中国科学院微生物研究所、中国微生物学会主办
文章信息
- 祝洁, 杨晶, 罗云孜
- Zhu Jie, Yang Jing, Luo Yunzi
- 改造肠道微生物在疾病诊断与治疗中的应用
- Applications of engineered intestinal bacteria in disease diagnosis and treatment
- 生物工程学报, 2019, 35(12): 2350-2366
- Chinese Journal of Biotechnology, 2019, 35(12): 2350-2366
- 10.13345/j.cjb.190277
-
文章历史
- Received: June 18, 2019
- Accepted: September 27, 2019
2. 四川大学 华西医院消化内科,四川 成都 610041
2. Department of Gastroenterology, West China Hospital, Sichuan University, Chengdu 610041, Sichuan, China
人体肠道内微生物具有丰富的多样性,种类包括了细菌、病毒、真菌和原生动物等[1]。其中仅细菌数量就高达100万亿个,因此肠道微生物也被称作人类的第二个基因组[2]。随着宏基因组测序与生物信息学的发展,越来越多的数据表明,肠道内微生物与宿主之间存在着密不可分的关系。宿主通过饮食等调节肠道内微生物群的平衡[3],肠道微生物从肠道内未分解的物质中获取所需能量,供其生长,同时也参与宿主体内的物质代谢、营养吸收、生理和免疫防御过程[4]。在此基础上,人类开启了人类微生物组计划,旨在揭示两者之间的关系[5]。伴随着研究的深入,人类健康和疾病与肠道微生物间的关系逐渐被探讨以及研究。合成生物学在此基础上利用基因合成、编辑以及调控等手段结合工程学来定向改造细菌,构建新的人工系统。合成生物学为肠道微生物改造提供“工具”,同时通过设计基因线路构建以肠道微生物为载体,靶向分泌表达治疗因子的工程菌,以及能够响应环境并且对其进行调控的“智能微生物”。改造后的肠道微生物也被称为工程菌,工程菌具有能够在肠道内定植、稳定持续给药等优点,近年来,在医学应用方面从实验室到临床阶段不断有新的成果涌现。但随之也出现菌株的安全性、工具的短缺以及基因线路的复杂性等问题。本文从肠道微生物与疾病之间的关系出发,阐述合成生物学工具构建以及基因回路设计,总结目前利用合成生物学设计和改造肠道微生物在疾病诊断与治疗中的应用,并对现有的问题加以讨论并且提出展望。
1 肠道微生物与疾病之间关系的研究现状系统生物学整合了基因组学、转录组学、蛋白组学以及代谢组学等高通量组学,把研究目标从单个分子或细胞转移至整个细胞或生物体内的基因调控以及整体与体外环境间相互作用和信号转导[6]。近年来,系统生物学的发展逐步揭示出肠道微生物与疾病之间的关系。研究表明,健康状态下我们的肠道菌群处于平衡状态,但当人体处于疾病状态时,如肠道炎症、代谢疾病甚至精神疾病等,肠道内菌群的组成也会改变。肠道微生物通过参与人体代谢影响健康,其自身能够帮助人类代谢人体内无法完全消化的纤维素[7],能够为宿主代谢提供酶,如肠道微生物中存在尿酸降解酶,可替代人体内缺乏的尿酸氧化酶,将尿酸代谢成二氧化碳和水,从而避免因尿酸升高导致的高尿酸血症[8],肠道微生物还通过合成宿主自身不能合成的维生素B和维生素K等为宿主提供营养,其产生的短链脂肪酸对人体也有诸多益处[9]。人们将这一类对人体有益的菌称为益生菌,目前大多数的工程菌都是基于益生菌进行改造的。
肠道微生物参与疾病治疗最初是直接通过粪便移植的方式来进行的,这种方法是将健康人群的肠道微生物菌群移植到非健康人群中来进行疾病治疗。最早记录利用粪便治疗肠炎和腹泻的方法是中国东晋时期的《肘后备急方》一书[10]。国外最早的记录是1958年,美国科罗拉多大学医学院外科医生Eiseman利用健康人群粪便治愈肠炎患者[11]。尽管利用健康患者粪便治疗疾病的方式出现已久,但由于粪便移植治疗不具有靶向性,并且其作用机制尚不明确,粪便中的病毒和杂菌较多,使得治疗效果因人而异,因此也限制了该治疗方式的推广[12-13]。
合成生物学利用天然或合成的生物元件来设计基因线路,从而实现程序化的细胞行为。它利用肠道微生物定植在肠道内与宿主相互作用这一特点,对微生物进行定向改造,为研究微生物群之间的结构-功能关系、设计新的生物疗法提供了新思路[14],同时也使微生物靶向治疗疾病成为可能。近年来,改造后的细菌已广泛地用于治疗肥胖、糖尿病和结肠炎等疾病的研究中[15-17]。除单纯的治疗外,科学家还将研究重点放到疾病的预防和诊断上。通过设计能够感知肠道微环境相关变化的回路,对人体内健康状态进行检测和诊断[18] (图 1)。

|
| 图 1 改造肠道微生物对疾病进行诊断与治疗(工程菌内基因回路由感应元件、基因元件以及报告元件三部分组成。在接收到体外信号后,通过体内基因回路计算操控工程菌的行为,表达报告因子用以诊断,治疗因子用以治疗,毒性因子用以杀死病原菌) Fig. 1 Engineering of Intestinal microbiota for the diagnosis and treatment of diseases. The circle in engineered bacteria is composed of sensors, gene circuits, and actuators. After receiving in vitro signals, by modulating the behavior of engineered bacteria through in vivo genetic loop calculation, the engineered bacteria would express reporters for diagnosis, therapeutics for diseases treatment, and toxics for killing cancer cells or pathogens. NAPEs: N-acylphosphatidylethanolamines; GLP-1: glucagon-like peptide; IL10: interleukin10; RBS: ribosome binding site; LacZ: LacZ; GFP: green fluorescent protein; CheZ: a motivity protein that drives the bacteria. |
| |
合成生物学为改造肠道微生物提供手段,一方面为处于开发初级阶段的微生物提供载体质粒和用于编辑改造的工具。另一方面结合电路学设计更为复杂的调控路径来改造和调控肠道微生物。
2.1 编辑工具肠道菌群种类丰富,但大部分还有待研究,因此需要构建合适的系统以及工具对其进行编辑和改造。目前大多数改造对象为益生菌,或者通过合成生物学构建“合成益生菌”。合成生物学的作用是提供工具,用于编辑微生物。每一种菌都含有特定的“工具箱”,用以在体内进行基因编辑。常见的肠道微生物有大肠杆菌、乳酸杆菌、拟杆菌、双歧杆菌以及枯草芽胞杆菌等,它们均已有较为成熟的编辑工具[14, 19]。大肠杆菌作为基础的底盘细胞,已经有大量的可供选择的操作系统用于基因的表达和编辑。乳酸杆菌中以pWV01、pSH71、pAMβ-1等穿梭质粒为主,可进行治疗因子的表达与调控[14, 20]。其他几种菌的研究目前都还处于起步阶段,拥有为数不多的操作系统。例如pNBU2质粒适用于拟杆菌[21],而双歧杆菌中应用较多的是大肠杆菌-双歧杆菌穿梭质粒,如pBV220以及pBBAD/Xs[20, 22],pDG1730质粒则应用于枯草芽胞杆菌[19]。基因的表达通过设计启动子实现,一方面可以通过设计不同强度的启动子库实现不同强度物质表达,另一方面则是设计诱导型启动子,利用单组分或双组分信号转导调节系统诱导下游基因的表达。最常见的就是乳酸杆菌中的NICE和sppIP/IP-673诱导系统[23-24],而大肠杆菌以化学物诱导为主[14]。
2.2 回路设计合成生物学能够对工具进行复杂的“组装”与利用。结合电路学,设计回路用以调控物质在微生物体内的表达。简单的线性回路设计单纯以分泌治疗物质为主,为使得物质的表达和分泌更为可控,工程菌体内的回路设计从原来的单一线路,被设计成能对肠道疾病信号作出反应的智能微生物,在其体内形成可调控的细胞信号转导网络[25]。工程微生物的信号网络主要由感应元件、计算元件以及报告元件3个模块组成。感应元件感应标记物并以此激活下游开关,目前应用的感应物有一氧化氮(Nitric oxide,NO)、海藻糖(Fucose)、硫代硫酸盐(Thiosulfate)、连四硫酸盐(Tetrathionate)、乳糖(Lactose)、无水四环素(Anhydrotetracycline,ATC)、阿拉伯半乳糖(Arabinogalactan)、异丙基硫代半乳糖苷(Isopropyl β-D-thiogalactoside,IPTG)和鼠李糖(Rhamnose)等[18]。逻辑线路在信号网络系统中充当计算元件,对接收的信号进行计算与处理,以控制细菌的行为。报告元件主要为下游蛋白的表达,包括治疗因子的分泌——疾病治疗、荧光蛋白的表达——疾病诊断以及驱动蛋白的表达——控制细菌行为等。Din等在合成路线中添加了正负反馈调节路线,使得细菌具有鲁棒振荡动力学特征。通过添加转录因子(LuxR)和N-酰基高丝氨酸内酯(AHL)调控噬菌体裂解基因的合成,当AHL达到一定阈值后,细菌裂解释放药物,细菌死亡数量下降,等待下一轮扩增裂解给药。这种振荡式的给药方式减轻了工程菌的负担[26]。在靶向性调控方面,科学家通过改造肠道微生物的趋向性,让菌能够在特定部位表达药物或细胞因子[27]。振荡器利用模块化的部件和基因电路对细菌进行编程设计,以精确控制治疗物质的响应、表达和传递,有实验表明综合对细胞振荡性给药以及趋向性调控行为两种手段对肠道微生物进行改造,改造后的肠道菌有80%能够靶向肿瘤细胞,并且分泌治疗性多肽[28-29]。疾病的检测信号弱会影响诊断结果,Bonnet实验室通过前期摸索,利用细菌的无限复制与表达从而扩大分子检测的敏感性,构建了用于检测疾病的全细胞传感器,为解决这一问题提供了新的思路[30]。除前面提到的感应元件,该实验室前期还探索出通过化学诱导二聚体(CID)系统来研究和控制生物系统的方法[31],为疾病检测提供新的入手点。
