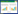中国科学院微生物研究所、中国微生物学会主办
文章信息
- 吴美玉, 阮靖华, 钟伯雄
- Wu Meiyu, Ruan Jinghua, Zhong Boxiong
- 人表皮生长因子的研究进展
- Progress in human epidermal growth factor research
- 生物工程学报, 2020, 36(12): 2813-2823
- Chinese Journal of Biotechnology, 2020, 36(12): 2813-2823
- 10.13345/j.cjb.200209
-
文章历史
- Received: April 14, 2020
- Accepted: August 11, 2020
- Published: August 31, 2020
最初的人表皮细胞生长因子(Human epidermal growth factor,hEGF)是从人的尿液中提取的,但是提取率很低,后来人们尝试利用化学合成法获取hEGF,但依然无法满足要求。随着基因技术的发展,人们利用基因工程技术在不同表达系统中获得具有生物活性的hEGF,包括大肠杆菌Escherichia coli[1]、豆科植物花生[2]、毕赤酵母Pichia pastoris[3]和家蚕[4],逐渐满足hEGF的需求量,使得hEGF的应用研究不断得到了扩展。hEGF是由53个氨基酸组成的单链小分子酸性多肽,具有多种生物学功能,尤其对细胞的增殖、分化和迁移作用显著,从而在器官发生发育[5]和伤口修复[6]中扮演重要角色。近年来,hEGF主要在溃疡和皮肤伤口愈合领域受到越来越多的关注[7]。人们通过改变hEGF的剂型或与其他材料结合来不断完善hEGF的应用[8]。尽管hEGF应用前景较好,但仍需要不断得到优化,从而真正满足临床治疗要求。本文对hEGF的基本特性、作用机制进行简要概述,重点综合近几年来hEGF在胃肠溃疡愈合和伤口修复病理过程中的研究进展,以及在肿瘤的发生发展中所扮演的角色,以期为人们关于hEGF的相关研究提供辅助信息,也为家蚕丝腺生物反应器表达hEGF的研究提供参考。
1 hEGF基因和蛋白的结构与特性表皮生长因子最早发现于啮齿动物[9]。由于该物质能够直接刺激表皮生长和角化,故被命名为表皮生长因子,简称EGF。成熟的hEGF来源于其前体(hEGF precursor)的水解释放。hEGF前体基因全长约110 kb (GenBank,NC_000004.12),位于人类染色体4q25-q27,包括24个外显子[10],如图 1所示,该基因的外显子与低密度脂蛋白(LDL)受体基因[11]和TGF-α前体基因同源[12];而外显子6–9、15和17–19编码8个独立的EGF-like基序,为EGF家族成员的共有序列。其中,除了转化生长因子(TGF-α)和肝素结合性EGF (HBEGF)之外,双调蛋白(AREG)[13]、β纤维素(BTC)[14]、上调蛋白(EREG)和表观基因(EPGN)与EGF一样,均位于人类第4号染色体(见表 1)。hEGF前体蛋白是由1 207个氨基酸组成的Ⅰ型跨膜糖蛋白[15],理论分子量约为134 kDa。由于含有多个糖基化位点[16],随着糖基化水平差异,hEGF前体大小也不一样。在结构上,hEGF前体分为N端延伸区、EGF-like基序、短的近膜茎、疏水区以及C末端[17],其中,疏水区即跨膜结构域,用于固定hEGF前体于细胞膜表面。
|
| 图 1 hEGF基因结构示意图[18] Fig. 1 Schematic view of hEGF gene. The hEGF precursor gene has about 110 kb and 24 exons. Some exons encoded protein segments that are homologuous to sequences in other proteins, like LDL receptor, EGF-like repeats and TGF-α precursor. Mature hEGF was coded by exon 20 and 21. Modified from Zeng FH, et al[18]. |
| |
| EGF gene family members | EGF | TGF-α | AR | BTC | EPR | HBEGF | EPGN |
| Chromosomal localizations | 4q25-q27 | 2p13.3 | 4q13.3 | 4q13.3 | 4q13.3 | 5q31.3 | 4q13.3 |
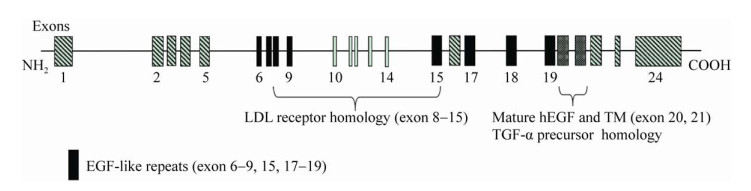
细胞在合成hEGF过程中,首先合成不溶性的hEGF前体,被C末端跨膜结构域锁定在细胞膜表面,经膜表面的蛋白酶分别切割其N端和C端的Arg-Asn和Arg-His连接键[19],从而释放出“可溶性” hEGF,即成熟的hEGF (以下简称hEGF)。hEGF由前体基因外显子20和21所编码(图 1),蛋白分子量大小约为6.2 kDa,等电点pI为4.6;对热、酸、碱稳定,耐受胰蛋白酶和胃蛋白酶等多种酶类。hEGF氨基酸序列中含有6个半胱氨酸残基(图 2),形成的3对分子内二硫键(C6-C20,C14-C31和C33-C42)是hEGF的活性中心;同时,分子内产生了A、B、C三个环,被认为是hEGF结构中最稳定的区域[20]。
