中国科学院微生物研究所、中国微生物学会主办
文章信息
- 陈媛媛, 张保财, 吴德光, 李锋, 宋浩
- Chen Yuanyuan, Zhang Baocai, Wu Deguang, Li Feng, Song Hao
- 产电微生物的筛选方法研究进展
- Research progress in screening method of exoelectrogens
- 生物工程学报, 2020, 36(12): 2719-2731
- Chinese Journal of Biotechnology, 2020, 36(12): 2719-2731
- 10.13345/j.cjb.200176
-
文章历史
- Received: March 31, 2020
- Accepted: May 25, 2020
- Published: June 5, 2020
2. 天津大学 系统生物工程教育部重点实验室 合成生物学前沿科学中心,天津 300072;
3. 天津大学 天津化学化工协同创新中心合成生物学研究平台,天津 300072;
4. 茅台学院,贵州 遵义 564501
2. Frontier Science Center for Synthetic Biology, Key Laboratory of Systems Bioengineering (Ministry of Education), Tianjin University, Tianjin 300072, China;
3. Synthetic Biology Research Platform, Collaborative Innovation Center of Chemical Science and Engineering (Tianjin), School of Chemical Engineering and Technology, Tianjin University, Tianjin 300072, China;
4. Moutai Institute, Zunyi 564501, Guizhou, China
随着全球不可再生石化燃料的消耗和环境污染的加剧,新型可再生的绿色能源成为当前研究的热点。产电微生物通过自身的呼吸代谢氧化底物、抽提环境有机物中的电子,将化学能转化为电能,其广泛存在于海泥、河床等淤泥沉积物中。基于产电微生物胞外电子传递开发的微生物电催化系统,在清洁能源与环境修复中将发挥重要作用[1-3]。
在自然界中,包括地杆菌属、希瓦氏菌属和假单胞菌属在内的大多数微生物都能够进行胞外电子传递,但电子传递效率较低,这已经成为限制电活性微生物工业化应用的主要瓶颈[1-3]。随着微生物学和分子生物学学科的不断发展,电子传递机制被不断解析,新的遗传育种技术不断涌现,在一定程度上提高了产电微生物的电子传递速率。但由于菌株的自然属性不同,电子传递机制和效率的差异巨大,难以从根本上扭转现有产电菌株环境适应性差、产电效率低、稳定性不足的缺陷,从而限制了产电菌在不同温度、电场、压力、盐度、酸碱度等恶劣复杂环境中的应用。从天然环境中挖掘产电效率高、培养方式简单、环境适应性强、遗传操作方便的产电菌,是当前产电微生物工业化应用中的主要策略[4-6]。因此,高效的产电菌株筛选方法成为快速挖掘性能优异产电微生物的核心技术。本文首先阐述了产电微生物的种类和胞外电子传递机制,总结回顾了现有产电微生物的筛选鉴定方法,并对其研究前景进行了总结展望。
1 产电微生物的种类目前报道的产电微生物有很多种,包括细菌、真菌和古生菌。其中,绝大部分产电微生物是细菌,主要分布在变形菌门、厚壁菌门、酸杆菌门和放线菌门;部分真菌(酵母属和毕赤酵母属)、古生菌也具有释放电子的能力。表 1汇总了当前筛选鉴定的典型产电菌。
| Phylum | Class | Order | Family | Species | References |
| Proteobacteria | α-proteobacteria | Rhodospirillales | Acetobacteraceae | Acidiphilium cryptum | [7] |
| Rhizobiales | Brucellaceae | Ochrobactrum anthropi YZ-1 | [8] | ||
| Bradyrhizobiaceae | Rhodopseudomonas palustris TIE-1 | [9] | |||
| β-proteobacteria | Burkholderiales | Comamonadaceae | Comamonas denitrificans | [10] | |
| Comamonas testosteroni | [11] | ||||
| Rhodoferax ferrireducens | [12] | ||||
| γ-proteobacteria | Aeromonadales | Aeromonadaceae | Aeromonas hydrophila | [13] | |
| Enterobacterales | Enterobacteriaceae | Citrobacter freundii | [14] | ||
| Citrobacter sp. SX-1 | [15] | ||||
| Enterobacter cloacae | [16] | ||||
| Klebsiella pneumoniae | [17] | ||||
| Klebsiella sp. ME17 | [18] | ||||
| Alteromonadales | Shewanellaceae | Shewanella decolorationis | [19] | ||
| Shewanella oneidensis MR-1 | [20] | ||||
| Shewanella frigidimarina | [21] | ||||
| Shewanella algae E-1 | [22] | ||||
| Pseudomonadales | Pseudomonadaceae | Pseudomonas aeruginosa ZH1 | [23] | ||
| Aeromonadales | Aeromonadaceae | Tolumonas osonensis | [24] | ||
| δ-proteobacteria | Desulfobacterales | Desulfobulbaceae | Desulfobulbus propionicus | [25] | |
| Geobacter sulfurreducens | [26] | ||||
| Geobacter metallireducens | [27] | ||||
| ε-proteobacteria | Campylobacterales | Campylobacteraceae | Arcobacter butzleri ED-1 | [28] | |
| Firmicutes | Clostridia | Clostridiales | Clostridiaceae | Clostridium acetobutylicum | [29] |
| Clostridiales | Peptococcaceae | Desulfitobacterium hafniense | [30] | ||
| Thermincola ferriacetica | [31] | ||||
| Thermincola potens JR | [32] | ||||
| Bacilli | Bacillales | Paenibacillaceae | Paenibacillus sp. | [33] | |
| Lactobacillales | Enterococcaceae | Enterococcus avium | [34] | ||
| Acidobacteria | Holophagae | Holophagales | Holophagaceae | Geothrix fermentans | [35] |