3 改造肠道微生物参与疾病的诊断微生物具有无限复制的特点,能够将检测到的信号无限放大,有利于增大疾病监测的敏感性。同时肠道微生物可以直接通过粪便提取,具有不会对人体造成二次伤害的优势。基于这两点优势,肠道微生物在疾病的检测与诊断中具有良好的应用前景。
3.1 炎症诊断炎症性肠病是一种慢性炎症肠道疾病,分为克罗恩病(Crohn’s disease,CD)和溃疡性结肠炎(Ulcerative Colitis,UC),临床症状为腹痛和腹胀。与腹泻和结肠炎的症状相似,需要用结肠镜取病理组织来对其进行检测,该病检测操作麻烦且对病人造成不适感。连四硫酸盐Tetrathionate是一类炎症的标志物,Kristina实验室建立了一种基于口服给药和流式细胞检测的方法无创检测结肠炎症(结肠炎)。该实验室在大肠杆菌Nissle1917 (Escherichia coli Nissle1917,EcN)中构建了硫代硫酸盐传感器,在结肠炎状态下,激活肠炎小鼠的硫代硫酸盐传感器,刺激下游表达荧光蛋白,取粪便样品对粪便进行流式细胞检测,通过荧光强度来反映肠道健康状态[32]。Pamela实验室前期构建了能够在肠道内感知、记忆并作出相应报告的大肠杆菌遗传记忆系统,该系统由触发元件和记忆元件两部分组成。触发元件的λCro基因受四环素诱导型启动子调控,而记忆元件则来源于噬菌体中的cI/Cro区域,在四环素的调控下,使细胞处于不同状态[33],从而记录肠道疾病状态。在此基础上,作者还将该系统应用于改造E. coli strain PAS638,使其可以感应肠道内连四硫酸盐,并检测体内炎症状态[25]。但是目前可供检测的信号分子仍然有限,Bonnet实验室设计的新受体,通过单结构域抗体的二聚化来激活开关,该实验室证明了单域VHH抗体与咖啡因结合后会形成二聚体,导致转录抑制因子LexA的单体DNA结合域(DBD)被激活,通过表达荧光蛋白来反映激活状态[34-35]。通过抗体或者特定设计配体蛋白这一方法为生物感应器提供了新的方向。
3.2 霍乱检测与诊断霍乱由胃肠道感染霍乱弧菌引起,情况严重可致死。Holowko等将霍乱弧菌中用于感应自身群体的蛋白异源表达至大肠杆菌中,同时通过计算器模拟其体内行为系统,从而达到诊断霍乱的目的[36]。该系统也成功应用于抑制肠道内其他有害菌(如沙门氏菌)的生长[37]。Mao等设计了一株乳酸乳杆菌,在检测肠道中霍乱弧菌的群体感应信号的同时,触发报告基因的表达,使其更易在粪便样本中检测,方便了疾病的监测[38]。改造益生菌还被用于干扰细菌间的通讯以抑制霍乱弧菌的毒性[39],从而预防霍乱。
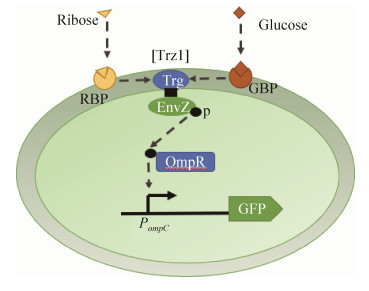
3.3 癌症癌细胞生长和繁殖速度很快,需要大量的葡萄糖提供能量。葡萄糖通过影响肿瘤的生长及代谢从而影响肿瘤的趋向性[40]。葡萄糖的浓度代表肿瘤的生存能力。癌细胞选择有氧糖酵解作为葡萄糖代谢的主要方式[41]。Panteli等设计了一个葡萄糖敏感细菌,细菌体内含Trz1葡萄糖受体,该受体与肿瘤微环境中的糖结合时,触发体内绿色荧光蛋白(Green fluorescent protein,GFP)的表达[42](图 2),利用荧光信号来实时反映出肿瘤的生存能力。该实验将Trz1受体的葡萄糖传感能力与细菌的肿瘤靶向能力结合起来,进一步还可以通过不同的局部微环境将重组蛋白药物直接递送到肿瘤的不同区域,达到靶向治疗的效果。Bonnet等利用类似原理检测到了糖尿病人尿液中的糖类成分,用于糖尿病检测[30]。细菌具有定植于肿瘤的倾向性,Bhatia实验室开发了一种利用细菌对肝转移进行检测的改造肠道微生物;他们证实了构建的微生物EcN能够在肿瘤内富集,其细菌浓度远远大于正常细胞,改造后的EcN在肿瘤细胞定植后,会大量表达LacZ蛋白,该蛋白可将一个底物切割成可以在尿液中检测到的小分子,从而达到无创检测肿瘤的目的[43]。
|
| 图 2 改造菌检测实体肿瘤细胞环境中葡萄糖浓度的空间分布情况(融合受体蛋白Trz1结合趋化受体Trg的核糖/葡萄糖/半乳糖的识别域和信号转导域EnvZ,将糖转化为光信号输出。核糖和葡萄糖分子在膜内分别与核糖结合蛋白(Ribose binding protein,RBP)或葡萄糖/半乳糖结合蛋白(Galactose-binding protein,GBP)结合。在结合配体与Trz1间的物质相互作用,导致Trz1内膜(Inner membrane,IM)构象的改变,使磷转移到细胞质渗透孔蛋白调节剂(Osmoporin regulator,OmpR)中,磷酸化的OmpR作为转录激活子作用于启动子POmpC,从而激活绿色荧光蛋白(GFP)的表达) Fig. 2 Spatial distribution detection of glucose concentration in solid tumor using engineered bacteria. The Trz1 fusion receptor combines the ribose/glucose/galactose sensing domain of Trg chemotaxis receptor and the signal transduction domain of EnvZ osmoporin, which transduce sugar into fluorescence signal. Ribose and glucose molecules bind to the ribose binding protein (RBP) and the glucose/galactose binding protein (GBP), respectively, in the membrane. The ligand-binding protein complex then interacts with the periplasmic portion of the Trz1 receptor, which causes conformational change in Trz1 across the inner-membrane (IM) and phosphorus transfer to cytoplasmic osmoporin regulator, OmpR. The phosphorylated OmpR then acts as a transcriptional activator on the osmoporin promoter, PompC, to activate the expression of the green fluorescent protein (GFP). |
| |
合成生物学还能够为疾病的检测提供记录装置。哥伦比亚大学Wang实验室结合CRISPR/Cas编辑技术,通过CRISPR在不同时间段的spacer插入位点来记录这些细菌与肠道内环境发生相互作用的时间[44]。这一系统可用来监测炎症、感染以及癌症等疾病的发展,同时可提高疾病检测的时效性。在检测技术的改进方面,卢冠达实验室通过结合传感器构建了“细菌药丸”,细菌在检测到胃部出血时会发出荧光信号,传感器接受到荧光信号后,通过无线信号传输到电子设备上,从而实现在不取出粪便的情况下实时检测胃肠道内的疾病[45],极大地降低了疾病检测的难度。这些工具也为后续研究利用肠道微生物对疾病检测和诊断的技术奠定了基础。
4 改造肠道微生物参与疾病的治疗改造肠道微生物不仅能够对疾病进行诊断,也能够参与疾病治疗。改造肠道微生物参与疾病治疗的研究主要从两个方面入手,一方面通过细胞分泌治疗因子用于靶向疾病组织发挥作用或者参与宿主代谢,另一方面通过运输将宿主内毒性因子运送至工程菌体内将其代谢成无毒物质。目前参与的疾病研究包括全身性代谢疾病(肥胖和糖尿病等)、炎症、细菌性感染以及免疫类疾病等。
4.1 慢性全身性代谢性疾病肥胖和糖尿病之间存在密切关系,过度肥胖还会引起2型糖尿病以及心血管疾病。N-acylethanolamides (NAEs)是一类进食时,由小肠中脂质衍生的厌食性信号分子,N-acylphosphatidylethanolamines (NAPEs)是它的前体物质。Sean实验室分别在2014年和2018年证明将表达NAPEs的工程菌EcN添加到高脂肪饮食小鼠的饮用水中,肥胖小鼠模型的体重增加明显受到抑制[46-47]。后期的实验结果表明,Sean实验室构建的工程EcN还有降低肝脏炎症和缓解纤维化早期症状的作用。胰高血糖素样肽-1 (GLP-1)是由肠细胞产生的肠促胰岛素激素,在葡萄糖存在下刺激肠上皮细胞转化为胰岛素分泌细胞,从而促进胰岛素分泌。研究证明,改造乳酸菌分泌GLP-1,将肠道细胞重新编程为葡萄糖诱导性胰岛素分泌细胞可以改善糖尿病大鼠模型中的高血糖症状[48]。改造干酪乳杆菌分泌GLP-1还能使大鼠中血清低密度脂蛋白胆固醇、甘油三酯和富含甘油三酯的脂蛋白胆固醇显著降低[49]。这些数据表明,微生物分泌重组促胰岛素能够有效缓解胆固醇代谢和饮食引起的血脂异常以及胰岛素敏感性代谢功能障碍。组织中积累的吡咯并喹啉醌(Pyrroloquinoline quinone,PQQ)可防止肝脏和全身氧化损伤,联合短链脂肪酸(Short chain fatty acid,SCFAs)可以降低高脂血症[50]。PQQ的浓度随着SCFAs而变化。果糖脱氢酶(Fructose dehydrogenase,fdh)能够将果糖转化为5-酮-d-果糖,甘露醇-2-脱氢酶(Mannitol-2- dehydrogenase,mtlK)可以将果糖转化为甘露醇。Chaudhari等构建可以表达mtlK和fdh酶的工程EcN,发现小鼠体内的SCFAs和PQQ均出现上升,体重和血液内葡萄糖浓度均有所降低。该方法提高了PQQ和SCFAs的产量,既能改善肥胖症状又可以辅助增强二甲双胍的血糖控制和胃肠的耐受性[51]。