2010年,Huang等[21]利用核磁共振法(NMR)测定hEGF的结构(PDB ID:2kv4)。hEGF的二级结构由一个β转角连接的双层反向平行的β折叠(氨基酸残基19–21和30–32)和2个α螺旋组成,一个位于hEGF的N端(Leu8-Gly12),另一个则位于C端(Leu47-Glu51)。Huang等的实验结果显示C端结构域对hEGF与受体的结合具有重要作用,其中起关键作用的一个氨基酸残基是Leu47。hEGF的C端α螺旋周围是一个疏水区,由氨基酸残基Ile38的脂肪侧链与Tyr44、Leu47和Lys48相互作用而形成,Leu47位于疏水区的核心。当hEGF处于游离状态时,C端形成一个疏水核,当hEGF与EGFR形成复合物时,hEGF发生构象改变,C端疏水核被破坏,Leu47暴露出来,从而与EGFR结合,启动下游一系列信号转导。
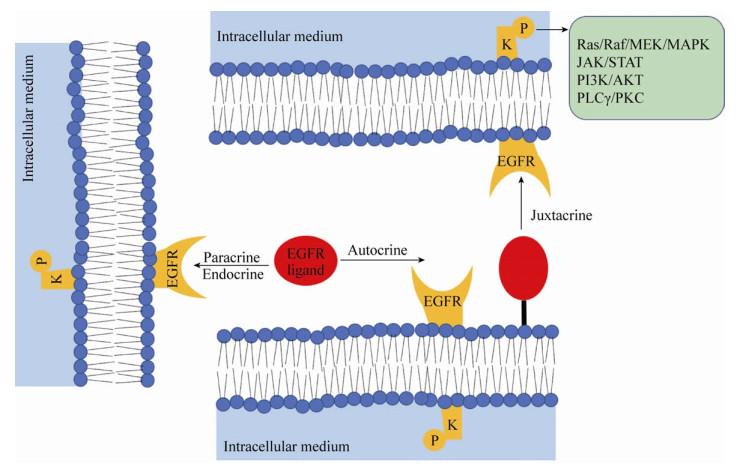
2 hEGF的作用机制及其生物学效应hEGF主要通过与EGF受体(Epidermal growth factor receptor,EGFR)结合发挥生物学功能。EGFR基因[22]全长约110 kb,位于人染色体7p12-14,编码1 210个氨基酸,是一种蛋白分子量为170 kDa的Ⅰ型跨膜糖蛋白[23]。EGFR属于EGFR家族,也被称为ErbB受体或Ⅰ型受体。该家族由4种跨膜受体组成,包括EGFR (ErbB1/HER1)、ErbB2/HER2、ErbB3/HER3和ErbB4/HER4[24-25]。在结构上,EGFR受体主要分为3个部分[26-27]:胞外区、跨膜区和胞内区,其中,胞外区富含半胱氨酸,为高度糖基化的配体结合区。hEGF与EGFR结合的方式有多种,如图 3所示,包括自分泌(Autocrine)、近分泌(Juxtacrine)、内分泌(Endocrine)和旁分泌(Paracrine)[17]。hEGF与EGFR结合后,诱导EGFR形成同源或异源二聚体,激活受体酪氨酸激酶(RTK),从而引起EGFR的C末端结构域中特异的酪氨酸残基磷酸化;磷酸化的酪氨酸残基成为第二信使分子,招募含有SH2 (Src同源2)结构域和PTB (磷酸化酪氨酸结合)结构域的信号分子进行组装,将胞外信号传入细胞内,激活下游一系列多功能化的信号通路,包括Ras/Raf/MEK/ MAPK、JAK/STAT、PI3K/AKT和PLCγ/PKC,主要参与细胞的增殖、分化和存活[28-30]。
|
| 图 3 EGFR配体激活EGFR的不同形式[17] Fig. 3 Different forms of activating EGFR via EGFR ligands. The autocrine form occurs in the same cell; the paracrine and endocrine form in a neighbour and distant cell; the juxtacrine form in a neighbour cell when the ligand is anchored to the cell membrane. Modified from Schneider MR, et al[17]. |
| |
hEGF广泛存在于人体各种组织和体液中,包括血液、乳汁、胃液、羊水等,调节着多种生理和病理过程。如上所述,hEGF与体内EGFR结合后,激活下游信号通路,产生多种生物学效应,包括:对不同来源的多种细胞,比如表皮细胞、上皮细胞和成纤维细胞,均有强烈的促分裂活性,促进细胞内DNA、RNA和蛋白质的合成,能够加速组织的增殖与分化;促进胚胎细胞的增殖与分化,影响器官的发生发育;促进间质细胞的增殖,比如角膜内皮细胞,对角膜相关疾病具有一定的修复治疗价值。另外,hEGF还可以刺激细胞内细胞因子和胶原蛋白酶的产生,有利于伤口修复;抑制胃酸分泌[31],保护胃黏膜免受胃酸侵蚀;影响干细胞逆分化,比如以诱导神经嵴干细胞分化为神经细胞和黑色素细胞[32];增加物质的转运[33-34]和糖代谢等。
3 hEGF的应用研究hEGF广泛存在于人体各种组织和体液中,通过与EGFR结合,激活下游信号通路,参与多种生理和病理调节过程。hEGF具有强烈的促细胞增殖和分化活性,并能刺激细胞内细胞因子的产生,与细胞因子发挥协同作用,有利于体内组织和皮肤的再生和修复,从而在胃肠溃疡和伤口修复领域具有重要价值,尤其随着生物材料研究的兴起而备受关注。然而,基于hEGF促进细胞活和抑制细胞凋亡的效应,其在肿瘤病理过程中也产生了不可忽视的影响。
3.1 hEGF与胃肠溃疡胃溃疡是消化性溃疡的一种,也是困扰多数人的一种常见疾病,发生在胃内壁,主要与胃黏膜受损有关。作为有效的促分裂活性分子,hEGF能够促进胃肠黏膜上皮细胞的增殖分化[35],加速胃黏膜的再生;同时,hEGF具有抑制胃酸分泌以及增加黏膜血流量的作用,进一步改善对胃溃疡的治疗。另外,由于hEGF具有耐酸和蛋白酶的特点,可以长时间作用于黏膜细胞。因此,hEGF在胃肠道溃疡疾病中扮演着重要角色。1991年,Sullivan等[36]首次采用持续性注射hEGF治愈了一名患有类似坏死性肠炎的8个月大的危重患儿。Brzozowski等[37]研究表明,使用EGF、HGF或bFGF对大鼠进行局部治疗,可显著降低胃酸分泌,加快溃疡愈合的速度,并增加胃的血流量和溃疡边缘新血管的生成;Bang等[38]利用EGF内喷雾剂有效地治疗了动物模型的胃溃疡过程中的胃出血,并恢复了胃黏膜厚度。然而,考虑到hEGF促进上皮细胞异常增殖和肿瘤发生的可能,hEGF应用受到一定限制。Mira等[39]研制了一种重组hEGF益生菌(EGF-EcN),能够精准传送hEGF到靶位点,促进EGFR发挥积极作用,在治疗鼠肠溃疡的同时并未加重相关的结肠癌,从而有望成为一种有前景的治疗胃肠溃疡的方案。关于hEGF治疗胃肠道疾病的研究大部分还停留在动物模型阶段,临床用于治疗胃肠道溃疡等疾病的治疗还有待进一步探索,以确保其有效性和安全性。