| Actinobacteria | Micrococcales | Micrococcaceae | Kocuria rhizophila P2-A-5 | [36] |
此外,在淤泥沉积物中发现的一种丝状多细胞微生物(电缆细菌),也具有胞外电子转移能力,该菌属于变形菌门的脱硫球茎菌科。电缆细菌连接沉积物表面与深处缺氧层,通过自身丝状结构的长距离导电,耦合表层还原与深层氧化,从而构成完整的氧化还原反应,这种微生物能够调节沉积物中氧分子和硫化物的转移,在富含硫酸盐的自然环境中广泛存在[37]。
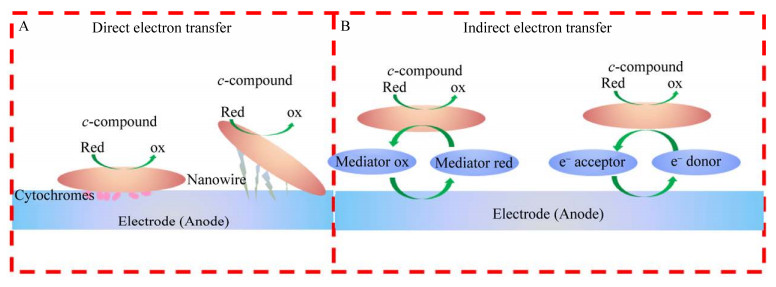
2 产电微生物的电子传递机制目前,产电细胞有两种胞外电子传递机制获得广泛认可,分别是基于细胞色素蛋白或导电纳米线的直接电子传递(图 1A)和自身代谢分泌或环境中氧化还原介体化合物(黄素类、吩嗪类、醌类等)介导的间接电子传递(图 1B)[38-39]。
|
| 图 1 产电微生物胞外电子转移机制(A:直接电子转移机制,通过c-型细胞色素和菌毛进行电子传递[38];B:间接电子转移机制,利用可溶性电子传递体介导电子转移[38]) Fig. 1 Mechanisms of transfer extracellular electrons in microbial exoelectrogens. (A) Direct electron transfer (DET). Electrons transport across the cell membranes through the c-type cytochromes and pili[38]. (B) Indirect electron transfer (IET). Electrons transfer between the external electron acceptor/donor mediated by soluble shuttle molecules[38]. |
| |
产电微生物在电子受体(铁矿物、电极等)表面附着并形成生物膜,细胞通过色素蛋白或导电纳米线将胞内代谢产生的电子传递到电子受体。希瓦氏菌、地杆菌等大多数产电菌株都具有传递电子的c-型细胞色素蛋白系统[40],目前发现有近百种导电色素蛋白。希瓦氏菌胞内代谢产生的还原态烟酰胺腺嘌呤二核苷(Nicotinamide adenine dinucleotide,NADH)通过NADH脱氢酶、甲基萘醌脱氢酶将电子传递到内膜色素蛋白CymA,经过Fcc3和STC色素蛋白跨过周质空间,传递到外膜色素蛋白复合体MtrCAB,最后通过外膜外侧的MtrC、OmcA、MtrF等色素蛋白传递到胞外电子受体[41]。硫还原地杆菌Geobacter sulfurreducens主要利用OmcE、OmcS、OmcB等色素蛋白进行电子的近距离接触式传递[42]。
除了利用色素蛋白直接传递电子外,部分产电微生物还可通过“导电鞭毛”介导电子的远距离传递。Tan等[43]在G. sulfurreducens表面发现一种富含芳香族氨基酸、长数十微米的菌毛结构,这种结构的电导率与金属相当,能够将远离电极细胞的电子快速传递到电子受体,但关于导电菌毛的导电机制目前尚有争论[44-45]。此外,Barchinger等[46]发现希瓦氏菌并不具备“导电菌毛”,希瓦氏菌表面的凸起实则是自身细胞膜的外突囊泡,只有地杆菌具有真正的纳米导线[47]。
2.2 间接电子传递在远离电子受体的情况下,产电微生物可以利用自身代谢分泌的或环境中的氧化还原介体介导电子间接传递[48]。希瓦氏菌、地杆菌利用自身合成的核黄素、黄素单核苷酸等黄素类化合物介导电子传递。Lin等[49]和Yang等[50]通过加强希瓦氏菌核黄素的合成分泌,大幅提高了希瓦氏菌的电子传递效率;假单胞菌利用吩嗪-1-羧酸、吩嗪-1-甲酰胺等吩嗪类化合物介导电子传递,Yong等[51]通过种群感应提高了铜绿假单胞菌Pseudomonas aeruginosa中吩嗪-1-羧酸的合成,将电流密度提升了1.6倍;此外,大部分产电微生物能够利用环境腐殖质中的蒽醌-2, 6-二磺酸等醌类化合物介导电子传递[48]。
核黄素等电子传递载体除了通过穿梭于色素蛋白与电极间,以双电子机制介导电子传递外,还可作为蛋白辅因子与外膜色素蛋白结合为复合体,以单电子机制直接传递电子。在希瓦氏菌中,核黄素与OmcA结合、黄素单核苷酸与MtrA结合,复合体的电子传递速率相比于双电子传递提高103–105倍[52-53]。基于此,Yu等[54]将维生素B2吸附固定在以碳玻璃为基底的石墨烯电极表面,维生素B2作为辅因子促进了细胞色素与电极间的电子传递,从而将G. sulfurreducens的胞外电子传递速率提高了200%。
3 产电微生物的筛选检测方法 3.1 电化学富集筛选法 3.1.1 常规微生物燃料电池筛选法基于产电微生物的亲电极性,在分离产电微生物与非产电微生物时,使用微生物燃料电池(Microbial fuel cell,MFC)进行电化学富集是最常用的方法。将接种源加入到微生物燃料电池中,当电池平稳运行后从阳极生物膜中分离菌株,使用磷酸盐缓冲液将阳极的菌体洗脱,经过逐级稀释涂板,即可得到单菌株。经过电池富集后得到产电菌株后,仍需对菌株的产电性能进行再次确认。Yang等[55]从微生物燃料电池阳极生物膜中分离出一株产电菌,该菌能够在厌氧条件下生长,具有还原蒽醌-2, 6-二磺酸盐(Anthraquinone-2, 6-disulfonate,AQDS)的能力。You等[56]将活性污泥接种到微生物燃料电池中,电流输出持续稳定后,从阳极生物膜分离得到一株具有产电活性的巨大芽孢杆菌Bacillus megaterium LLD-1。Naradasu等[34]利用电化学富集法从人肠道中筛选出鸟肠球菌Enterococcus avium和肺炎克雷伯氏菌Klebsiella pneumoniae两株产电微生物。
除了双室微生物燃料电池外,还可以利用U型管微生物燃料电池来富集产电菌。U型管微生物燃料电池由直管的阳极室和U型的阴极室构成,两个腔室之间通过质子交换膜分隔开。这种构造使悬浮液中的产电菌在亲电极性和重力的双重作用下,能更快地沉积并形成生物膜,从而快速富集产电微生物[8]。这种方法适用于从少量样品中快速高精度地筛选产电微生物。
3.1.2 微型微生物燃料电池阵列筛选法通过微生物燃料电池电化学系统对菌株产电活性的分析通常更加准确可靠,但常规的燃料电池体积较大、成本高,不适合开展大规模筛选实验,因此需要开发高通量微生物燃料电池筛选模型。Hou等[57]开发了一种微型微生物燃料电池组,用于高通量表征微生物的产电能力,微生物燃料电池组由24个单独的微型电池集成阵列,每个微型电池具有独立的阳极室和阴极室(图 2A),每个腔室的电能输出相差在8%以内。与普通的H型微生物燃料电池相比,这种微型电池组对于试剂和样品的消耗更少,并且可以同时进行多组平行实验,具有便捷高效、准确性高、可靠性强的特征。

|
| 图 2 微型微生物燃料电池阵列(A:微型微生物燃料电池阵列组装示意图[57];B:6孔微型微生物燃料电池阵列示意图,该阵列由五个功能层组成,包括两个电极层和两个电极室层以及质子交换膜[58];C:纸基64孔传感阵列组装示意图,包括MFC阵列的组装步骤示意图,单个MFC的横截面示意图,以及废弃的纸基传感阵列的处理方式[59]) Fig. 2 Microfabricated microbial fuel cell array. (A) Illustration of the MFC array and its assembly steps[57]. (B) Schematic diagram of the 6-well miniature MFC array system. MFC array was composed of five functional layers, including two electrode layers, and two chamber layers and proton exchange membrane[58]. (C) The schematic of 64-well papertronic sensing array, including the procedure of assembling the MFC, the schematic of cross-section of a single MFC, the handling method of the array that has been used[59]. |