4.2 炎症性疾病IL-12、IL-10、IL-27、IL35等是常见用于抑制炎症的因子。改造肠道微生物,使其分泌抗炎因子是常见的肠道微生物改造方式之一。Kotula等利用重组乳酸乳球菌Lactococcus lactis在小鼠模型[28]中治疗IBD。在该研究中,使用表达重组IL-10的L. lactis灌胃IL-10 (-/-)小鼠可以成功预防该小鼠的结肠炎,使结肠炎的发生率降低了50%。Miyoshi实验室在前期构建了在乳酸乳球菌中分泌和表达IL-10的两种质粒系统,第一个系统基于应激诱导控制表达系统(SICE),用于在粘膜表面产生和传递蛋白,第二个系统则是改造菌株使菌株分泌纤连蛋白结合蛋白A (Fibronectin binding protein A,FnBPA),该蛋白能使菌株具有黏附功能,通过黏附细胞直接向宿主细胞传递IL-10的cDNA盒,该盒位于真核DNA表达载体(pValac)中[52-53]。除乳酸乳球菌外,双歧杆菌也用作安全载体分泌IL-10,用以治疗小鼠结肠炎[22]。类似的各种炎症因子也相继被应用到工程菌中,Hanson等改造乳酸菌在肠道内粘膜层分泌IL-27减轻结肠炎[54]。双歧杆菌作为IL-12的口服载体也用于研究治疗Balb/c小鼠心肌炎[55]。乳酸乳球菌工程菌L. lactis-IL35分泌IL-35也能有效降低慢性阻塞性肺疾病的发生率和严重程度[56],NO是克罗恩病的肠道内标志物,Bentley通过设计运动回路以及添加驱动蛋白来改造益生菌EcN,改造后的EcN能够在NO升高处富集并表达粒细胞巨噬细胞集落刺激因子(Macrophage colony stimulating factor,GMCSF)。GMCSF首先进入胞浆,然后通过TolAⅢ形成的孔释放到细胞外。激活成熟粒细胞及单核巨噬细胞从而提高抗感染和免疫功能,缓解克罗恩病炎症症状[57] (图 3),该实验方法也为改造肠道微生物的临床设计做了铺垫。

|
| 图 3 检测肠道炎症标志物一氧化氮NO用于治疗炎症性肠病IBD (NO存在下,CheZ蛋白表达驱动非运动型细胞定向移动,T7pol蛋白表达诱导T7启动子,激活下游粒细胞巨噬细胞集落刺激因子GMCSF和成孔蛋白TolAⅢ的表达,TolAⅢ在细胞膜表面形成孔,帮助GMCSF排出至细胞外行使功能) Fig. 3 Detection of intestinal inflammatory marker NO for the IBD treatment. In the presence of NO, CheZ protein drives the directional movement of non-sporty cells. T7pol protein induces the T7 promoter, thereby activating the expression of downstream GMCSF and pore forming protein TolAⅢ. TolAⅢ forms pores on the surface of the cell membrane and helps GMCSF move to extracellular regions. |
| |
志贺氏菌病是一种急性肠侵袭性疾病,全世界有数百万人感染。以乳酸乳球菌作为疫苗载体,传递保守的抗原蛋白粘膜外膜蛋白A (OmpA),能够有效诱导全身以及粘膜免疫[58]。Paton等设计了可与毒素结合的益生菌以预防和治疗肠毒素大肠杆菌引起的腹泻[59]。类似的益生菌也可与肠道受体竞争,结合霍乱毒素从而预防感染霍乱[60]。利用细菌的群体感应分子使工程化细菌识别并抑制致病菌是目前的研究热点。绿脓杆菌是常见的条件致病菌,Gupta和Hwang先后改造Escherichia coli用以消灭绿脓杆菌。前者在大肠杆菌中设计了一个感应绿脓杆菌分泌AHL蛋白的回路,当该蛋白浓度达到一定量时即可启动工程菌表达Copy蛋白从而抑制绿脓杆菌的生长。后者在此基础上添加了动力蛋白CheZ,使得大肠杆菌在感应到AHL蛋白过量表达时便迁移至绿脓杆菌附近,杀死绿脓杆菌[61-62]。Jayaraman等改造工程化大肠杆菌的群体感应分子CAI-1,使其特异性地检测霍乱弧菌,并通过表达裂解蛋白YebF-Art-085进行响应,从而自我裂解以释放杀伤蛋白Art-085达到杀死霍乱弧菌的目的[63]。
4.4 免疫类疾病 4.4.1 HIVCyanovirin-N (CV-N)是一种独特的氨基酸蛋白,在低纳摩尔浓度下可杀灭艾滋病毒HIV-1和HIV-2、猿猴免疫缺陷病毒和猫免疫缺陷病毒,其对物理化学降解具有极强的抵抗力。尽管CV-N灭活HIV的确切机制尚未完全阐明,但已有研究证实CV-N可以干扰病毒与其进入细胞必需的靶细胞受体间相互作用。在链球菌Streptococcus中表达强效的HIV灭活蛋白CV-N并分泌于菌体表面,作为一种可能的途径局部递送CV-N,可有效捕获HIV病毒粒子以防止其性传播[64]。高度定植的大肠杆菌菌株EcN,可用以表达HIV-gp41-溶血素A,这种杂合肽可以阻断HIV与靶细胞融合并进入靶细胞,发挥抗HIV作用[65]。
4.4.2 癌症肿瘤的异质性使癌症难以治疗,许多小分子癌症药物能够靶向肿瘤周围并快速裂解细胞,但难以深入肿瘤区域。在前期检测的基础上,Adachi等开发出可特异性分泌药物或抗性因子的微生物载体,乳酸菌工程菌GLBL101c表达分泌的HPV16-E7能够引起宫颈瘤CIN3的消退,通过将点突变插入核糖体结合位点以修饰HPV16衍生的E7基因,构建表达分泌该基因的IGMKK16E7菌株,强化了E7的表达[66-67]。内皮抑素基因是一种抗血管生成基因,南京大学Li等在益生菌中构建了将内皮抑素基因转运至实体瘤的递送系统,仅在肿瘤部位表达该基因,成功抑制了肿瘤的生长,同时将肿瘤相关抗原递送至抗原呈递细胞以引发抗肿瘤免疫[68]。Anderson等为细菌配备群体感应(Qs)开关,只有当细菌数量达到阈值密度时才会激活效应基因表达,达到在肿瘤部位进行治疗的目的[69]。由于工程益生菌的易操控性以及低毒性,许多研究将其作为传递小分子物质的载体[70-71],再加上其特殊的定植能力,常被用作后续抗肿瘤药物的载体[72]。
5 现状与发展以上所有研究表明,随着对宿主-肠道微生物之间的相互作用机制以及合成生物基因电路设计的不断深入理解,有望集成各种方法以系统、精确的方式发展工程微生物疗法。可操作菌株以及设计工具的发展将使我们能够针对更多人类疾病作出相应调整,本文将目前参与到疾病诊断与治疗中的改造肠道微生物作了一个总结,如表 1所示。
| Strains | Applications | Purposes | Mechanism | References | |
| E. coli Niss-le 1917 (EcN) | Obesity | T | Expressing NAPE, fructose dehydrogenase (fdh) | [46, 51, 86] | |
| EcN | Phenylketonuria | T | Degrading Phe in serum | [74] | |
| EcN | Hyperammonemia | T | Producing l-arg and consumed NH3 in an in vitro system | [75] | |
| EcN | human Immunodeficiency virus (HIV) | T | Secreting an HIV fusion inhibitor peptide | [65] | |
| EcN | Cancer | D | Generating a high-contrast urine signal | [43] | |
| EcN | Diabetes | D | A genetic switch to detect pathological biomarkers | [30] | |
| EcN | Inflammatory | D | Thiosulfate and tetrathionate sensor | [65] | |
| EcN | ethanol-induced Oxidative damage & hyperlipidemia | T | Secreting pyrroloquinoline quinone (PQQ) | [50] | |
| EcN | Cholerae | T | Expressing the autoinducer-molecule cholera autoinducer 1 (CAI-1) | [39] | |
| EcN | Infection-Salmonella | T | Expressing and secreting the antimicrobial peptide, Microcin J25 | [87] | |
| EcN | Nutritions | P | Expressing eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), | [88] | |
| E. coli | Cholerae | T | Inhibiting virulence of Vibrio cholerae | [39] | |
| E. coli | Inflammatory bowel disease (IBD) | D & T | Co-expressing the pore forming protein TolAIII with the biologic, granulocyte macrophage-colony stimulating factor (GM-CSF), and recording an inflammatory response | [57] | |
| E. coli strain JE3513 | Infection-Pseudomonas aeruginosa | D & T | Sensing and killing pathogen | [61-62] | |
| Lb. casei | HIV | T | Secreting single-chain variable fragment (scFv) and other HIV-1 antigen | [89] | |
| Lb. casei | Infection | T | Displaying albumin-binding domain variants against Shiga toxin 1 B subunit | [90] | |
