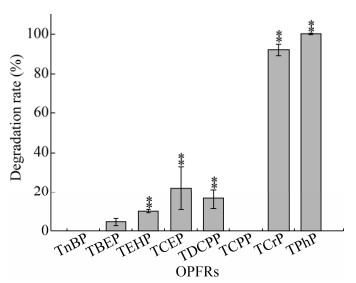
2.4 混合菌群YC-BJ1对OPFRs的降解将混合菌群YC-BJ1接种到含有100 mg/L不同的OPFRs的TEM培养基中,培养4 d后检测底物降解,降解率结果见图 3。混合菌群YC-BJ1表现出明显的底物特异和偏好,能够高效降解芳基磷酸酯,对TPhP和TCrP的降解率均在90%以上,对含氯磷酸酯的降解效率较低,对TCEP和TDCPP的降解率在20%左右,除了TEHP (10%降解),几乎不能降解任何的烷基磷酸酯。在本研究的实验条件下,未检测到混合菌群YC-BJ1对TnBP、TBEP和TCPP的显著降解。因为该菌群是在单一底物TPhP的胁迫下富集的,所以对芳基磷酸酯表现出了较明显的底物偏好。
|
| 图 3 混合菌群YC-BJ1对不同有机磷阻燃剂的降解 Fig. 3 OPFRs degradation by the consortium YC-BJ1 within 4 days. The initial concentration of each OPFRs is 100 mg/L. Each data point represents the mean of three replicates. Double asterisks (**) indicate a statistical significance of P≤0.01 (One-way ANOVA) compared with the uninoculated control. |
| |
通过16S rRNA基因V3+V4区域高通量测序分析混和菌群YC-BJ1的群落结构,α多样性指数见表 4。所有样品的OTUs覆盖率都在0.999 9以上,结合稀释曲线(图 4A),说明测序结果能够真实地反映样品的群落结构。在菌属水平上,混合菌群YC-BJ1由生丝微菌属Hyphomicrobium (38.80%)、金黄杆菌属Chryseobacterium (17.57%)和鞘氨醇盒菌属Sphingopyxis (17.46%)主导(图 4B)。其余含量较多(> 4%)的菌属有产碱杆菌属Alcaligenes、Sphingomonas、丛毛单胞菌属Comamonas以及无色杆菌属Achromobacter。

|
| 图 4 混合菌群YC-BJ1的物种多样性及其组成 Fig. 4 Diversity and composition of the bacterial consortium YC-BJ1. (A) Rarefaction curve of observed OTUs (≥97% sequence identity) over the number of sequences. (B) Relative abundance of genus. The Y-axis represents the genera over 1% relative abundance, with other genera classified as "Others" and the unclassified genera referred to as "Unclassified". The uncultured bacterium clone JG30-KF-CM45 was simplified as "Uncultured". |
| |
| Samples | Number of sequences | Observed OTUs | Chao1 | ACE | Simpson | Shannon | Coverage |
| YC-BJ1_01 | 57 257 | 41 | 42.50 | 43.327 2 | 0.154 2 | 2.202 2 | 0.999 9 |
| YC-BJ1_02 | 56 818 | 40 | 40.00 | 40.399 3 | 0.152 8 | 2.212 8 | 1 |
| YC-BJ1_03 | 57 214 | 40 | 40.33 | 41.104 3 | 0.151 9 | 2.228 6 | 1 |
目前已报道的OPFRs降解纯菌不多。在有葡萄糖作为补充碳源时,菌株Roseobacter sp. YS-57能够在3 d内将0.5 mg/L的TCrP和TPhP降解99%以上[38]。Sphingomonas sp. TDK1和Sphingobium sp. TCM1能够利用TPhP、TCrP、TCEP和TDCPP作为唯一磷源生长,培养6 h可以将20 µmol/L的TCEP和TDCPP完全降解,遗憾的是,文章中没有提供对TCrP和TPhP的降解率数据[39]。一株Brevibacillus brevis能够降解3 µmol/L的TPhP,降解率为92.1%[41]。一株分离自广西省的Sphingomonas sp.能够利用TnBP作为唯一碳源生长,培养63 h对100 mg/L底物的降解率能够达到90%,同时也是目前已报道的唯一一株能够将OPFRs作为唯一碳源的菌[40]。因此,混合菌群YC-BJ1中包含未见报道的OPFRs降解菌,这也是我们下一步的研究计划,希望能够从中分离到新的OPFRs的高效降解菌,进行单菌或者多菌协同降解试验。
3 结论从北京垃圾场渗滤液中富集到一个混合菌群YC-BJ1,能够高效降解芳基有机磷阻燃剂,可以耐受广泛的环境变化(15–40 ℃, pH 5.0–12.0, 0%–4%盐度),在OPFRs降解机理的揭示和其污染环境的生物修复中具有巨大的潜能。本研究只使用HPLC和GC检测了底物的减少,未来的研究需要借助质谱检测降解中间产物并推测可能的降解通路,从混合菌群中分离纯化OPFRs高效降解菌也是下一步的研究重点。
| [1] |
International Programme on Chemical Safety. Environmental health criteria 218. Flame retardants: tris(2-butoxyethyl) phosphate, tris(2-ethylhexyl) phosphate and tetrakis (hydroxymethyl) phosphonium salts. Geneva: World Health Organization, 2000.