3.2 hEGF与伤口修复hEGF通过自分泌、旁分泌等途径作用于细胞,激活MAPK、AKT等信号通路,参与细胞的增殖和存活,对表皮细胞、成纤维细胞和上皮细胞等多种类型的细胞具有强大的活性,有利于肉芽组织形成和皮肤创面上皮化;同时,hEGF还可以提高一些内源性生长因子(血管内皮生长因子、血小板衍生生长因子、TGF-α)的合成以及胶原蛋白的合成[40],加快了伤口愈合的速度。hEGF作为伤口愈合剂已被广泛研究,用于治疗糖尿病性溃疡和包括烧伤在内的外科伤口修复。
糖尿病性溃疡是最常见的糖尿病并发症,其中,糖尿病足溃疡(DFU)甚至会导致截肢残疾和早期死亡。在动物模型上,Zhang等[41]探讨了EGF对兔糖尿病足溃疡(DFU)伤口的愈合作用,结果表明EGF可以加速伤口愈合过程,而且外源性EGF可能会上调新生组织中EGF mRNA的表达。在临床研究中,EGF治疗糖尿病足溃疡的疗效也已得到证实。Singla等[42]报道了rhEGF可以缩短糖尿病足溃疡愈合时间;一项包括4项随机对照试验和294例患者的荟萃分析(Meta分析)显示,rhEGF能提高创面愈合率且创面愈合率是对照组的4倍以上[43]。近几年,Xu等[44]也证明了hEGF对糖尿病足溃疡伤口的愈合作用,且与aFGF联合使用时作用更显著;Park等[45]利用rhEGF新型喷雾剂对糖尿病足溃疡患者喷洒,可导致更快的治愈速度和更高的完全治愈率。除了基于对细胞增殖、生长的作用外,hEGF促进溃疡愈合的作用还可能是由于减少氧化应激和恢复患者的全身氧化还原平衡[46-48]。因此,EGF是一种很有前途的治疗糖尿病溃疡的药物。而关于hEGF用于外科伤口的治疗研究,也已有多项研究予以了证实。Alemdaroğlu等[49]和Hong等[50]开发出含有EGF的软膏制剂用于治疗烧伤伤口。不过,软膏形式的EGF用在湿润的伤口区域比较困难,通透性也差,从而降低了治疗效果。2019年,Lu等[51]制备了含有视黄酸可变形脂质体(TRA DLs)和EGF阳离子可变形脂质体(EGF CDLs)的双重脂质体药膏,被认为是用于烧伤治疗的有希望的局部治疗剂,实验证实两种脂质体具有高药物截留效率和持续的药物释放行为,与游离药物相比,表现出优异的皮肤渗透性。
然而,无论是溃疡还是其他类型的伤口,释放的EGF都容易受到创面环境中高度活跃的蛋白酶(比如肽酶)影响,导致有效剂量减少、生物活性差[52-53]。因此,许多研究工作致力于开发有效的EGF传递系统,以提高EGF的生物利用度和治疗效果。例如,已经开发了聚合物贴剂、水凝胶和纳米纤维等,用于EGF的长期受控递送,但EGF的生物利用度依然有限。Kim[54]和Kim等[55]均将EGF与透明质酸(HA)结合制成贴剂用于改善伤口的愈合,延长了EGF的释放和停留时间,提高了其稳定性。Wang等[56]利用聚己内酯和透明质酸制成包裹EGF的纳米纤维支架,能够控制生长因子释放的能力,促进皮肤的增强再生,被认为可以用作伤口愈合的皮肤组织工程。在治疗糖尿病溃疡研究中,为了进一步提高溃疡愈合能力,Choi等[57]开发了结构稳定的hEGF (ST-EGF)和bFGF (ST-EGF)新材料,将ST-EGF和ST-EGF加载到生物相容性载体透明质酸胶原敷料(HCD)基质上,结果证明该系统在室温下长期稳定,可以有效促进糖尿病溃疡创面的愈合。
近几年来,关于hEGF与蚕丝蛋白结合制成伤口敷料的研究比较火热。蚕丝具有良好的生物相容性、生物降解性、高吸水性、低免疫原性和优良的力学性能[58-59]。多项研究将hEGF混合于脱胶后的蚕丝丝素蛋白溶液中再进行纺丝或制膜[60-61];或者采用涂层的方法,将制好的丝素蛋白材料浸泡于hEGF溶液,最终获得hEGF功能化材料[62]。然而,在这些方法中,hEGF会随着时间的流逝而减少,需要通过更换伤口敷料进行更新,以提供足够浓度的hEGF。为了解决这些问题,研究人员利用转基因技术培育出含有hEGF-丝素蛋白偶联基因序列的转基因家蚕[4],提取丝素融合蛋白进行制膜,所得的hEGF功能化丝膜延长了hEGF的作用时间,在动物模型上显著加速了伤口的愈合。hEGF功能化蚕丝材料在伤口敷料的研究方面显示出巨大潜力。
可见,hEGF在伤口修复方面具有巨大的潜力,而关于将其与其他生长因子联合使用或新剂型、新材料的研发将得到进一步探索,以提高hEGF治疗效率,同时还需要更大样本的临床试验数据来验证hEGF的有效性和安全性。
3.3 hEGF与肿瘤EGF作为一种具有强烈促有丝分裂活性的因子,能刺激多种组织细胞分裂和增生,除了修复受损的组织,在肿瘤发生发展过程中也产生着重要影响。EGF通过与EGFR结合,激活各种下游信号分子,促进肿瘤细胞存活、生长和发展[63]。其中,PI3K信号通路参与细胞的抗凋亡,通过作用于Bcl-2家族促进细胞生长,使前凋亡因子Bad和Procaspase 9磷酸化而抑制细胞凋亡,从而促进肿瘤细胞的增生[64]。除了促进癌细胞的增殖,hEGF还参与癌细胞的转移过程。比如,Bracher等[65]发现黑色素瘤细胞分泌的高水平EGF可以通过直接刺激淋巴管内皮细胞、诱导血管内皮生长因子C (VEGF-C)的表达以及增加肿瘤细胞的活性这3种方式来介导其对黑色素瘤淋巴结的转移。尽管hEGF在肿瘤病理过程中扮演着负面角色,但是研究发现hEGF的存在对于癌症的治疗也具有一定的意义。比如,Hao等[66]研究发现EGF和TGF-α可用于评估疾病进展的指标,为临床治疗提供依据;Bracher等[65]发现hEGF可能成为预测淋巴结转移的一种新的预后标志物,对新疗法和随访策略的制定至关重要;Chen等[67]在非小细胞肺癌研究过程中,发现呼气冷凝物中EGF水平的检测对肺癌的诊断、疾病监测和预后也具有重要意义。