| Lb. casei | IBD | T | Expressing superoxide dismutase (SOD) | [91] | |
| Lb. plantarum | Immune | T | Expressing the highly immunogenic tetanus toxin C-terminal Fragment (TTFC) as antigen | [92] | |
| Lb. plantarum | Respiratory allergies | T | Expressing a major Japanese cedar pollen allergen Cry j 1 suppressed nasal clinical symptoms | [93] | |
| Lb. plantarum | Inflammation | T | Expressing interleukin 10 (IL-10) | [94] | |
| Lactobacillus | Infection-s. pneumoniae | T | Producing the intracellular antigen PspA | [95] | |
| Lactococcus lactis | Immune | T | Expressing rotavirus VP8 | [96] | |
| L. lactis | Inflammations | T | Expressing IL2, IL6 | [97] | |
| L. lactis | Immune | T | Expressing BLG protein—cow β-lactoglobulin | [98] | |
| L. lactis | Avian flu | T | Expressing chicken 2 (chIL-2) together with avian influenza hemagglutinin (H5) | [98-99] | |
| L. lactis | Infection-Leishmania major | T | Expressing the protective Leishmania antigen, LACK (LAC) and IL-12. | [100-101] | |
| L. lactis | Diarrhea | T | Expressing dendritic cell-Targeting peptide fused with porcine epidemic diarrhea virus (PEDV) COE antigen | [102] | |
| L. lactis | Infection-Clostridium perfringens | T | Expressing the toxoid of C. perfringens α-toxin | [103] | |
| L. lactis | IBD | T | Expressing anti-tumor necrosis factor α (TNFα) antibodies, IL-17, IL-23,IL12 and IL-10 | [104] | |
| L. lactis | IBD | T | Binding different chemokines and neutralize CXCL8 production | [105] | |
| L. lactis | IBD | T | Secreting biologically active heme oxygenase-1 (HO-1) | [106] | |
| L. lactis | Diabetes type Ⅰ | T | Expressing fusion protein HSP65-6P277 or glutamic acid decarboxylase or tyrosine phosphatase-like protein | [107-109] | |
| L. lactis | Cancer | T | Producing catalase | [110] | |
| L. lactis | Cancer | T | Expressing human papillomavirus 16 antigen E7 (HPV16 E7) | [111] | |
| L. lactis | Cancer | D | Expressing attachment to breast cancer MDAMB232 cells | [112] | |
| L. lactis | Infection | D & T | Expressing anti-enterococcal peptides | [113] | |
| L. lactis | Cholesterol | T | Overexpressing bile salt hydrolase | [114] | |
| L. lactis | Glucose tolerance | T | Expressing glucagon-like peptide-1 (GLP-1), insulin | [115-116] | |
| L. acidophilus | Influenza | T | Expressing the highly pathogenic avian influenza virus protein hemagglutinin (HA)-1 | [117] | |
| L. paracasei | Diabetes type Ⅰ | T | Expressing GLP-2 | [115] | |
| Bifidobacterium longum | Cancer | T | Production of Tumstatin, inhibiting proliferation & inducing apoptosis of tumorous vascular endothelial cells | [118] | |
| Bifidobacterium longum | Cancer | T | Production of an enzyme to convert pro-drug 5-fluorocytosine to the toxic 5-FU within tumors | [119] | |
| Bifidobacterium longum | Infection | T | Displaying Salmonella-antigen | [118] | |
| Bifidobacterium longum | Ulcerative colitis(UC) | T | Expressing alpha-melanocyte-stimulating hormone, manganese superoxide dismutase (rhMnSOD) | [120-121] | |
| Bacillus subtilis | Infection-Helicobacterpylori | T | Display of H. pylori urease B protein on spore coat | [122] | |
| Bacillus subtilis | IBD | T | Producing 4, 4ʹ-diaponeurosporene | [123] | |
| T: therapies; D: diagnostics; P: prophylaxis. | |||||
研究发现肠道微生物在人体健康中占据着重要的位置,各个国家也相继开发出用肠道微生物(益生菌)来进行疾病治疗的方案。Rebiotix公司利用粪便移植来治疗艰难梭状菌感染。加拿大政府批准了一种治疗住院病人艰难梭菌感染的益生菌专利配方[73]。这都为微生物制剂的市场应用打开了大门。许多利用微生物组治疗疾病的方案都已进入临床阶段。Assembly公司是发现和开发口服活微生物生物治疗产品的临床阶段领导者,利用肠道细菌的自然进化功能,在疾病治疗领域提供临床效益。该公司开发的ABI-M201由一组确定的肠道共生菌群组成,用于治疗溃疡性结肠炎,目前在临床一期阶段。相较于肠道微生物组,利用合成生物学改造肠道微生物治疗疾病才起步不久,Synlogic是较早将合成生物学利用到微生物中,使其行使治疗功能的公司。该公司设计改造的SYNB1020可用于治疗由肝硬化和尿素循环障碍(UCD)引起的高血氨症,此项研究目前处于1b/2a期临床试验。用于治疗苯丙酮尿症的SYN1618现已获得美国食品与药物管理局(FDA)治疗苯丙酮尿症(PKU)的快速通道[74-75]。Synlogic公司还与Abbvie公司合作,准备研发一系列治疗肠道炎症等的工程菌。Intrexon的ActoBio系列药物通过对乳酸菌进行改造用于治疗糖尿病以及口腔疾病,改造菌株如表 2所示。同时ActoBio也将研究目标放在代谢疾病以及肠道炎症疾病的治疗上。除以上两家公司,陆续也有公司尝试利用合成生物学改造肠道微生物来治疗疾病。Ernest Pharmaceuticals公司着重于改造能够在肿瘤中定植的沙门氏菌,用于靶向治疗癌症。Trayer Biotherapeutics公司通过改造乳酸菌,使其能够治疗苯丙氨酸代谢紊乱。ActoGenix与Intrexon合作通过改造乳酸链球菌使其生产出一种或多种治疗性的肽和蛋白质[76]。Blue Turtle Bio公司通过改造细菌来治疗癌症[77]。总体来说,利用合成生物学制造具有治疗疾病效果的菌株药物目前在医学领域初露锋芒[78],吸引着越来越多的公司加入其中。
| Strains | Applications | Clinic phase | Companies |
| EcN | Hyperammonemia-Urea cycle disorders | PhaseⅠ/Ⅱ | Synlogic |
| EcN | PKU | PhaseⅠ | Synlogic |
| L. lactis | Type I diabetes | PhaseⅠb/Ⅱa | Intrexon |
| L. lactis | Oral mucositis | PhaseⅠclinical trial | Intrexon |