|
| [2] |
International Programme on Chemical Safety. Environmental health criteria 209. Flame retardants: tris(chloropropyl) phosphate and tris(2-chloroethyl) phosphate. Geneva: World Health Organization, 1998.
|
| [3] |
International Programme on Chemical Safety. Environmental health criteria 192. flame retardants: a general introduction. Geneva: World Health Organization, 1997.
|
| [4] |
Wei GL, Li DQ, Zhuo MN, et al. Organophosphorus flame retardants and plasticizers: sources, occurrence, toxicity and human exposure. Environ Pollut, 2015, 196: 29-46. DOI:10.1016/j.envpol.2014.09.012 |
| [5] |
Li TY, Bao LJ, Wu CC, et al. Organophosphate flame retardants emitted from thermal treatment and open burning of e-waste. J Hazard Mater, 2019, 367: 390-396. DOI:10.1016/j.jhazmat.2018.12.041 |
| [6] |
van der Veen I, de Boer J. Phosphorus flame retardants: properties, production, environmental occurrence, toxicity and analysis. Chemosphere, 2012, 88(10): 1119-1153. DOI:10.1016/j.chemosphere.2012.03.067 |
| [7] |
Chen MQ, Jiang JY, Gan ZW, et al. Grain size distribution and exposure evaluation of organophosphorus and brominated flame retardants in indoor and outdoor dust and PM10 from Chengdu, China. J Hazard Mater, 2019, 365: 280-288. DOI:10.1016/j.jhazmat.2018.10.082 |
| [8] |
He CT, Zheng J, Qiao L, et al. Occurrence of organophosphorus flame retardants in indoor dust in multiple microenvironments of southern China and implications for human exposure. Chemosphere, 2015, 133: 47-52. DOI:10.1016/j.chemosphere.2015.03.043 |
| [9] |
Lee S, Jeong W, Kannan K, et al. Occurrence and exposure assessment of organophosphate flame retardants (OPFRs) through the consumption of drinking water in Korea. Water Res, 2016, 103: 182-188. DOI:10.1016/j.watres.2016.07.034 |
| [10] |
Shi YL, Gao LH, Li WH, et al. Occurrence, distribution and seasonal variation of organophosphate flame retardants and plasticizers in urban surface water in Beijing, China. Environ Pollut, 2016, 209: 1-10. DOI:10.1016/j.envpol.2015.11.008 |
| [11] |
Wang RM, Tang JH, Xie ZY, et al. Occurrence and spatial distribution of organophosphate ester flame retardants and plasticizers in 40 rivers draining into the Bohai Sea, North China. Environ Pollut, 2015, 198: 172-178. DOI:10.1016/j.envpol.2014.12.037 |
| [12] |
Wolschke H, Sühring R, Xie ZY, et al. Organophosphorus flame retardants and plasticizers in the aquatic environment: a case study of the Elbe River, Germany. Environ Pollut, 2015, 206: 488-493. DOI:10.1016/j.envpol.2015.08.002 |
| [13] |
Zhong MY, Tang JH, Mi LJ, et al. Occurrence and spatial distribution of organophosphorus flame retardants and plasticizers in the Bohai and Yellow Seas, China. Mar Pollut Bull, 2017, 121(1/2): 331-338. |
| [14] |
Faiz Y, Siddique N, He H, et al. Occurrence and profile of organophosphorus compounds in fine and coarse particulate matter from two urban areas of China and Pakistan. Environ Pollut, 2018, 233: 26-34. DOI:10.1016/j.envpol.2017.09.091 |
| [15] |
Salamova A, Ma YN, Venier M, et al. High levels of organophosphate flame retardants in the great lakes atmosphere. Environ Sci Technol Lett, 2014, 1(1): 8-14. |
| [16] |