目前,关于肿瘤的治疗还是以靶向hEGF受体为主。作为第一个被鉴定到的并与癌症相关的RTK (受体酪氨酸激酶)[24],EGFR在肿瘤过程中的影响至关重要[68]。因此,以EGFR为靶点的肿瘤治疗成为研究热点,比如,EGFR酪氨酸激酶抑制剂(TKIs)[69]吉非替尼和厄洛替尼等,单克隆抗体西妥昔单抗[70]以及抗体药物偶联物ABT-414[71]等。而通过EGF治疗肿瘤的研究也在进行中,比如,Le等[72]利用含有姜黄素的EGF (EGF)偶联脂质体(EGF-LP-Cur)靶向人胰腺癌细胞系中的EGFR,发现该脂质体可以增强姜黄素对人胰腺癌细胞的抗肿瘤活性。尽管一些治疗方法已在肿瘤治疗中取得一定进展,但是其耐药性仍是一个难题[73],而将耐药性最小化或消除掉成为癌症治疗中的重要目标[74]。
4 总结hEGF是较早被发现的具有促进有丝分裂活性的分子,同EGF家族成员一样,均是由前体酶解而来。成熟的hEGF仅含有53个氨基酸,但内部结构牢固,理化性质稳定。在作用机制方面,hEGF主要通过与EGFR结合激活下游多种信号通路而发挥效应,主要表现在对多种细胞具有显著的增殖、迁移等作用。在组织再生和皮肤伤口修复领域,hEGF作为有效的愈合因子,对溃疡、烧伤和外科伤口的治疗具有重要价值,尤其在制备功能性生物材料方面显示出更广泛的应用前景。在肿瘤治疗过程中,作为一种诊断或预后标志物,hEGF对癌症的治疗具有重要意义。虽然人们对于EGF已有较清楚的认识,但仍需要进一步探索,比如,关于前体蛋白的活性和作用机理中复杂的信号网络的研究等。在治疗过程中,由于hEGF半衰期比较短,且容易在受损环境中受多种因素影响,从而易发生降解,需要反复使用或增大剂量才能达到治疗效果,这就可能由于hEGF过度刺激细胞异常增殖而导致细胞增生和肥大,甚至引发肿瘤。基于这种担忧,已有研究人员研制了一种重组hEGF益生菌(EGF-ECN),能够精确稳定地传递EGF到达靶点,解决了口服剂型受消化系统影响而致吸收效率低的问题,在治愈结肠炎的同时并未加重相关癌症的发生[39],为将来研发更有效的药物传递系统提供了参考。类似地,在体外治疗皮肤伤口过程中,由于高浓度hEGF会促进胶原蛋白的高度表达,从而有可能形成纤维化和疤痕。对于这种现象,hEGF合适剂量的控制可能是一个关键点,而上面所描述的能够维持药物稳定并实现药物缓释的复合生物材料有可能改善这一问题。而在肿瘤治疗中,耐药性的解决是一个重要任务,新型抗癌药物有待进一步研究。相信随着科学技术的发展和人们对于EGF的深入认识,我们所担忧的各种问题都将得到改善。
| [1] |
Shams D, Alizadeh M, Azari S, et al. High expression level of human epidermal growth factor (hEGF) using a well-designed fusion protein-tagged construct in E. coli. Bratisl Lek Listy, 2019, 120(10): 757-763. |
| [2] |
Yao QS, Yu ZP, Liu P, et al. High efficient expression and purification of human epidermal growth factor in Arachis hypogaea L. Int J Mol Sci, 2019, 20(8): 2045. DOI:10.3390/ijms20082045 |
| [3] |
Eissazadeh S, Moeini H, Dezfouli MG, et al. Production of recombinant human epidermal growth factor in Pichia pastoris. Braz J Microbiol, 2017, 48(2): 286. |
| [4] |
Bienert M, Hoss M, Bartneck M, et al. Growth factor-functionalized silk membranes support wound healing in vitro. Biomed Mater, 2017, 12(4): 045023. DOI:10.1088/1748-605X/aa7695 |
| [5] |
Wolf CJ, Belair DG, Becker CM, et al. Development of an organotypic stem cell model for the study of human embryonic palatal fusion. Birth Defects Res, 2018, 110(17): 1322-1334. DOI:10.1002/bdr2.1394 |
| [6] |
