中国科学院微生物研究所、中国微生物学会主办
文章信息
- 万家悦, 贺希格都楞, 彭位华, 安玲瑶, 江琼, 杨佳敏, 朱诚
- Wan Jiayue, Bao Hexigeduleng, Peng Weihua, An Lingyao, Jiang Qiong, Yang Jiamin, Zhu Cheng
- 玉米与番茄间作对土壤镉吸收的影响
- Effects of intercropping on cadmium uptake by maize and tomato
- 生物工程学报, 2020, 36(3): 518-528
- Chinese Journal of Biotechnology, 2020, 36(3): 518-528
- 10.13345/j.cjb.190116
-
文章历史
- Received: March 29, 2019
- Accepted: June 12, 2019
- Published: November 21, 2019
With economic development, the exploitation of mineral resources, as well as the widespread use of pesticides and fertilizers containing heavy metals, has resulted in an alarming increase in heavy metal pollution[1]. According to a recent survey of farmland soils in China (covering 5.48 million hm2 in key polluted areas of 24 provinces and cities), farmland polluted by heavy metals exceeds 80%[2]. Cadmium (Cd) is one of the most serious sources of pollution among the heavy metals recorded in the farmland soils. This heavy metal is characterized by having a long holding time, poor mobility, and being difficult to decompose in soil. High levels of Cd are harmful to human health, resulting in damage to the intestine, stomach, lungs, circulatory system and the nervous system[3]. Therefore, in order to control and improve the status of Cd pollution in farmland, and to ensure food security and sustainable agricultural development, it is important to urgently explore efficient and safe soil remediation methods which are easy to be used.
Although there exist soil remediation methods such as physical repair, chemical repair and bioremediation, current practices have disadvantages such as having poor repair effects, single effects and secondary pollution. Intercropping, a traditional intensive cultivation model used in China, has been established as a suitable method to improve land productivity[4]. Previous studies have shown that intercropping can change the uptake of heavy metals by changing soil properties, including changes in plant root exudates, soil enzyme activities, soil microbes and soil pH. These changes affect bioavailability and mobility of heavy metals in soil, thus affecting the uptake of heavy metals by plants and improving the remediation efficiency of soil heavy metal pollution[5-6]. For example, under Cd and Pb stress, intercropping of leguminous crops such as Maize (Zea mays), Mucuna (Mucuna pruriens), Okra (Abelmoschus esculentus) and Kenaf (Hibiscus cannabinus) with legumes significantly increased the uptake of Cd[7-8]. Intercropping of broad bean and sedge reduced the effective Cd, Pb and Zn content in the soil and improved the quality of the broad beans[9]. Under the intercropping method, the content of heavy metals in tobacco leaves was greatly reduced, playing an important and positive role in production[10]. The use of phytoremediation practices is cost-effective and environmentally friendly, and they have been the subject of numerous studies in recent years, focusing on the remediation of heavy metal contamination in soil. However, limitations to long-term remediation are also associated with this method.
In this study, two common plants (tomato and maize) grown in the Yangtze River Delta were selected as the target species. Tomato (Lycopersicon esculentum. var. Zhongshu 4) is a relatively high accumulator of Cd, while maize (Zea mays L. var. Jinzhumi) is a relatively low accumulator of Cd. The differences and mechanisms of Cd uptake by crops under different planting modes were studied by measuring Cd content, soil pH, soil enzyme activity and root microbial diversity. Our results may provide a reference for further exploration of the effects of intercropping on the uptake of heavy metals by plants, and for the use of crops which have a high rate of heavy metal accumulation to remediate heavy metal contaminated soils.
1 Materials and methods 1.1 Plants and soilThe pot experiment was conducted in the city of Hangzhou, Zhejiang province, China, from April to August. Two plant species were used in the experiment: (1) tomato (Lycopersicon esculentum var. Zhongshu 4), and (2) maize (Zea mays L. var. Jinzhumi). Plant seeds were purchased from the Hangzhou Seeds Company. In order to strictly control the heavy metal content, moisture, nutrients, temperature and light conditions, and to avoid outside interference, a pot experiment was carried out using three cropping modes: monoculture, restricted intercropping and intercropping[11].