| Salmonella | Liver cancer | PhaseⅠ | Ernest Pharmaceuticals |
| Salmonella | Breast cancer | PhaseⅠ | Ernest Pharmaceuticals |
癫痫、自闭症、抑郁症等与脑神经相关的疾病也与肠道微生物相关,而合成生物学的快速发展也推动了疾病与肠道微生物之间具体关系的探究[79-81]。但是微生物与肠道内环境之间相互作用机制尚不明确,阻碍了工程菌的推广与应用。由于应激源和病原体的反应复杂,合成生物系统启动的反应必须考虑这种复杂性和细微差别,才能发挥作用,因此需要开发对刺激作出多方面反应的系统。Wang等将基因电路作为研究重点,通过设计组装不同的逻辑门元件形成基因逻辑门电路,从而使合成生物能够根据多种输入组合而非单一输入启动生物编程[82-83]。然而许多肠道菌目前还缺乏成熟有效的编辑工具。面对更复杂的治疗系统时,还须考虑工程菌的稳定性和安全性。改造后的微生物具有较大的环境压力,负担体内合成线路运作的同时还需同肠道内原生菌株竞争营养,“抢夺”生态地位,以保证改造微生物在肠道内的治疗时间[84]。此外,工程菌达到治疗效果后,如何从体内及环境中去除也是亟待解决的问题。改造后的菌株仍属于活菌,可能引起环境中的基因污染或转移的安全性问题。针对这一问题,Synlogic公司开发的工程菌只能存在于低氧环境,且依赖于胸苷营养物而生存,一旦该物质浓度较低,工程菌便会自动死亡,此方法提高了工程菌的安全性[75]。Chan等设计的“Deadman”和“Passcode”杀毒开关也采用了类似方法,这一方法需要输入一个或连续输入多个分子配体维持对毒素的抑制,但其序列的不稳定性也容易导致自毁序列的过早激活[85]。由于肠道微生物的特殊性,还需开发更稳定的合成工具用以解决菌株及其工具的安全性问题。
7 展望合成生物学和肠道微生物都是近年来快速发展的领域,肠道微生物治疗疾病具有定向给药、可复制、操作简单灵活等优点,合成生物学也可以为遗传学家、生态学家、计算生物学家和临床医生提供强大的“工具箱”,为实现微生物疗法提供多样化的工具和技术手段。合成生物学与肠道微生物的结合,为人类的疾病治疗开创了新思路。随着科学技术的发展,合成生物学有望形成一套系统性、强调控性的细菌控制回路,通过精准的设计和模块的构建使肠道微生物改造朝着更具有靶向性、安全性的方向前进。
| [1] |
Joukar F, Mavaddati S, Mansour-Ghanaei F, et al. Gut microbiota as a positive potential therapeutic factor in carcinogenesis:an overview of microbiota-targeted therapy. J Gastrointest Cancer, 2019. DOI:10.1007/s12029-019-00237-6 |
| [2] |
Angelucci F, Cechova K, Amlerova J, et al. Antibiotics, gut microbiota, and Alzheimer's disease. J Neuroinflamm, 2019, 16(1): e13005. |
| [3] |
Walker AW, Ince J, Duncan SH, et al. Dominant and diet-responsive groups of bacteria within the human colonic microbiota. ISME J, 2011, 5(2): 220-230. DOI:10.1038/ismej.2010.118 |
| [4] |
Elzinga J, van der Oost J, de Vos WM, et al. The use of defined microbial communities to model host-microbe interactions in the human gut. Microbiol Mol Biol Rev, 2019, 83(2): 00054-18. |
| [5] |
人类微生物组计划[EB/OL].[2019-06-21]. https://baike.baidu.com/item/%E4%BA%BA%E7%B1%BB%E5%BE%AE%E7%94%9F%E7%89%A9%E7%BB%84%E8%AE%A1%E5%88%92/10745945?fr=aladdin.
|
| [6] |
Ju JQ, Wei P. Signaling network-based functional cell design. Chin J Biotech, 2017, 33(3): 386-392 (in Chinese). 鞠见齐, 魏平. 基于信号网络的功能细胞设计. 生物工程学报, 2017, 33(3): 386-392. |
| [7] |
Gibson GR, Roberfroid MB. Dietary modulation of the human colonic microbiota:introducing the concept of prebiotics. J Nutr, 1995, 125(6): 1401-1412. DOI:10.1093/jn/125.6.1401 |
| [8] |
Poehlein A, Solano JDM, Bengelsdorf FR, et al. Draft genome sequence of purine-degrading Clostridium cylindrosporum HC-1 (DSM 605). Genome Announc, 2015, 3(4): e00917-15. |
| [9] |
Jandhyala SM, Talukdar R, Subramanyam C, et al. Role of the normal gut microbiota. World J Gastroenterol, 2015, 21(29): 8787-8803. DOI:10.3748/wjg.v21.i29.8787 |
| [10] |
Zhang FM, Luo WS, Shi Y, et al. Should we standardize the 1, 700-year-old fecal microbiota transplantation?. Am J Gastroenterol, 2012, 107(11): 1755. |
| [11] |
Eiseman B, Silen W, Bascom GS, et al. Fecal enema as an adjunct in the treatment of pseudomembranous enterocolitis. Surgery, 1958, 44(5): 854-859. |
| [12] |
Kelly CR, Kahn S, Kashyap P, et al. Update on fecal microbiota transplantation 2015:indications, methodologies, mechanisms, and outlook. Gastroenterology, 2015, 149(1): 223-237. DOI:10.1053/j.gastro.2015.05.008 |
| [13] |
Imdad A, Nicholson MR, Tanner-Smith EE, et al. Fecal transplantation for treatment of inflammatory bowel disease. Cochrane Database Syst Rev, 2018, 11: CD012774. |
| [14] |
Bober JR, Beisel CL, Nair NU. Synthetic biology approaches to engineer probiotics and members of the human microbiota for biomedical applications. Annu Rev Biomed Eng, 2018, 20: 277-300. DOI:10.1146/annurev-bioeng-062117-121019 |
| [15] |
Steidler L, Hans W, Schotte L, et al. Treatment of murine colitis by Lactococcus lactis secreting interleukin-10. Science, 2000, 289(5483): 1352-1355. DOI:10.1126/science.289.5483.1352 |
| [16] |
Duan FP, Curtis KL, March JC. Secretion of insulinotropic proteins by commensal bacteria:rewiring the gut to treat diabetes. Appl Environ Microbiol, 2008, 74(23): 7437-7438. DOI:10.1128/AEM.01019-08 |
| [17] |
Ganjayi MS, Balaji M, Sreenivasulu D, et al. Recent Developments in the Prevention of Obesity by Using Microorganisms//Buddolla A, ed. Recent Developments in Applied Microbiology and Biochemistry. New York: Elsevier Inc., 2019: 47-60.
|
| [18] |
Landry BP, Tabor JJ. Engineering diagnostic and therapeutic gut bacteria. Microbiol Spectr, 2017, 5(5), doi: 10.1128/microbiolspec.BAD-0020-2017.