Ternullo S, Basnet P, Holsæter AM, et al. Deformable liposomes for skin therapy with human epidermal growth factor: The effect of liposomal surface charge. Eur J Pharm Sci, 2018, 125: 163-171. DOI:10.1016/j.ejps.2018.10.005 |
| [7] |
Chouhan D, Mandal BB. Silk biomaterials in wound healing and skin regeneration therapeutics: From bench to bedside. Acta Biomater, 2020, 103: 24-51. DOI:10.1016/j.actbio.2019.11.050 |
| [8] |
Qiang WD, Zhou TT, Lan XX, et al. A new nanoscale transdermal drug delivery system: oil body-linked oleosin-hEGF improves skin regeneration to accelerate wound healing. J Nanobiotechnol, 2018, 16(1): 62. |
| [9] |
Cohen S. Isolation of a mouse submaxillary gland protein accelerating incisor eruption and eyelid opening in the new-born animal. J Biol Chem, 1962, 237: 1555-1562. |
| [10] |
Bell GI, Fong NM, Stempien MM, et al. Human epidermal growth factor precursor: cDNA sequence, expression in vitro and gene organization. Nucleic Acids Res, 1986, 14(21): 8427-8446. DOI:10.1093/nar/14.21.8427 |
| [11] |
Galicia-Garcia U, Benito-Vicente A, Uribe KB, et al. Mutation type classification and pathogenicity assignment of sixteen missense variants located in the EGF-precursor homology domain of the LDLR. Sci Rep, 2020, 10: 1727-1727. DOI:10.1038/s41598-020-58734-9 |
| [12] |
Minder P, Bayha E, Becker-Pauly C, et al. Meprinα transactivates the epidermal growth factor receptor (EGFR) via ligand shedding, thereby enhancing colorectal cancer cell proliferation and migration. J Biol Chem, 2012, 287(42): 35201-35211. DOI:10.1074/jbc.M112.368910 |
| [13] |
Sisto M, Lorusso L, Ingravallo G, et al. Exocrine gland morphogenesis: Insights into the role of amphiregulin from development to disease. Arch Immunol Ther Exp (Warsz), 2017, 65(6): 477-499. DOI:10.1007/s00005-017-0478-2 |
| [14] |
Rush JS, Peterson JL, Ceresa BP. Betacellulin (BTC) biases the EGFR to dimerize with ErbB3. Mol Pharmacol, 2018, 94(6): 1382-1390. DOI:10.1124/mol.118.113399 |
| [15] |
Mroczkowski B, Reich M, Chen K, et al. Recombinant human epidermal growth factor precursor is a glycosylated membrane protein with biological activity. Mol Cell Biol, 1989, 9(7): 2771-2778. DOI:10.1128/MCB.9.7.2771 |
| [16] |
Harvey BM, Haltiwanger RS. Regulation of notch function by O-glycosylation//Borggrefe T, Giaimo B, Eds. Molecular Mechanisms of Notch Signaling. Cham: Springer, 2018, 1066: 59–78.