| |
随着精密仪器制造技术的发展,制造体积更小的微生物燃料电池得以实现。Mukherjee等[58]开发了一种微型微生物燃料电池组,用于快速分析筛选产电微生物。这种微型燃料电池的阴极和阳极体积分别只有1.5 μL,分别由阴极电极层、阳极电极层、质子交换膜、阴极室层和阳极室层5个部分组成(图 2B),每个独立电池的开路电压相差不到1.4%,是目前最小的微生物燃料电池。
然而,微型电池组的运行离不开复杂的外线路控制系统以及多通道进料系统,这使电池组的清洁维护工作耗时费力。为此,Tahernia等[59]设计了一种用于筛选电活性微生物的64孔传感装置,整个传感阵列由6层纸基材料组成,依次是阴极封层、阴极接线层、MFC阴极室、MFC阳极室、阳极接线层、阳极封层(图 2C)。这种一次性的纸基生物传感阵列,利用纸的毛细虹吸作用控制流体,无需使用外部管道即可自动吸取样品溶液,阵列中连接各个微孔的线路集成在纸层上,无需复杂的控制系统,从而实现了平行组间的同步快速测量。
3.2 高通量筛选法 3.2.1 电致变色方法利用微生物燃料电池进行电化学分离鉴定产电微生物是目前常用的方法,但是常规的基于电压筛选的方法耗时低效,微型燃料电池阵列需要特殊的设备,在其并未大规模商业化应用前,难以实现广泛应用,因此需要有效、廉价和高通量的方法来鉴定产电菌。Yuan等[60]开发了一种基于三氧化钨(WO3)纳米探针的高通量筛选方法。WO3具有电致变色活性,在钠离子和钾离子的作用下,其能够被产电微生物还原,生成蓝色的钠钨青铜。在筛选的过程中,将菌液与WO3溶液混合置于适宜的温度下,体系颜色的深浅和变化的快慢,可指示微生物的电子转移能力(图 3A)。这种方法操作简便、显色迅速、灵敏度高且成本低廉,适合大规模筛选,并且还可以根据颜色的深浅程度判断菌株产电能力的强弱,此技术在产电微生物的高通量筛选鉴定方面具有广阔的应用前景[61]。

|
| 图 3 电致变色法和比色分析法工作流程示意图(A:电致变色法的鉴定流程图,合成的三氧化钨纳米簇与菌体混合置于96孔板中,反应后扫描96孔板.利用软件分析每个孔的灰度,根据其灰度值即可以判断菌株的电活性[60];B:比色分析法的示意图,细菌的过氧化物酶催化TMB产生可测的颜色变化,根据此特征鉴定产电菌[65]) Fig. 3 Schematic diagram of the workflow for the methods of electrochromism and colorimetric assay. (A) Schematic diagram of the workflow for the method of electrochromism. The synthesized WO3 nanocluster and bacteria were mixed in 96-well plate then, scanned and analyzed. According to the gray-scale value analyzed by software, the electrical activity of the strain can be evaluated[60]. (B) Schematic diagram of the workflow for the method of colorimetric assay, the bacterial peroxidase-catalyzed oxidation of chromogen (TMB) resulted in a measurable color change, identifying the exoelectrogens on the base of the character[65]. |
| |
利用WO3纳米探针高通量筛选方法,He等[62]从沉积物中分离并鉴定出一种新的产电菌,并命名为球形芽孢杆菌Lysinibacillus sphaericus D-8,该菌在以乳酸作为电子供体的情况下,其输出功率达到92 mW/m2。Yang等[63]基于WO3纳米探针的电致变色原理,开发了检测环境中产电微生物种群数量的新方法,该方法结合WO3纳米探针和最大可能数计数法快速估算环境中产电微生物种群数量,已成功用于检测淡水湖泊水底沉积物和市政污水中的产电微生物种群分布。
利用WO3纳米探针开发的产电菌筛选方法可实现菌株的高通量筛选,但其操作步骤冗长。为进一步将筛选步骤集成,Marques等[64]开发了一种纸基电活性细菌检测平台,首先对纸张进行蜡印刷,形成亚毫米级别的亲水孔阵列,经过蜡印的纸张浸入WO3纳米探针分散液中,使纸张上的每个亲水孔挂上WO3纳米探针。在筛菌的过程中,样品稀释液中的产电微生物进入亲水孔中,与WO3纳米探针发生显色反应,利用数字照片对纸张的亲水孔颜色进行视觉比对和色光三原色分析,筛选出样品中的产电微生物。基于上述方法构建的高通量纸基产电菌检测平台,具有简单便捷、快速可靠、成本低廉的优势。
3.2.2 比色分析方法比色分析筛选法基于有机化合物在产电微生物作用下的颜色反应,通过体系溶液的吸光度鉴定筛选产电菌,可对所筛的菌株进行准确的定量分析。根据比色分析筛选法,Zhou等[65]利用产电微生物细胞膜上的c-型细胞色素的辣根过氧化物酶活性氧化3, 3′, 5, 5′-四甲基联苯胺(3, 3′, 5, 5′-tetramethylbenzidine,TMB),使其颜色变为蓝色(图 3B),根据颜色的变化鉴别细菌的胞外电子转移能力,结合酶标仪可实现在短时间内对产电菌株进行快速、高通量的鉴定筛选。在此基础上,Wen等[66]开发了一种免疫磁捕获技术(Immunomagnetic capture,IMC),将c-型细胞色素蛋白的特异性抗体固定在磁珠上,希瓦氏菌的c-型细胞色素蛋白与磁珠上的特异性抗体结合形成复合物,在磁珠的吸附作用下,将菌株从环境中分离。由于c-型细胞色素蛋白与辣根过氧化物酶具有相似的氧化活性,可利用TMB对分离的菌株进一步开展比色分析,筛选电活性高的希瓦氏菌株。利用IMC可快速地从复杂环境中分离富集目的菌株,消除样品基质对目标物的影响,同时保持微生物的活性。该方法具有高灵敏度、低毒性的特点,对于快速检测目标微生物具有广泛的应用前景。
另外,基于偶氮染料的生物脱色反应,可利用偶氮染料甲基橙对产电微生物进行高通量筛选。Xiao等[67]利用甲基橙评估了希瓦氏菌及其色素蛋白基因缺失突变株的胞外电子转移能力,发现甲基橙的脱色反应速率与细胞的电子转移能力正相关。与TMB相比较,甲基橙价格低廉,具有明显的成本优势,并且这种筛选鉴定方法还可探究其他因素对其胞外电子转移的影响,应用范围广,检测成本低。
3.2.3 电化学发光分析针对产电微生物筛选过程中灵敏度低和选择性差的问题,电化学发光分析技术(Electrochemiluminescence,ECL)以高灵敏度和高选择性得到了广泛研究[68]。在联吡啶钌-三丙胺(Ru(bpy)32+-TprA)体系中,产电菌的胞外电子转移会促使Ru(bpy)32+•形成,从而引发体系的光信号变化(图 4A)。基于此,You等[69]开发了鉴定不同电活性类型微生物的方法,发现联吡啶钌-三丙胺的发光强度与细胞浓度之间具有线性关系。另外,这些具有电化学发光活性的复合物对细胞的还原、氧化呈现不同的光信号。因此,利用电化学发光原理开发的高通量筛选技术,不仅可以分析菌株的胞外还原能力,还可以评估菌株的胞外氧化能力。

|