|
| [19] |
Guiziou S, Sauveplane V, Chang HJ, et al. A part toolbox to tune genetic expression in Bacillus subtilis. Nucleic Acids Res, 2016, 44(15): 7495-7508. |
| [20] |
Li X, Fu GF, Fan YR, et al. Bifidobacterium adolescentis as a delivery system of endostatin for cancer gene therapy:selective inhibitor of angiogenesis and hypoxic tumor growth. Cancer Gene Ther, 2003, 10(2): 105-111. DOI:10.1038/sj.cgt.7700530 |
| [21] |
Mimee M, Tucker AC, Voigt CA, et al. Programming a human commensal bacterium, Bacteroides thetaiotaomicron, to sense and respond to stimuli in the murine gut microbiota. Cell Syst, 2015, 1(1): 52-71. |
| [22] |
Yao J, Wang JY, Lai MG, et al. Treatment of mice with dextran sulfate sodium-induced colitis with human interleukin 10 secreted by transformed Bifidobacterium longum. Mol Pharmaceutics, 2011, 8(2): 488-497. DOI:10.1021/mp100331r |
| [23] |
Topp S, Reynoso CMK, Seeliger JC, et al. Synthetic riboswitches that induce gene expression in diverse bacterial species. Appl Environ Microbiol, 2010, 76(23): 7881-7884. DOI:10.1128/AEM.01537-10 |
| [24] |
Mierau I, Kleerebezem M. 10 years of the nisin-controlled gene expression system (NICE) in Lactococcus lactis. Appl Microbiol Biotechnol, 2005, 68(6): 705-717. DOI:10.1007/s00253-005-0107-6 |
| [25] |
Riglar DT, Giessen TW, Baym M, et al. Engineered bacteria can function in the mammalian gut long-term as live diagnostics of inflammation. Nat Biotechnol, 2017, 35(7): 653-658. DOI:10.1038/nbt.3879 |
| [26] |
Din MO, Danino T, Prindle A, et al. Synchronized cycles of bacterial lysis for in vivo delivery. Nature, 2016, 536(7614): 81-85. DOI:10.1038/nature18930 |
| [27] |
Park JS, Rhau B, Hermann A, et al. Synthetic control of mammalian-cell motility by engineering chemotaxis to an orthogonal bioinert chemical signal. Proc Natl Acad Sci USA, 2014, 111(16): 5896-5901. DOI:10.1073/pnas.1402087111 |
| [28] |
Huh JH, Kittleson JT, Arkin AP, et al. Modular design of a synthetic payload delivery device. ACS Synth Biol, 2013, 2(8): 418-424. DOI:10.1021/sb300107h |
| [29] |
Reeves AZ, Spears WE, Du J, et al. Engineering Escherichia coli into a protein delivery system for mammalian cells. ACS Synth Biol, 2015, 4(5): 644-654. DOI:10.1021/acssynbio.5b00002 |
| [30] |
Courbet A, Endy D, Renard E, et al. Detection of pathological biomarkers in human clinical samples via amplifying genetic switches and logic gates. Sci Trans Med, 2015, 7(289): 289ra83. DOI:10.1126/scitranslmed.aaa3601 |
| [31] |
Lesne J, Chang HJ, de Visch A, et al. Structural basis for chemically-induced homodimerization of a single domain antibody. Sci Rep, 2019, 9: 1840. DOI:10.1038/s41598-019-38752-y |
| [32] |
Daeffler KN, Galley JD, Sheth RU, et al. Engineering bacterial thiosulfate and tetrathionate sensors for detecting gut inflammation. Mol Syst Biol, 2017, 13(4): 923. |
| [33] |
Kotula JW, Kerns SJ, Shaket LA, et al. Programmable bacteria detect and record an environmental signal in the mammalian gut. Proc Natl Acad Sci USA, 2014, 111(13): 4838-4843. DOI:10.1073/pnas.1321321111 |
| [34] |
Chang HJ, Mayonove P, Zavala A, et al. A modular receptor platform to expand the sensing repertoire of bacteria. ACS Synth Biol, 2018, 7(1): 166-175. |
| [35] |
Bazin I, Tria SA, Hayat A, et al. New biorecognition molecules in biosensors for the detection of toxins. Biosens Bioelectron, 2017, 87: 285-298. DOI:10.1016/j.bios.2016.06.083 |
| [36] |
Holowko MB, Wang HJ, Jayaraman P, et al. Biosensing Vibrio cholerae with genetically engineered Escherichia coli. ACS Synth Biol, 2016, 5(11): 1275-1283. DOI:10.1021/acssynbio.6b00079 |
| [37] |
Palmer JD, Piattelli E, McCormick BA, et al. Engineered probiotic for the inhibition of Salmonella via tetrathionate-induced production of microcin H47. ACS Infect Dis, 2018, 4(1): 39-45. DOI:10.1021/acsinfecdis.7b00114 |
| [38] |
Mao N, Cubillos-Ruiz A, Cameron DE, et al. Probiotic strains detect and suppress cholera in mice. Sci Transl Med, 2018, 10(445): eaao2586. DOI:10.1126/scitranslmed.aao2586 |
| [39] |
Duan FP, March JC. Engineered bacterial communication prevents Vibrio cholerae virulence in an infant mouse model. Proc Natl Acad Sci USA, 2010, 107(25): 11260-11264. DOI:10.1073/pnas.1001294107 |
| [40] |
Tubiana M, Pejovic MH, Koscielny S, et al. Growth rate, kinetics of tumor cell proliferation and long-term outcome in human breast cancer. Int J Cancer, 1989, 44(1): 17-22. |
| [41] |
Jiang B. Aerobic glycolysis and high level of lactate in cancer metabolism and microenvironment. Genes Dis, 2017, 4(1): 25-27. |
| [42] |
Panteli JT, Forbes NS. Engineered bacteria detect spatial profiles in glucose concentration within solid tumor cell masses. Biotechnol Bioeng, 2016, 113(11): 2474-2484. DOI:10.1002/bit.26006 |
| [43] |
Danino T, Prindle A, Kwong GA, et al. Programmable probiotics for detection of cancer in urine. Sci Transl Med, 2015, 7(289): 289ra84. DOI:10.1126/scitranslmed.aaa3519 |
| [44] |
Sheth RU, Yim SS, Wu FL, et al. Multiplex recording of cellular events over time on CRISPR biological tape. Science, 2017, 358(6369): 1457-1461. DOI:10.1126/science.aao0958 |
| [45] |
Mimee M, Nadeau P, Hayward A, et al. An ingestible bacterial-electronic system to monitor gastrointestinal health. Science, 2018, 360(6391): 915-918. DOI:10.1126/science.aas9315 |
| [46] |
Chen ZY, Guo LL, Zhang YQ, et al. Incorporation of therapeutically modified bacteria into gut microbiota inhibits obesity. J Clin Invest, 2014, 124(8): 3391-3406. DOI:10.1172/JCI72517 |
| [47] |
May-Zhang LS, Chen ZY, Dosoky NS, et al. Administration of N-acyl-phosphatidylethanolamine expressing bacteria to low density lipoprotein receptor-/- mice improves indices of cardiometabolic disease. Sci Rep, 2019, 9: 420. DOI:10.1038/s41598-018-37373-1 |
| [48] |
Lin Y, Krogh-Andersen K, Pelletier J, et al. Oral delivery of pentameric glucagon-like peptide-1 by recombinant Lactobacillus in diabetic rats. PLoS ONE, 2016, 11(9): e0162733. DOI:10.1371/journal.pone.0162733 |
| [49] |
Ryan PM, Patterson E, Kent RM, et al. Recombinant incretin-secreting microbe improves metabolic dysfunction in high-fat diet fed rodents. Sci Rep, 2017, 7: 13523. DOI:10.1038/s41598-017-14010-x |
| [50] |
Singh AK, Pandey SK, Kumar GN. Pyrroloquinoline quinone-secreting probiotic Escherichia coli Nissle 1917 ameliorates ethanol-induced oxidative damage and hyperlipidemia in rats. Alcohol Clin Exp Res, 2014, 38(7): 2127-2137. DOI:10.1111/acer.12456 |
| [51] |
Somabhai CA, Raghuvanshi R, Nareshkumar G. Genetically engineered Escherichia coli nissle 1917 synbiotics reduce metabolic effects induced by chronic consumption of dietary fructose. PLoS ONE, 2016, 11(10): e0164860. DOI:10.1371/journal.pone.0164860 |
| [52] |
Zurita-Turk M, Del Carmen S, Santos ACG, et al. Lactococcus lactis carrying the pValac DNA expression vector coding for IL-10 reduces inflammation in a murine model of experimental colitis. BMC Biotechnol, 2014, 14: 73. DOI:10.1186/1472-6750-14-73 |
| [53] |
Del Carmen S, Martín Rosique R, Saraiva T, et al. Protective effects of lactococci strains delivering either IL-10 protein or cDNA in a TNBS-induced chronic colitis model. J Clin Gastroenterol, 2014, 48(Suppl 1): S12-S17. |
| [54] |
Hanson ML, Hixon JA, Li WQ, et al. Oral delivery of IL-27 recombinant bacteria attenuates immune colitis in mice. Gastroenterology, 2014, 146(1): 210-221.e13. DOI:10.1053/j.gastro.2013.09.060 |
| [55] |
Yu ZJ, Huang Z, Sao CW, et al. Bifidobacterium as an oral delivery carrier of interleukin-12 for the treatment of Coxsackie virus B3-induced myocarditis in the Balb/c mice. Int Immunopharmacol, 2012, 12(1): 125-130. DOI:10.1016/j.intimp.2011.10.022 |