Wang Y, Wu XW, Zhang QN, et al. Organophosphate esters in sediment cores from coastal Laizhou Bay of the Bohai Sea, China. Sci Total Environ, 2017, 607-608: 103-108. DOI:10.1016/j.scitotenv.2017.06.259 |
| [17] |
Yadav IC, Devi NL, Li J, et al. Concentration and spatial distribution of organophosphate esters in the soil-sediment profile of Kathmandu Valley, Nepal: implication for risk assessment. Sci Total Environ, 2018, 613-614: 502-512. DOI:10.1016/j.scitotenv.2017.09.039 |
| [18] |
Papachlimitzou A, Barber JL, Losada S, et al. Organophosphorus flame retardants (PFRs) and plasticisers in harbour porpoises (Phocoena phocoena) stranded or bycaught in the UK during 2012. Mar Pollut Bull, 2015, 98(1/2): 328-334. |
| [19] |
Li J, Xie ZY, Mi WY, et al. Organophosphate esters in air, snow, and seawater in the North Atlantic and the Arctic. Environ Sci Technol, 2017, 51(12): 6887-6896. DOI:10.1021/acs.est.7b01289 |
| [20] |
M ller A, Sturm R, Xie ZY, et al. Organophosphorus flame retardants and plasticizers in airborne particles over the Northern Pacific and Indian Ocean toward the polar regions: evidence for global occurrence. Environ Sci Technol, 2012, 46(6): 3127-3134. DOI:10.1021/es204272v |
| [21] |
Sun LW, Xu WB, Peng T, et al. Developmental exposure of zebrafish larvae to organophosphate flame retardants causes neurotoxicity. Neurotoxicol Teratol, 2016, 55: 16-22. DOI:10.1016/j.ntt.2016.03.003 |
| [22] |
Liu XS, Ji K, Choi K. Endocrine disruption potentials of organophosphate flame retardants and related mechanisms in H295R and MVLN cell lines and in zebrafish. Aquat Toxicol, 2012, 114-115: 173-181. DOI:10.1016/j.aquatox.2012.02.019 |
| [23] |
McGee SP, Konstantinov A, Stapleton HM, et al. Aryl phosphate esters within a major pentaBDE replacement product induce cardiotoxicity in developing zebrafish embryos: potential role of the aryl hydrocarbon receptor. Toxicol Sci, 2013, 133(1): 144-156. DOI:10.1093/toxsci/kft020 |
| [24] |
Isales GM, Hipszer RA, Raftery TD, et al. Triphenyl phosphate-induced developmental toxicity in zebrafish: potential role of the retinoic acid receptor. Aquat Toxicol, 2015, 161: 221-230. DOI:10.1016/j.aquatox.2015.02.009 |
| [25] |
Wang QW, Lam JCW, Han J, et al. Developmental exposure to the organophosphorus flame retardant tris(1, 3-dichloro-2-propyl) phosphate: estrogenic activity, endocrine disruption and reproductive effects on zebrafish. Aquat Toxicol, 2015, 160: 163-171. DOI:10.1016/j.aquatox.2015.01.014 |
| [26] |
Yuan SL, Li H, Dang Y, et al. Effects of triphenyl phosphate on growth, reproduction and transcription of genes of Daphnia magna. Aquat Toxicol, 2018, 195: 58-66. DOI:10.1016/j.aquatox.2017.12.009 |
| [27] |
Xiang P, Liu RY, Li C, et al. Effects of organophosphorus flame retardant TDCPP on normal human corneal epithelial cells: implications for human health. Environ Pollut, 2017, 230: 22-30. DOI:10.1016/j.envpol.2017.06.036 |
| [28] |
Liu J, Ye JS, Chen YF, et al. UV-driven hydroxyl radical oxidation of tris(2-chloroethyl) phosphate: intermediate products and residual toxicity. Chemosphere, 2018, 190: 225-233. DOI:10.1016/j.chemosphere.2017.09.111 |
| [29] |
Song QY, Feng YP, Liu GG, et al. Degradation of the flame retardant triphenyl phosphate by ferrous ion-activated hydrogen peroxide and persulfate: kinetics, pathways, and mechanisms. Chem Eng J, 2019, 361: 929-936. DOI:10.1016/j.cej.2018.12.140 |