| 图 4 电化学发光和生物激光打印原理示意图(A:电化学发光识别产电菌的机制示意图,具有胞外还原能力的细菌增强ECL的发射信号,具有胞外氧化能力的细菌降低ECL强度[69];B:生物激光打印(BioLP)示意图,载体由透明石英载体、能量转换层和液体细胞培养物等三层组成,接收载体为琼脂平板[73]) Fig. 4 Schematic diagram of the mechanisms of ECL and BioLP. (A) Schematic diagram of the mechanism of ECL for identification the exoelectrogens. The bacteria with the ability of extracellular reduction enhance the signal of ECL emission, however, the bacteria with the ability of extracellular oxidation reduce intensity of the ECL[69]. (B) Biological laser printing (BioLP) schematic. The launch support consists of three layers: a transparent quartz layer, an energy conversion layer and the liquid cell culture. And the receiving support is an agar plate[73]. |
| |
除了上述的化学方法外,电泳分析等物理方法在电活性菌株筛选中也发挥着重要作用。产电微生物的细胞表面极化率与细胞色素蛋白和胞外电子转移密切相关,细胞色素蛋白缺失、胞外电子转移削弱会导致细胞表面极化率下降。Wang等[70]利用微流体系统研究了细胞的电化学特性,将产电菌置于直流电场下的介电电泳微流体中,不同菌株的捕获电压和线性电动迁移率有所差异,利用计算机模拟计算菌株表面极化率,以此来分析检测菌株的产电活性。此外,脂多糖或离子通道的存在也会影响细胞的表面极化率,利用介电电泳技术,也可对细胞进行表型分析。
3.2.5 生物激光打印法在产电菌的筛选过程中,微生物的纯化分离是限制筛选效率的主要瓶颈。菌株的生物激光打印(Biological laser printing,BioLP)技术能够以微米级精度直接从液体中将活细胞转移至接收基表面,从而实现在复杂的环境中快速分离纯化菌株[71]。在此方法中,将涂覆样品浓缩液的二氧化钛涂层载玻片倒置放入BioLP设备中(图 4B),在瞬时激光放热下,与二氧化钛层直接接触的细胞培养基薄层一部分汽化,剩余未汽化的液体向下落在琼脂板上形成等距液滴阵列,利用连续的激光脉冲,可以将样品逐滴加入到收集板上,形成单细胞斑点,大多数印刷斑点至多含一个细胞,从而能够快速、高通量地分离纯化菌株[72]。Ringeisen等[73]使用BioLP技术从两种复杂的培养物中成功分离出单个菌落,细菌被打印在含AQDS的琼脂平板上,根据AQDS与产电微生物的显色反应(AQDS遇到产电微生物颜色由无色变为亮红色),可直接筛选到样品中的产电微生物。
4 总结与展望在过去的几年中,随着微生物电催化系统在清洁能源与环境修复中的作用越发重要,针对产电微生物的挖掘改造逐渐成为当前的研究热点。然而目前的理论基础和技术方法难以弥补现有产电微生物的自然属性缺陷,从而限制了产电菌在恶劣复杂环境中的应用。针对这一难题,研究者基于产电菌的种源分布和电子传递机制开发建立了多种快速、高通量的产电菌株筛选技术,从海泥、河床等淤泥沉积物中高效筛选到了多种性能优异的产电微生物。然而,这些方法大多是以菌株的分离培养为基础,这往往会漏筛生长条件苛刻(严格厌氧、低温等)或无法分离培养的产电菌,难以获得极端环境中的电活性微生物[74]。
展望未来,为实现对复杂环境中产电微生物菌群的系统分析,高效筛选多种产电微生物,促进微生物电催化系统的广泛应用和快速发展,未来可以从以下几个方面深入研究。(1)基于宏基因组学对环境中电活性微生物菌群系统分析,针对菌群特征开发与之匹配的筛选方法[75-76];(2)研究电活性微生物菌群特征,突破传统的针对单一菌株的筛选模式,开发高效便捷的产电微生物菌群筛选技术;(3)对所筛菌株的遗传信息进行深入分析,挖掘、鉴定其功能元件,利用合成生物学模块化工程策略,从元件挖掘、模块组装、线路设计、底盘适配4个维度,从拓宽并提高底物利用效率、增加胞内还原力和电子池、优化导电细胞色素系统、提高导电载体的生物合成与传递、构建导电生物膜5个角度,强化产电菌的电子传递速率,此外结合模式化产电菌,克服产电菌株在复杂环境中的自然缺陷,构建高效人工产电系统[77-78]。
| [1] |
Logan BE. Exoelectrogenic bacteria that power microbial fuel cells. Nat Rev Microbiol, 2009, 7(5): 375-381. DOI:10.1038/nrmicro2113 |
| [2] |
Lovley DR. Bug juice: harvesting electricity with microorganisms. Nat Rev Microbiol, 2006, 4(7): 497-508. DOI:10.1038/nrmicro1442 |
| [3] |
Shi L, Dong HL, Reguera G, et al. Extracellular electron transfer mechanisms between microorganisms and minerals. Nat Rev Microbiol, 2016, 14(10): 651-622. DOI:10.1038/nrmicro.2016.93 |
| [4] |
Kumar A, Hsu LHH, Kavanagh P, et al. The ins and outs of microorganism-electrode electron transfer reactions. Nat Rev Chem, 2017, 1(3): 0024. |
| [5] |
Hartshorne RS, Reardon CL, Ross D, et al. Characterization of an electron conduit between bacteria and the extracellular environment. Proc Natl Acad Sci USA, 2009, 106(52): 22169-22174. DOI:10.1073/pnas.0900086106 |
| [6] |
Logan BE, Rossi R, Ragab A, et al. Electroactive microorganisms in bioelectrochemical systems. Nat Rev Microbiol, 2019, 17(5): 307-319. DOI:10.1038/s41579-019-0173-x |
| [7] |
Borole AP, O'neill H, Tsouris C, et al. A microbial fuel cell operating at low pH using the acidophile Acidiphilium cryptum. Biotechnol Lett, 2008, 30(8): 1367-1372. DOI:10.1007/s10529-008-9700-y |
| [8] |
Zuo Y, Xing DF, Regan JM, et al. Isolation of the exoelectrogenic bacterium Ochrobactrum anthropi YZ-1 by using a U-Tube microbial fuel cell. Appl Environ Microbiol, 2008, 74(10): 3130-3137. DOI:10.1128/AEM.02732-07 |
| [9] |
Bose A, Gardel EJ, Vidoudez C, et al. Electron uptake by iron-oxidizing phototrophic bacteria. Nat commun, 2014, 5: 3391. DOI:10.1038/ncomms4391 |
| [10] |