|
| [17] |
Schneider MR, Wolf E. The epidermal growth factor receptor ligands at a glance. J Cell Physiol, 2009, 218(3): 460-466. DOI:10.1002/jcp.21635 |
| [18] |
Zeng FH, Harris RC. Epidermal growth factor, from gene organization to bedside. Semin Cell Dev Biol, 2014, 28: 2-11. DOI:10.1016/j.semcdb.2014.01.011 |
| [19] |
Le Gall SM, Auger R, Dreux C, et al. Regulated cell surface pro-EGF ectodomain shedding is a zinc metalloprotease-dependent process. J Biol Chem, 2003, 278(46): 45255-45268. DOI:10.1074/jbc.M307745200 |
| [20] |
Watts CR, Lovas S, Murphy RF. Molecular dynamics simulations of epidermal growth factor and transforming growth factor-alpha structures in water. Proteins, 1998, 33(3): 396-407. DOI:10.1002/(SICI)1097-0134(19981115)33:3<396::AID-PROT8>3.0.CO;2-I |
| [21] |
Huang HW, Mohan SK, Yu C. The NMR solution structure of human epidermal growth factor (hEGF) at physiological pH and its interactions with suramin. Biochem Biophys Res Commun, 2010, 402(4): 705-710. DOI:10.1016/j.bbrc.2010.10.089 |
| [22] |
Gazzeri S. Nuclear EGFR: a new mode of oncogenic signalling in cancer. Biol Aujourd'hui, 2018, 212(1/2): 27-33. |
| [23] |
Ullrich A, Coussens L, Hayflick JS, et al. Human epidermal growth factor receptor cDNA sequence and aberrant expression of the amplified gene in A431 epidermoid carcinoma cells. Nature, 1984, 309(5967): 418-425. DOI:10.1038/309418a0 |
| [24] |
Wang ZX. ErbB receptors and cancer//Wang Z, Ed. ErbB Receptor Signaling. New York: Humana Press, 2017, 1652: 3–35.
|
| [25] |
Wang W, Zhao HF, Yao TF, et al. Advanced development of ErbB family-targeted therapies in osteosarcoma treatment. Invest New Drugs, 2019, 37(1): 175-183. DOI:10.1007/s10637-018-0684-8 |
| [26] |
Kovacs E, Zorn JA, Huang YJ, et al. A structural perspective on the regulation of the epidermal growth factor receptor. Annu Rev Biochem, 2015, 84: 739-764. DOI:10.1146/annurev-biochem-060614-034402 |
| [27] |
Coussens L, Yang-Feng TL, Liao YC, et al. Tyrosine kinase receptor with extensive homology to EGF receptor shares chromosomal location with neu oncogene. Science, 1985, 230(4730): 1132-1139. DOI:10.1126/science.2999974 |
| [28] |
Yoshida E, Kurita M, Eto K, et al. Methylmercury promotes prostacyclin release from cultured human brain microvascular endothelial cells via induction of cyclooxygenase-2 through activation of the EGFR-p38 MAPK pathway by inhibiting protein tyrosine phosphatase 1B activity. Toxicology, 2017, 392: 40-46. DOI:10.1016/j.tox.2017.09.013 |
| [29] |
Donohoe F, Wilkinson M, Baxter E, et al. Mitogen-activated protein kinase (MAPK) and obesity-related cancer. Int J Mol Sci, 2020, 21(4): 1241. DOI:10.3390/ijms21041241 |
| [30] |
Bandaranayake RM, Ungureanu D, Shan YB, et al. Crystal structures of the JAK2 pseudokinase domain and the pathogenic mutant V617F. Nat Struct Mol Biol, 2012, 19(8): 754-759. DOI:10.1038/nsmb.2348 |
| [31] |
Koskenpato K, Ainola M, Przybyla B, et al. Diminished salivary epidermal growth factor secretion: a link between Sjögren's syndrome and autoimmune gastritis?. Scand J Rheumatol, 2016, 45(2): 118-121. |
| [32] |
Garcez RC, Teixeira BL, dos Santos Schmitt S, et al. Epidermal growth factor (EGF) promotes the in vitro differentiation of neural crest cells to neurons and melanocytes. Cell Mol Neurobiol, 2009, 29(8): 1087-1091. DOI:10.1007/s10571-009-9406-2 |
| [33] |
Zou ZG, Rios FJ, Montezano AC, et al. TRPM7, magnesium, and signaling. Int J Mol Sci, 2019, 20(8): 1877. DOI:10.3390/ijms20081877 |
| [34] |
Ortega B, Dey JM, Gardella AR, et al. Antibody-mediated inhibition of EGFR reduces phosphate excretion and induces hyperphosphatemia and mild hypomagnesemia in mice. Physiol Rep, 2017, 5(5): e13176. DOI:10.14814/phy2.13176 |
| [35] |
Trinh NT, Privé A, Maillé E, et al. EGF and K+ channel activity control normal and cystic fibrosis bronchial epithelia repair. Am J Physiol Lung Cell Mol Physiol, 2008, 295(5): L866-L880. DOI:10.1152/ajplung.90224.2008 |