| [56] |
Maddaloni M, Kochetkova I, Hoffman C, et al. Delivery of IL-35 by Lactococcus lactis ameliorates collagen-induced arthritis in mice. Front Immunol, 2018, 9: 2691. DOI:10.3389/fimmu.2018.02691 |
| [57] |
McKay R, Ghodasra M, Schardt J, et al. A platform of genetically engineered bacteria as vehicles for localized delivery of therapeutics:Toward applications for Crohn's disease. Bioeng Transl Med, 2018, 3(3): 209-221. DOI:10.1002/btm2.10113 |
| [58] |
Yagnik B, Sharma D, Padh H, et al. Oral immunization with LacVax® OmpA induces protective immune response against Shigella flexneri 2a ATCC 12022 in a murine model. Vaccine, 2019, 37(23): 3097-3105. DOI:10.1016/j.vaccine.2019.04.053 |
| [59] |
Paton AW, Jennings MP, Morona R, et al. Recombinant probiotics for treatment and prevention of enterotoxigenic Escherichia coli diarrhea. Gastroenterology, 2005, 128(5): 1219-1228. DOI:10.1053/j.gastro.2005.01.050 |
| [60] |
Focareta A, Paton JC, Morona R, et al. A recombinant probiotic for treatment and prevention of cholera. Gastroenterology, 2006, 130(6): 1688-1695. DOI:10.1053/j.gastro.2006.02.005 |
| [61] |
Gupta S, Bram EE, Weiss R. Genetically programmable pathogen sense and destroy. ACS Synth Biol, 2013, 2(12): 715-723. DOI:10.1021/sb4000417 |
| [62] |
Hwang IY, Tan MH, Koh E, et al. Reprogramming microbes to be pathogen-seeking killers. ACS Synth Biol, 2014, 3(4): 228-237. DOI:10.1021/sb400077j |
| [63] |
Jayaraman P, Holowko MB, Yeoh JW, et al. Repurposing a two-component system-based biosensor for the killing of Vibrio cholerae. ACS Synth Biol, 2017, 6(7): 1403-1415. DOI:10.1021/acssynbio.7b00058 |
| [64] |
Giomarelli B, Provvedi R, Meacci F, et al. The microbicide cyanovirin-N expressed on the surface of commensal bacterium Streptococcus gordonii captures HIV-1. Aids, 2002, 16(10): 1351-1356. DOI:10.1097/00002030-200207050-00006 |
| [65] |
Rao S, Hu S, McHugh L, et al. Toward a live microbial microbicide for HIV:commensal bacteria secreting an HIV fusion inhibitor peptide. Proc Natl Acad Sci USA, 2005, 102(34): 11993-11998. DOI:10.1073/pnas.0504881102 |
| [66] |
Adachi K, Kawana K, Yokoyama T, et al. Oral immunization with a Lactobacillus casei vaccine expressing human papillomavirus (HPV) type 16 E7 is an effective strategy to induce mucosal cytotoxic lymphocytes against HPV16 E7. Vaccine, 2010, 28(16): 2810-2817. DOI:10.1016/j.vaccine.2010.02.005 |
| [67] |
Komatsu A, Igimi S, Kawana K. Optimization of human papillomavirus (HPV) type 16 E7-expressing lactobacillus-based vaccine for induction of mucosal E7-specific IFNγ-producing cells. Vaccine, 2018, 36(24): 3423-3426. DOI:10.1016/j.vaccine.2018.05.009 |
| [68] |
Xu X, Hegazy WA, Guo LJ, et al. Effective cancer vaccine platform based on attenuated Salmonella and a type Ⅲ secretion system. Cancer Res, 2014, 74(21): 6260-6270. DOI:10.1158/0008-5472.CAN-14-1169 |
| [69] |
Anderson JC, Clarke EJ, Arkin AP, et al. Environmentally controlled invasion of cancer cells by engineered bacteria. J Mol Biol, 2006, 355(4): 619-627. DOI:10.1016/j.jmb.2005.10.076 |
| [70] |
Mu ZP, Zou ZN, Yang Y, et al. A genetically engineered Escherichia coli that senses and degrades tetracycline antibiotic residue. Synth Syst Biotechnol, 2018, 3(3): 196-203. DOI:10.1016/j.synbio.2018.05.001 |
| [71] |
Zahirović A, Lunder M. Microbial delivery vehicles for allergens and allergen-derived peptides in immunotherapy of allergic diseases. Front Microbiol, 2018, 9: 1449. DOI:10.3389/fmicb.2018.01449 |
| [72] |
Nallar SC, Xu DQ, Kalvakolanu DV. Bacteria and genetically modified bacteria as cancer therapeutics:Current advances and challenges. Cytokine, 2017, 89: 160-172. DOI:10.1016/j.cyto.2016.01.002 |
| [73] |
Goldenberg JZ, Yap C, Lytvyn L, et al. Probiotics for the prevention of Clostridium difficile-associated diarrhea in adults and children. Cochrane Database Syst Rev, 2017, 12: CD006095. |
| [74] |
Isabella VM, Ha BN, Castillo MJ, et al. Development of a synthetic live bacterial therapeutic for the human metabolic disease phenylketonuria. Nat Biotechnol, 2018, 36(9): 857-864. DOI:10.1038/nbt.4222 |
| [75] |
Kurtz CB, Millet YA, Puurunen MK, et al. An engineered E. coli Nissle improves hyperammonemia and survival in mice and shows dose-dependent exposure in healthy humans. Sci Transl Med, 2019, 11(475): eaau7975. DOI:10.1126/scitranslmed.aau7975 |
| [76] |
全球40家创新公司竞逐国内微生物组产业即将走向应用爆发期[EB/OL]. (2018-01-31)[2019-06-21]. https://www.cn-healthcare.com/articlewm/20180131/content-1022166.html.
|
| [77] |
Pipeline of Blue Turtle Bio[EB/OL].[2019-06-21]. http://blueturtlebio.com/blog.html.
|
| [78] |
武田、辉瑞等纷纷进军微生物组学领域竟是看中这些潜力![EB/OL]. (2019-01-28)[2019-06-21]. https://med.sina.com/article_detail_103_2_59928.html.
|
| [79] |
He Z, Cui BT, Zhang T, et al. Fecal microbiota transplantation cured epilepsy in a case with Crohn's disease:The first report. World J Gastroenterol, 2017, 23(19): 3565-3568. DOI:10.3748/wjg.v23.i19.3565 |
| [80] |
Forssberg H. Microbiome programming of brain development:implications for neurodevelopmental disorders. Dev Med Child Neurol, 2019, 61(7): 744-749. DOI:10.1111/dmcn.14208 |
| [81] |
Dinan TG, Cryan JF. Gut microbes and depression:still waiting for Godot. Brain Behav Immun, 2019, 79: 1-2. DOI:10.1016/j.bbi.2019.02.007 |
| [82] |
Shis DL, Hussain F, Meinhardt S, et al. Modular, multi-input transcriptional logic gating with orthogonal LacI/GalR family chimeras. ACS Synth Biol, 2014, 3(9): 645-651. DOI:10.1021/sb500262f |
| [83] |
Wang LQ, Qian K, Huang Y, et al. SynBioLGDB:a resource for experimentally validated logic gates in synthetic biology. Sci Rep, 2015, 5: 8090. DOI:10.1038/srep08090 |
| [84] |
Krumbeck JA, Marsteller NL, Frese SA, et al. Characterization of the ecological role of genes mediating acid resistance in Lactobacillus reuteri during colonization of the gastrointestinal tract. Environ Microbiol, 2016, 18(7): 2172-2184. DOI:10.1111/1462-2920.13108 |
| [85] |
Chan CTY, Lee JW, Cameron DE, et al. 'Deadman' and 'Passcode' microbial kill switches for bacterial containment. Nat Chem Biol, 2016, 12(2): 82-86. DOI:10.1038/nchembio.1979 |
| [86] |
Dosoky NS, Guo LL, Chen ZY, et al. Dietary fatty acids control the species of N-acyl-phosphatidylethanolamines synthesized by therapeutically modified bacteria in the intestinal tract. ACS Infect Dis, 2018, 4(1): 3-13. DOI:10.1021/acsinfecdis.7b00127 |
| [87] |
Forkus B, Ritter S, Vlysidis M, et al. Antimicrobial probiotics reduce Salmonella enterica in turkey gastrointestinal tracts. Sci Rep, 2017, 7: 40695. DOI:10.1038/srep40695 |
| [88] |
Amiri-Jami M, Abdelhamid AG, Hazaa M, et al. Recombinant production of omega-3 fatty acids by probiotic Escherichia coli Nissle 1917. FEMS Microbiol Lett, 2015, 362(20): fnv166. DOI:10.1093/femsle/fnv166 |
| [89] |
Wang M, Gao ZQ, Zhang YG, et al. Lactic acid bacteria as mucosal delivery vehicles:a realistic therapeutic option. Appl Microbiol Biotechnol, 2016, 100(13): 5691-5701. DOI:10.1007/s00253-016-7557-x |
| [90] |
Zadravec P, Marečková L, Petroková H, et al. Development of recombinant Lactococcus lactis displaying albumin-binding domain variants against Shiga toxin 1 B Subunit. PLoS ONE, 2016, 11(9): e0162625. DOI:10.1371/journal.pone.0162625 |
| [91] |
Watterlot L, Rochat T, Sokol H, et al. Intragastric administration of a superoxide dismutase-producing recombinant Lactobacillus casei BL23 strain attenuates DSS colitis in mice. Int J Food Microbiol, 2010, 144(1): 35-41. DOI:10.1016/j.ijfoodmicro.2010.03.037 |