Xing DF, Cheng SA, Logan BE, et al. Isolation of the exoelectrogenic denitrifying bacterium Comamonas denitrificans based on dilution to extinction. Appl Microbiol Biotechnol, 2010, 85(5): 1575-1587. DOI:10.1007/s00253-009-2240-0 |
| [11] |
Yu YY, Wu YC, Cao B, et al. Adjustable bidirectional extracellular electron transfer between Comamonas testosteroni biofilms and electrode via distinct electron mediators. Electrochem Commun, 2015, 59: 43-47. DOI:10.1016/j.elecom.2015.07.007 |
| [12] |
Chaudhuri SK, Lovley DR. Electricity generation by direct oxidation of glucose in mediatorless microbial fuel cells. Nat Biotechnol, 2003, 21(10): 1229-1232. DOI:10.1038/nbt867 |
| [13] |
Pham CA, Jung SJ, Phung NT, et al. A novel electrochemically active and Fe(Ⅲ)-reducing bacterium phylogenetically related to Aeromonas hydrophila, isolated from a microbial fuel cell. FEMS Microbiol Lett, 2003, 223(1): 129-134. DOI:10.1016/S0378-1097(03)00354-9 |
| [14] |
Huang JJ, Zhu NW, Cao YL, et al. Exoelectrogenic bacterium phylogenetically related to Citrobacter freundii, isolated from anodic biofilm of a microbial fuel cell. Appl Biochem Biotechnol, 2015, 175(4): 1879-1891. |
| [15] |
Xu S, Liu H. New exoelectrogen Citrobacter sp. SX-1 isolated from a microbial fuel cell. J App Microbiol, 2011, 111(5): 1108-1115. DOI:10.1111/j.1365-2672.2011.05129.x |
| [16] |
Cournet A, Délia ML, Bergel A, et al. Electrochemical reduction of oxygen catalyzed by a wide range of bacteria including Gram-positive. Electrochem Commun, 2010, 12(4): 505-508. |
| [17] |
Deng LF, Li FB, Zhou SG, et al. A study of electron-shuttle mechanism in Klebsiella pneumoniae based-microbial fuel cells. Chin Sci Bull, 2010, 55(1): 99-104. |
| [18] |
Xia X, Cao XX, Liang P, et al. Electricity generation from glucose by a Klebsiella sp. in microbial fuel cells. Appl Microbiol Biotechnol, 2010, 87(1): 383-390. |
| [19] |
Yang YG, Sun GP, Guo J, et al. Differential biofilms characteristics of Shewanella decolorationis microbial fuel cells under open and closed circuit conditions. Bioresour Technol, 2011, 102(14): 7093-7098. DOI:10.1016/j.biortech.2011.04.073 |
| [20] |
El-Naggar MY, Wanger G, Leung KM, et al. Electrical transport along bacterial nanowires from Shewanella oneidensis MR-1. Proc Natl Acad Sci USA, 2010, 107(42): 18127-18131. DOI:10.1073/pnas.1004880107 |
| [21] |
Fitzgerald LA, Petersen ER, Leary DH, et al. Shewanella frigidimarina microbial fuel cells and the influence of divalent cations on current output. Biosens Bioelectron, 2013, 40(1): 102-109. DOI:10.1016/j.bios.2012.06.039 |
| [22] |
Huang YX, Li X, Zhao JY, et al. Isolation and characterization of a salt-tolerant exoelectrogenic strain Shewanella algae E-1. Microbiol China, 2020, 47(2): 351-361 (in Chinese). 黄亦馨, 李晓, 赵津莹, 等. 一株耐盐产电菌Shewanella algae E-1的分离及其产电特性分析. 微生物学通报, 2020, 47(2): 351-361. |
| [23] |
Nor MHM, Mubarak MFM, Elmi HSA, et al. Bioelectricity generation in microbial fuel cell using natural microflora and isolated pure culture bacteria from anaerobic palm oil mill effluent sludge. Bioresour Technol, 2015, 190: 458-465. DOI:10.1016/j.biortech.2015.02.103 |
| [24] |
Luo JM, Yang J, He HH, et al. A new electrochemically active bacterium phylogenetically related to Tolumonas osonensis and power performance in MFCs. Bioresour Technol, 2013, 139: 141-148. DOI:10.1016/j.biortech.2013.04.031 |
| [25] |
Holmes DE, Bond DR, Lovley DR. Electron transfer by Desulfobulbus propionicus to Fe(Ⅲ) and graphite electrodes. Appl Environ Microbiol, 2004, 70(2): 1234-1237. |