| [30] |
Hou R, Huang C, Rao KF, et al. Characterized in vitro metabolism kinetics of alkyl organophosphate esters in fish liver and intestinal microsomes. Environ Sci Technol, 2018, 52(5): 3202-3210. DOI:10.1021/acs.est.7b05825 |
| [31] |
Pickard MA, Whelihan JA, Westlake DWS. Utilization of triaryl phosphates by a mixed bacterial population. Can J Microbiol, 1975, 21(2): 140-145. DOI:10.1139/m75-021 |
| [32] |
Saeger VW, Hicks O, Kaley RG, et al. Environmental fate of selected phosphate esters. Environ Sci Technol, 1979, 13(7): 840-844. DOI:10.1021/es60155a010 |
| [33] |
Heitkamp MA, Huckins JN, Petty JD, et al. Fate and metabolism of isopropylphenyl diphenyl phosphate in freshwater sediments. Environ Sci Technol, 1984, 18(6): 434-439. DOI:10.1021/es00124a008 |
| [34] |
Heitkamp MA, Freeman JP, Cerniglia CE. Biodegradation of tert-butylphenyl diphenyl phosphate. Appl Environ Microbiol, 1986, 51(2): 316-322. |
| [35] |
Kawagoshi Y, Nakamura S, Fukunaga I. Degradation of organophosphoric esters in leachate from a sea-based solid waste disposal site. Chemosphere, 2002, 48(2): 219-225. DOI:10.1016/S0045-6535(02)00051-6 |
| [36] |
Takahashi S, Kawashima K, Kawasaki M, et al. Enrichment and characterization of chlorinated organophosphate ester-degrading mixed bacterial cultures. J Biosci Bioeng, 2008, 106(1): 27-32. DOI:10.1263/jbb.106.27 |
| [37] |
Jurgens SS, Helmus R, Waaijers SL, et al. Mineralisation and primary biodegradation of aromatic organophosphorus flame retardants in activated sludge. Chemosphere, 2014, 111: 238-242. DOI:10.1016/j.chemosphere.2014.04.016 |
| [38] |
Kawagoshi Y, Nakamura S, Nishio T, et al. Isolation of aryl-phosphate ester-degrading bacterium from leachate of a sea-based waste disposal site. J Biosci Bioeng, 2004, 98(6): 464-469. DOI:10.1016/S1389-1723(05)00313-0 |
| [39] |
Takahashi S, Satake I, Konuma I, et al. Isolation and identification of persistent chlorinated organophosphorus flame retardant-degrading bacteria. Appl Environ Microbiol, 2010, 76(15): 5292-5296. DOI:10.1128/AEM.00506-10 |
| [40] |
Liu J, Lin H, Dong YB, et al. Elucidating the biodegradation mechanism of tributyl phosphate (TBP) by Sphingomonas sp. isolated from TBP-contaminated mine Tailings. Environ Pollut, 2019, 250: 284-291. |
| [41] |
Wei K, Yin H, Peng H, et al. Bioremediation of triphenyl phosphate by Brevibacillus brevis: degradation characteristics and role of cytochrome P450 monooxygenase. Sci Total Environ, 2018, 627: 1389-1395. DOI:10.1016/j.scitotenv.2018.02.028 |
| [42] |
Caporaso JG, Kuczynski J, Stombaugh J, et al. QⅡME allows analysis of high-throughput community sequencing data. Nat Methods, 2010, 7: 335-336. DOI:10.1038/nmeth.f.303 |
| [43] |
Haritash AK, Kaushik CP. Biodegradation aspects of polycyclic aromatic hydrocarbons (PAHs): a review. J Hazard Mater, 2009, 169(1/3): 1-15. |
| [44] |
Muangchinda C, Rungsihiranrut A, Prombutara P, et al. 16S metagenomic analysis reveals adaptability of a mixed-PAH-degrading consortium isolated from crude oil-contaminated seawater to changing environmental conditions. J Hazard Mater, 2018, 357: 119-127. DOI:10.1016/j.jhazmat.2018.05.062 |
 2019, Vol. 35
2019, Vol. 35