| [92] |
Grangette C, Muller-Alouf H, Geoffroy M, et al. Protection against tetanus toxin after intragastric administration of two recombinant lactic acid bacteria:impact of strain viability and in vivo persistence. Vaccine, 2002, 20(27/28): 3304-3309. |
| [93] |
Ohkouchi K, Kawamoto S, Tatsugawa K, et al. Prophylactic effect of Lactobacillus oral vaccine expressing a Japanese cedar pollen allergen. J Biosci Bioeng, 2012, 113(4): 536-541. DOI:10.1016/j.jbiosc.2011.11.025 |
| [94] |
Grangette C, Nutten S, Palumbo E, et al. Enhanced antiinflammatory capacity of a Lactobacillus plantarum mutant synthesizing modified teichoic acids. Proc Natl Acad Sci USA, 2005, 102(29): 10321-10326. DOI:10.1073/pnas.0504084102 |
| [95] |
Hanniffy SB, Carter AT, Hitchin E, et al. Mucosal delivery of a pneumococcal vaccine using Lactococcus lactis affords protection against respiratory infection. J Infect Dis, 2007, 195(2): 185-193. |
| [96] |
Marelli B, Perez AR, Banchio C, et al. Oral immunization with live Lactococcus lactis expressing rotavirus VP8* subunit induces specific immune response in mice. J Virol Methods, 2011, 175(1): 28-37. DOI:10.1016/j.jviromet.2011.04.011 |
| [97] |
Steidler L, Robinson K, Chamberlain L, et al. Mucosal delivery of murine interleukin-2 (IL-2) and IL-6 by recombinant strains of Lactococcus lactis coexpressing antigen and cytokine. Infect Immun, 1998, 66(7): 3183-3189. |
| [98] |
Bermúdez-Humarán LG, Kharrat P, Chatel JM, et al. Lactococci and lactobacilli as mucosal delivery vectors for therapeutic proteins and DNA vaccines. Microb. Cell Fact., 2011, 10(Suppl 1): S4. DOI:10.1186/1475-2859-10-S1-S4 |
| [99] |
Szatraj K, Szczepankowska AK, Sączyńska V, et al. Expression of avian influenza haemagglutinin (H5) and chicken interleukin 2 (chIL-2) under control of the ptcB promoter in Lactococcus lactis. Acta Biochim Pol, 2014, 61(3): 609-614. |
| [100] |
Hugentobler F, Yam KK, Gillard J, et al. Immunization against Leishmania major infection using LACK-and IL-12-expressing Lactococcus lactis induces delay in footpad swelling. PLoS ONE, 2012, 7(2): e30945. DOI:10.1371/journal.pone.0030945 |
| [101] |
Hugentobler F, Di Roberto RB, Gillard J, et al. Oral immunization using live Lactococcus lactis co-expressing LACK and IL-12 protects BALB/c mice against Leishmania major infection. Vaccine, 2012, 30(39): 5726-5732. DOI:10.1016/j.vaccine.2012.07.004 |
| [102] |
Wang XN, Wang L, Huang XW, et al. Oral delivery of probiotics expressing dendritic cell-targeting peptide fused with porcine epidemic diarrhea virus COE antigen:a promising vaccine strategy against PEDV. Viruses, 2017, 9(11): 312. DOI:10.3390/v9110312 |
| [103] |
Gao XW, Ma YY, Wang Z, et al. Oral immunization of mice with a probiotic Lactobacillus casei constitutively expressing the α-toxoid induces protective immunity against Clostridium perfringens α-toxin. Virulence, 2019, 10(1): 166-179. DOI:10.1080/21505594.2019.1582975 |
| [104] |
Cano-Garrido O, Seras-Franzoso J, Garcia-Fruitós E. Lactic acid bacteria:reviewing the potential of a promising delivery live vector for biomedical purposes. Microb Cell Fact, 2015, 14: 137. DOI:10.1186/s12934-015-0313-6 |
| [105] |
Škrlec K, Janež AP, Rogelj B, et al. Evasin-displaying lactic acid bacteria bind different chemokines and neutralize CXCL8 production in Caco-2 cells. Microb Biotechnol, 2017, 10(6): 1732-1743. DOI:10.1111/1751-7915.12781 |
| [106] |
Shigemori S, Watanabe T, Kudoh K, et al. Oral delivery of Lactococcus lactis that secretes bioactive heme oxygenase-1 alleviates development of acute colitis in mice. Microb Cell Fact, 2015, 14: 189. DOI:10.1186/s12934-015-0378-2 |
| [107] |
Ma YJ, Liu JJ, Hou J, et al. Oral administration of recombinant Lactococcus lactis expressing HSP65 and tandemly repeated P277 reduces the incidence of type Ⅰ diabetes in non-obese diabetic mice. PLoS ONE, 2014, 9(8): e105701. DOI:10.1371/journal.pone.0105701 |
| [108] |
Robert S, Gysemans C, Takiishi T, et al. Oral delivery of glutamic acid decarboxylase (GAD)-65 and IL10 by Lactococcus lactis reverses diabetes in recent-onset NOD mice. Diabetes, 2014, 63(8): 2876-2887. DOI:10.2337/db13-1236 |
| [109] |
Robert S, van Huynegem K, Gysemans C, et al. Trimming of two major type 1 diabetes driving antigens, GAD65 and IA-2, allows for successful expression in Lactococcus lactis. Benef Microbes, 2015, 6(4): 591-601. DOI:10.3920/BM2014.0083 |
| [110] |
de Moreno de LeBlanc A, LeBlanc JG, Perdigón G, et al. Oral administration of a catalase-producing Lactococcus lactis can prevent a chemically induced colon cancer in mice. J Med Microbiol, 2008, 57(1): 100-105. DOI:10.1099/jmm.0.47403-0 |
| [111] |
Kawana K, Adachi K, Kojima S, et al. Oral vaccination against HPV E7 for treatment of cervical intraepithelial neoplasia grade 3 (CIN3) elicits E7-specific mucosal immunity in the cervix of CIN3 patients. Vaccine, 2014, 32(47): 6233-6239. DOI:10.1016/j.vaccine.2014.09.020 |
| [112] |
Baradaran A, Yusoff K, Shafee N, et al. Newcastle disease virus hemagglutinin neuraminidase as a potential cancer targeting agent. J Cancer, 2016, 7(4): 462-466. DOI:10.7150/jca.13566 |
| [113] |
Borrero J, Chen YQ, Dunny GM, et al. Modified lactic acid bacteria detect and inhibit multiresistant enterococci. ACS Synth Biol, 2015, 4(3): 299-306. DOI:10.1021/sb500090b |
| [114] |
Jones ML, Chen HM, Ouyang W, et al. Microencapsulated genetically engineered Lactobacillus plantarum 80 (pCBH1) for bile acid deconjugation and its implication in lowering cholesterol. J Biomed Biotechnol, 2004, 2004(1): 61-69. |
| [115] |
Duan FF, Liu JH, March JC. Engineered commensal bacteria reprogram intestinal cells into glucose-responsive insulin-secreting cells for the treatment of diabetes. Diabetes, 2015, 64(5): 1794-1803. DOI:10.2337/db14-0635 |
| [116] |
Holmes D. Genetically engineered Lactobacilli reprogram intestinal cells to secrete insulin and ameliorate hyperglycaemia. Nat Rev Endocrinol, 2015, 11(4): 192. |
| [117] |
Wang ZS, Yu QH, Gao JK, et al. Mucosal and systemic immune responses induced by recombinant Lactobacillus spp. expressing the hemagglutinin of the avian influenza virus H5N1. Clin Vaccine Immunol, 2012, 19(2): 174-179. DOI:10.1128/CVI.05618-11 |
| [118] |
Wei C, Xun AY, Wei XX, et al. Bifidobacteria expressing tumstatin protein for antitumor therapy in tumor-bearing mice. Technol Cancer Res Treat, 2016, 15(3): 498-508. DOI:10.1177/1533034615581977 |
| [119] |
Sasaki T, Fujimori M, Hamaji Y, et al. Genetically engineered Bifidobacterium longum for tumor-targeting enzyme-prodrug therapy of autochthonous mammary tumors in rats. Cancer Sci, 2006, 97(7): 649-657. DOI:10.1111/j.1349-7006.2006.00221.x |
| [120] |
Wei PJ, Yang Y, Liu ZB, et al. Oral Bifidobacterium longum expressing alpha-melanocyte-stimulating hormone to fight experimental colitis. Drug Deliv, 2016, 23(6): 2058-2064. DOI:10.3109/10717544.2015.1122672 |
| [121] |
Liu MG, Li SY, Zhang Q, et al. Oral engineered Bifidobacterium longum expressing rhMnSOD to suppress experimental colitis. Int Immunopharmacol, 2018, 57: 25-32. DOI:10.1016/j.intimp.2018.02.004 |
| [122] |
Zhou ZW, Gong ST, Li XM, et al. Expression of Helicobacter pylori urease B on the surface of Bacillus subtilis spores. J Med Microbiol, 2015, 64(1): 104-110. |
| [123] |
Jing YC, Liu HF, Xu WW, et al. Amelioration of the DSS-induced colitis in mice by pretreatment with 4, 4'-diaponeurosporene-producion Bacillus subtilis. Exp Ther Med, 2017, 14(6): 6069-6073. |
 2019, Vol. 35
2019, Vol. 35