| [26] |
Bond DR, Lovley DR. Electricity production by Geobacter sulfurreducens attached to electrodes. Appl Environ Microbiol, 2003, 69(3): 1548-1555. |
| [27] |
Lovley DR, Giovannoni SJ, White DC, et al. Geobacter metallireducens gen. nov. sp. nov., a microorganism capable of coupling the complete oxidation of organic compounds to the reduction of iron and other metals. Arch Microbiol, 1993, 159(4): 336-344. |
| [28] |
Fedorovich V, Knighton MC, Pagaling E, et al. Novel electrochemically active bacterium phylogenetically related to Arcobacter butzleri, isolated from a microbial fuel cell. Appl Environ Microbiol, 2009, 75(23): 7326-7334. DOI:10.1128/AEM.01345-09 |
| [29] |
Finch AS, Mackie TD, Sund CJ, et al. Metabolite analysis of Clostridium acetobutylicum: fermentation in a microbial fuel cell. Bioresour Technol, 2011, 102(1): 312-315. DOI:10.1016/j.biortech.2010.06.149 |
| [30] |
Milliken CE, May HD. Sustained generation of electricity by the spore-forming, Gram-positive, Desulfitobacterium hafniense strain DCB2. Appl Microbiol Biotechnol, 2007, 73: 1180-1189. DOI:10.1007/s00253-006-0564-6 |
| [31] |
Parameswaran P, Bry T, Popat SC, et al. Kinetic, electrochemical, and microscopic characterization of the thermophilic, anode-respiring bacterium Thermincola ferriacetica. Environ Sci Technol, 2013, 47(9): 4934-4940. DOI:10.1021/es400321c |
| [32] |
Wrighton KC, Thrash JC, Melnyk RA, et al. Evidence for direct electron transfer by a gram-positive bacterium isolated from a microbial fuel cell. Appl Environ Microbiol, 2011, 77(21): 7633-7639. DOI:10.1128/AEM.05365-11 |
| [33] |
Rowe AR, Yoshimura M, LaRowe DE, et al. In situ electrochemical enrichment and isolation of a magnetite-reducing bacterium from a high pH serpentinizing spring. Environ Microbiol, 2017, 19(6): 2272-2285. DOI:10.1111/1462-2920.13723 |
| [34] |
Naradasu D, Miran W, Sakamoto M, et al. Isolation and characterization of human gut bacteria capable of extracellular electron transport by electrochemical techniques. Front Microbiol, 2018, 9: 3267. |
| [35] |
Bond DR, Lovley DR. Evidence for involvement of an electron shuttle in electricity generation by Geothrix fermentans. Appl Environ Microbiol, 2005, 71(4): 2186-2189. |
| [36] |
Luo JM, Li M, Zhou MH, et al. Characterization of a novel strain phylogenetically related to Kocuria rhizophila and its chemical modification to improve performance of microbial fuel cells. Biosens Bioelectron, 2015, 69: 113-120. DOI:10.1016/j.bios.2015.02.025 |
| [37] |
Seitaj D, Schauer R, Sulu-Gambari F, et al. Cable bacteria generate a firewall against euxinia in seasonally hypoxic basins. Proc Natl Acad Sci USA, 2015, 112(43): 13278-13283. DOI:10.1073/pnas.1510152112 |
| [38] |
Sydow A, Krieg T, Mayer F, et al. Electroactive bacteria - molecular mechanisms and genetic tools. Appl Microbiol Biotechnol, 2014, 98(20): 8481-8495. DOI:10.1007/s00253-014-6005-z |
| [39] |
White GF, Edwards MJ, Gomez-Perez L, et al. Chapter three-mechanisms of bacterial extracellular electron exchange. Adv Microb Physiol, 2016, 68: 87-138. DOI:10.1016/bs.ampbs.2016.02.002 |
| [40] |
Firer-Sherwood M, Pulcu GS, Elliott SJ. Electrochemical interrogations of the Mtr cytochromes from Shewanella: opening a potential window. J Biol Inorg Chem, 2008, 13(6): 849-854. DOI:10.1007/s00775-008-0398-z |
| [41] |
Richardson DJ, Butt JN, Fredrickson JK, et al. The 'porin-cytochrome' model for microbe-to-mineral electron transfer. Mol Microbiol, 2012, 85(2): 201-212. |
| [42] |