| [36] |
Sullivan PB, Brueton MJ, Tabara ZB, et al. Epidermal growth factor in necrotising enteritis. Lancet, 1991, 338(8758): 53-54. |
| [37] |
Brzozowski T, Konturek PC, Konturek SJ, et al. Effect of local application of growth factors on gastric ulcer healing and mucosal expression of cyclooxygenase-1 and -2. Digestion, 2001, 64(1): 15-29. DOI:10.1159/000048835 |
| [38] |
Bang BW, Maeng JH, Kim MK, et al. Hemostatic action of EGF-endospray on mucosectomy-induced ulcer bleeding animal models. Biomed Mater Eng, 2015, 25(1): 101-109. |
| [39] |
Yu M, Kim J, Ahn JH, et al. Nononcogenic restoration of the intestinal barrier by E. coli-delivered human EGF. JCI Insight, 2019, 4(16): e125166. DOI:10.1172/jci.insight.125166 |
| [40] |
Kim YS, Lew DH, Tark KC, et al. Effect of recombinant human epidermal growth factor against cutaneous scar formation in murine full-thickness wound healing. J Korean Med Sci, 2010, 25(4): 589-596. DOI:10.3346/jkms.2010.25.4.589 |
| [41] |
Zhang JZ, Hu WX, Diao QX, et al. Therapeutic effect of the epidermal growth factor on diabetic foot ulcer and the underlying mechanisms. Exp Ther Med, 2019, 17(3): 1643-1648. |
| [42] |
Singla S, Garg R, Kumar A, et al. Efficacy of topical application of beta urogastrone (recombinant human epidermal growth factor) in Wagner's Grade 1 and 2 diabetic foot ulcers: Comparative analysis of 50 patients. J Nat Sci Biol Med, 2014, 5(2): 273-277. DOI:10.4103/0976-9668.136160 |
| [43] |
Yang SW, Geng ZJ, Ma K, et al. Efficacy of topical recombinant human epidermal growth factor for treatment of diabetic foot ulcer: A systematic review and meta-analysis. Int J Low Extrem Wounds, 2016, 15(2): 120-125. DOI:10.1177/1534734616645444 |
| [44] |
Xu JS, Min DH, Guo GH, et al. Experimental study of epidermal growth factor and acidic fibroblast growth factor in the treatment of diabetic foot wounds. Exp Ther Med, 2018, 15(6): 5365-5370. |
| [45] |
Park KH, Han SH, Hong JP, et al. Topical epidermal growth factor spray for the treatment of chronic diabetic foot ulcers: A phase Ⅲ multicenter, double-blind, randomized, placebo-controlled trial. Diabetes Res Clin Pract, 2018, 142: 335-344. DOI:10.1016/j.diabres.2018.06.002 |
| [46] |
Ojalvo AG, Acosta JB, Marí YM, et al. Healing enhancement of diabetic wounds by locally infiltrated epidermal growth factor is associated with systemic oxidative stress reduction. Int Wound J, 2017, 14(1): 214-225. DOI:10.1111/iwj.12592 |
| [47] |
Qi M, Zhou QH, Zeng WQ, et al. Growth factors in the pathogenesis of diabetic foot ulcers. Front Biosci (Landmark Ed), 2018, 23(1): 310-317. DOI:10.2741/4593 |
| [48] |
García-Ojalvo A, Acosta JB, Figueroa-Martínez A, et al. Systemic translation of locally infiltrated epidermal growth factor in diabetic lower extremity wounds. Int Wound J, 2019, 16(6): 1294-1303. DOI:10.1111/iwj.13189 |
| [49] |
Alemdaroğlu C, Degim Z, Celebi N, et al. Investigation of epidermal growth factor containing liposome formulation effects on burn wound healing. J Biomed Mater Res A, 2008, 85(1): 271-283. |
| [50] |
Hong JP, Kim YW, Jung HD, et al. The effect of various concentrations of human recombinant epidermal growth factor on split-thickness skin wounds. Int Wound J, 2006, 3(2): 123-132. |
| [51] |
Lu KJ, Wang W, Xu XL, et al. A dual deformable liposomal ointment functionalized with retinoic acid and epidermal growth factor for enhanced burn wound healing therapy. Biomater Sci, 2019, 7(6): 2372-2382. |
| [52] |
Wöltje M, Böbel M, Bienert M, et al. Functionalized silk fibers from transgenic silkworms for wound healing applications: Surface presentation of bioactive epidermal growth factor. J Biomed Mater Res A, 2018, 106(10): 2643-2652. |
| [53] |
Farokhi M, Mottaghitalab F, Fatahi Y, et al. Overview of silk fibroin use in wound dressings. Trends Biotechnol, 2018, 36(9): 907-922. |
| [54] |