Liu YM, Wang ZM, Liu J, et al. A trans-outer membrane porin-cytochrome protein complex for extracellular electron transfer by Geobacter sulfurreducens PCA. Environ Microbiol Rep, 2014, 6(6): 776-785. |
| [43] |
Tan Y, Adhikari RY, Malvankar NS, et al. Expressing the Geobacter metallireducens PilA in Geobacter sulfurreducens yields pili with exceptional conductivity. MBio, 2017, 8(1): e02203-16. |
| [44] |
Malvankar NS, Vargas M, Nevin KP, et al. Tunable metallic-like conductivity in microbial nanowire networks. Nat Nanotechnol, 2011, 6(9): 573-579. |
| [45] |
Vargas M, Malvankar NS, Tremblay PL, et al. Aromatic amino acids required for pili conductivity and long-range extracellular electron transport in Geobacter sulfurreducens. MBio, 2013, 4(2): e00105-13. |
| [46] |
Barchinger SE, Pirbadian S, Sambles C, et al. Regulation of gene expression in Shewanella oneidensis MR-1 during electron acceptor limitation and bacterial nanowire formation. Appl Environ Microbiol, 2016, 82(17): 5428-5443. |
| [47] |
Subramanian P, Pirbadian S, El-Naggar MY, et al. Ultrastructure of Shewanella oneidensis MR-1 nanowires revealed by electron cryotomography. Proc Natl Acad Sci USA, 2018, 115(14): E3246-E3255. |
| [48] |
Watanabe K, Manefield M, Lee M, et al. Electron shuttles in biotechnology. Curr Opin Biotechnol, 2009, 20(6): 633-641. |
| [49] |
Lin T, Ding WQ, Sun LM, et al. Engineered Shewanella oneidensis-reduced graphene oxide biohybrid with enhanced biosynthesis and transport of flavins enabled a highest bioelectricity output in microbial fuel cells. Nano Energy, 2018, 50: 639-648. |
| [50] |
Yang Y, Ding YZ, Hu YD, et al. Enhancing bidirectional electron transfer of Shewanella oneidensis by a synthetic flavin pathway. ACS Synth Biol, 2015, 4(7): 815-823. |
| [51] |
Yong YC, Yu YY, Li CM, et al. Bioelectricity enhancement via overexpression of quorum sensing system in Pseudomonas aeruginosa-inoculated microbial fuel cells. Biosens Bioelectron, 2011, 30(1): 87-92. |
| [52] |
Hong GY, Pachter R. Bound flavin-cytochrome model of extracellular electron transfer in Shewanella oneidensis: analysis by free energy molecular dynamics simulations. J Phys Chem B, 2016, 120(25): 5617-5624. |
| [53] |
Okamoto A, Kalathil S, Deng X, et al. Cell-secreted flavins bound to membrane cytochromes dictate electron transfer reactions to surfaces with diverse charge and pH. Sci Rep, 2014, 4: 5628. |
| [54] |
Yu SS, Cheng L, Chen JJ, et al. Framework of cytochrome/vitamin B2 linker/graphene for robust microbial electricity generation. ACS Appl Mater Interface, 2018, 10(41): 35090-35098. |
| [55] |
Yang GQ, Zhang J, Kwon SW, et al. Thauera humireducens sp. nov., a humus-reducing bacterium isolated from a microbial fuel cell. Int J Syst Evol Microbiol, 2013, 63(3): 873-878. |
| [56] |
You LX, Liu LD, Xiao Y, et al. Flavins mediate extracellular electron transfer in Gram-positive Bacillus megaterium strain LLD-1. Bioelectrochemistry, 2018, 119: 196-202. |
| [57] |
Hou HJ, Li L, Cho Y, et al. Microfabricated microbial fuel cell arrays reveal electrochemically active microbes. PLoS One, 2009, 4(8): e6570. |
| [58] |
Mukherjee S, Su SC, Panmanee W, et al. A microliter-scale microbial fuel cell array for bacterial electrogenic screening. Sensor Actuat A Phys, 2013, 201: 532-537. |
| [59] |
Tahernia M, Mohammadifar M, Hassett DJ, et al. A fully disposable 64-well papertronic sensing array for screening electroactive microorganisms. Nano Energy, 2019, 65: 104026. |
| [60] |