Kim H, Kong WH, Seong KY, et al. Hyaluronate-epidermal growth factor conjugate for skin wound healing and regeneration. Biomacromolecules, 2016, 17(11): 3694-3705. |
| [55] |
Kim YS, Sung DK, Kong WH, et al. Synergistic effects of hyaluronate-epidermal growth factor conjugate patch on chronic wound healing. Biomater Sci, 2018, 6(5): 1020-1030. |
| [56] |
Wang ZB, Qian YN, Li LH, et al. Evaluation of emulsion electrospun polycaprolactone/hyaluronan/ epidermal growth factor nanofibrous scaffolds for wound healing. J Biomater Appl, 2016, 30(6): 686-698. |
| [57] |
Choi SM, Lee KM, Kim HJ, et al. Effects of structurally stabilized EGF and bFGF on wound healing in type Ⅰ and type Ⅱ diabetic mice. Acta Biomater, 2018, 66: 325-334. |
| [58] |
Farokhi M, Mottaghitalab F, Ali Shokrgozar M, et al. Importance of dual delivery systems for bone tissue engineering. J Control Release, 2016, 225: 152-169. |
| [59] |
Farokhi M, Mottaghitalab F, Samani S, et al. Silk fibroin/hydroxyapatite composites for bone tissue engineering. Biotechnol Adv, 2018, 36(1): 68-91. |
| [60] |
Chouhan D, Janani G, Chakraborty B, et al. Functionalized PVA-silk blended nanofibrous mats promote diabetic wound healing via regulation of extracellular matrix and tissue remodelling. J Tissue Eng Regen Med, 2018, 12(3): e1559-e1570. |
| [61] |
Chouhan D, Chakraborty B, Nandi SK, et al. Role of non-mulberry silk fibroin in deposition and regulation of extracellular matrix towards accelerated wound healing. Acta Biomater, 2017, 48: 157-174. |
| [62] |
Gil ES, Panilaitis B, Bellas E, et al. Functionalized silk biomaterials for wound healing. Adv Healthc Mater, 2013, 2(1): 206-217. |
| [63] |
Wise R, Zolkiewska A. Metalloprotease-dependent activation of EGFR modulates CD44+/CD24– populations in triple negative breast cancer cells through the MEK/ERK pathway. Breast Cancer Res Treat, 2017, 166(2): 421-433. |
| [64] |
Jeyamohan S, Moorthy RK, Kannan MK, et al. Parthenolide induces apoptosis and autophagy through the suppression of PI3K/Akt signaling pathway in cervical cancer. Biotechnol Lett, 2016, 38(8): 1251-1260. |
| [65] |
Bracher A, Cardona AS, Tauber S, et al. Epidermal growth factor facilitates melanoma lymph node metastasis by influencing tumor lymphangiogenesis. J Invest Dermatol, 2013, 133(1): 230-238. |
| [66] |
Hao CF, Li ZL, Zhang XB, et al. Expression and clinical significance of EGF and TGF-α in chronic pancreatitis and pancreatic cancer. Minerva Endocrinol, 2018, 43(3): 253-258. |
| [67] |
Chen JL, Chen JR, Lv XD, et al. Epidermal growth factor in exhaled breath condensate as diagnostic method for non-small cell lung cancer. Technol Cancer Res Treat, 2019, 18: 1533033819872271. |
| [68] |
Sigismund S, Avanzato D, Lanzetti L. Emerging functions of the EGFR in cancer. Mol Oncol, 2018, 12(1): 3-20. |
| [69] |
Macdonald-Obermann JL, Pike LJ. Allosteric regulation of epidermal growth factor (EGF) receptor ligand binding by tyrosine kinase inhibitors. J Biol Chem, 2018, 293(35): 13401-13414. |
| [70] |
Mendelsohn J, Baselga J. Status of epidermal growth factor receptor antagonists in the biology and treatment of cancer. J Clin Oncol, 2003, 21(14): 2787-2799. |
| [71] |
Van Den Bent M, Gan HK, Lassman AB, et al. Efficacy of depatuxizumab mafodotin (ABT-414) monotherapy in patients with EGFR-amplified, recurrent glioblastoma: results from a multi-center, international study. Cancer Chemother Pharmacol, 2017, 80(6): 1209-1217. |
| [72] |
Le UM, Hartman A, Pillai G. Enhanced selective cellular uptake and cytotoxicity of epidermal growth factor-conjugated liposomes containing curcumin on EGFR-overexpressed pancreatic cancer cells. J Drug Target, 2018, 26(8): 676-683. |
| [73] |
McLoughlin EM, Gentzler RD. Epidermal growth factor receptor mutations. Thorac Surg Clin, 2020, 30(2): 127-136. |
| [74] |
Roskoski R Jr. Small molecule inhibitors targeting the EGFR/ErbB family of protein-tyrosine kinases in human cancers. Pharmacol Res, 2019, 139: 395-411. |
 2020, Vol. 36
2020, Vol. 36