Yuan SJ, Li WW, Cheng YY, et al. A plate-based electrochromic approach for the high-throughput detection of electrochemically active bacteria. Nat Protoc, 2014, 9(1): 112-119. |
| [61] |
Yuan SJ, He H, Sheng GP, et al. A photometric high-throughput method for identification of electrochemically active bacteria using a WO3 nanocluster probe. Sci Rep, 2013, 3: 1315. |
| [62] |
He H, Yuan SJ, Tong ZH, et al. Characterization of a new electrochemically active bacterium, Lysinibacillus sphaericus D-8, isolated with a WO3 nanocluster probe. Process Biochem, 2014, 49(2): 290-294. |
| [63] |
Yang ZC, Cheng YY, Zhang F, et al. Rapid detection and enumeration of exoelectrogenic bacteria in lake sediments and a wastewater treatment plant using a coupled WO3 nanoclusters and most probable number method. Environ Sci Technol Lett, 2016, 3(4): 133-137. |
| [64] |
Marques AC, Santos L, Costa MN, et al. Office paper platform for bioelectrochromic detection of electrochemically active bacteria using tungsten trioxide nanoprobes. Sci Rep, 2015, 5: 9910. |
| [65] |
Zhou SG, Wen JL, Chen JH, et al. Rapid measurement of microbial extracellular respiration ability using a high-throughput colorimetric assay. Environ Sci Tech Lett, 2015, 2(2): 26-30. |
| [66] |
Wen JL, Zhou SG, Chen JH. Colorimetric detection of Shewanella oneidensis based on immunomagnetic capture and bacterial intrinsic peroxidase activity. Sci Rep, 2014, 4: 5191. |
| [67] |
Xiao X, Liu QY, Li TT, et al. A high-throughput dye-reducing photometric assay for evaluating microbial exoelectrogenic ability. Bioresour Technol, 2017, 241: 743-749. |
| [68] |
Li LL, Chen Y, Zhu JJ. Recent advances in electrochemiluminescence analysis. Anal Chem, 2017, 89(1): 358-371. |
| [69] |
You LX, Chen NJ, Wang L, et al. Electrochemiluminescence for the identification of electrochemically active bacteria. Biosens Bioelectron, 2019, 137: 222-228. |
| [70] |
Wang QR, Jones AAD, Gralnick JA, et al. Microfluidic dielectrophoresis illuminates the relationship between microbial cell envelope polarizability and electrochemical activity. Sci Adv, 2019, 5(1): eaat5664. |
| [71] |
Ringeisen BR, Othon CM, Barron JA, et al. Jet-based methods to print living cells. J Biotechnol, 2006, 1(9): 930-948. |
| [72] |
Barron JA, Wu P, Ladouceur HD, et al. Biological laser printing: a novel technique for creating heterogeneous 3-dimensional cell patterns. Biomed Microdevices, 2004, 6(2): 139-147. |
| [73] |
Ringeisen BR, Lizewski SE, Fitzgerald LA, et al. Single cell isolation of bacteria from microbial fuel cells and potomac river sediment. Electroanalysis, 2010, 22(7/8): 875-882. |
| [74] |
Fortney NW, He SM, Converse BJ, et al. Investigating the composition and metabolic potential of microbial communities in Chocolate Pots hot springs. Front Microbiol, 2018, 9: 2075. |
| [75] |
Ishii SI, Suzuki S, Norden-Krichmar TM, et al. A novel metatranscriptomic approach to identify gene expression dynamics during extracellular electron transfer. Nat Commun, 2013, 4(1): 1601. |
| [76] |
Ishii SI, Suzuki S, Tenney A, et al. Microbial metabolic networks in a complex electrogenic biofilm recovered from a stimulus-induced metatranscriptomics approach. Sci Rep, 2015, 5: 14840. |
| [77] |
Li F, Wang L, Liu CG, et al. Engineering exoelectrogens by synthetic biology strategies. Curr Opin Electrochem, 2018, 10: 37-45. |
| [78] |
Teravest MA, Ajo-Franklin CM. Transforming exoelectrogens for biotechnology using synthetic biology. Biotechnol Bioeng, 2016, 113(4): 687-697. |
 2020, Vol. 36
2020, Vol. 36