Soil for the experiment was collected from the surface layer (0-20 cm) of an unpolluted paddy field in Hangzhou city. Physical and chemical properties of the soil were measured before planting (Table 1). The soil was sieved using a 2-mm grid sieve and air dried (composition of grain is 65% diameter 2-0.02 mm, 28.8% diameter 0.02-0.002 mm and 6.2% diameter less than 0.002 mm). According to the pre-experiment, we chose 4.00 mg/kg as the target concentration of soil Cd and the Cd-treated soil was prepared by manually mixing soil with a CdSO4 solution and letting it equilibrate for 30 days. To ensure Cd homogeneity, the soil was turned every 5 days during the 30-day period. Before planting, the Cd level of the soil was determined to be 3.70 mg/kg.
| pH | Organic matter (%) | Total nitrogen (%) | Available P (mg/kg) | Available K (mg/kg) | Total Cd (mg/kg) |
| 5.76 | 18.3 | 0.11 | 12.3 | 114 | 0.18 |
Nine kilograms of soil was added to one plastic rectangular pot (30 cm×15 cm×15 cm), in which one tomato and one maize plant were cultivated. The pots were separated into the three modes using the following methods (Fig. 1): (1) separated using a plastic (PE) barrier (monoculture), (2) separated using a nylon mesh (0.025 mm) (restricted intercro- pping), and (3) no separation (intercropping).

|
| Fig. 1 Root boxes used for the three planting patterns. |
| |
Seeds were germinated in soil mixed with vermiculite and peat (V:V=1:1). Three to five tomato seeds and two maize seeds were sown in each well, watered with deionized water and cultured at room temperature; seeds germinated after about one week. As maize grows slower than tomatoes, it was sown one week earlier. After the seedlings had grown to a height of about 5 cm, the most robust seedling in each well was kept and the remaining seedlings were removed. One month after tomato and maize plants began growing, transplanted the seedlings into a root box containing Cd-treated soil. One tomato and one maize plant were planted in each box.
Experiments were replicated four times in a randomized complete block design. The pots were located outside under a glass shelter to protect them from the rain. During the experiment, deionized water, compound fertilizer and pesticides were applied according to the weather and crop growth conditions, and all compound fertilizers and pesticides have been tested for Cd content below 0.05 mg/kg, which will not affect the experiment. Plants and soils were harvested two months later (July 3th), and rhizosphere soil was collected by shaking the roots. Soils from control pots (with no vegetation planted) were collected at the same time.
1.3 Sample processing and indicator testingAll plant samples were thoroughly washed with distilled water and then dried. The underground portion of the plants was immersed in EDTA solution for 20 min to sequester heavy metals adsorbed on the root surface. Plants were divided into roots, stems and leaves, and then weighed. Plant tissues were then oven-dried at 70 ℃ (until there was no change in weight) and ground using a stainless-steel grinder. Soil samples were air-dried, crushed and passed through a 0.082 mm sieve.
Take the dried plant and soil samples for digestion and all samples were digested with a mixture of HNO3-HClO4 (V:V=4:1) until completely clear[12]. Cd levels in plants and soils were determined by atomic absorption spectrometry (SLAARAA-M6). Soil pH was determined using a glass electrode in a 2:5 (W/V) soil: distilled water mixture. Acid phosphatase and urease activity determination were performed using the methods of Nannipieri[13] and Tabatabai[14].
1.4 Investigation of microorganism diversityDNA extraction of rhizosphere soil around the plants from the three planting patterns followed the methods of Zhou[15]. 16S rRNA gene fragments were PCR amplified following the procedure described by Muyzer[16]. The oligonucleotide primers were 5′-ATTACCGCGGCTGCTGG-3′ (forward primer) and 5′-CGCCCGCCGCGCGCGG CGGGCGGGGCGGGGGCACGGGGGGCCTACGGGAGGCAGCAG-3′ (reverse primer). The PCR reaction system contained Ex buffer (10×) 5.0 μL, dNTPs (2.5 mmol/L each) 5.0 μL, forward primer (10 μmol/L) 1 μL, reverse primer (10 μmol/L) 1 μL, Ex Taq DNA polymerase (TaKaRa) 0.5 μL, DNA template of about 50 ng, and 36.5 μL sterile distilled water. Cycling conditions were: one cycle of 94 ℃ for 3 min, followed by 35 cycles of 94 ℃ for 1 min, 55 ℃ for 1 min, 72 ℃ for 1.5 min, and one final cycle of 72 ℃ for 10 min. PCR products were checked prior to DGGE by electrophoresis in 1.5% (W/V) agarose gels stained with ethidium bromide (EB). All products were stored at 4 ℃.
The 16S rRNA gene fragments were separated using the DGGE system on an 8% (W/V) polyacryl- amide gel containing a linear gradient from 30% to 55% denaturant solution. Following polymerization with TEMED (Tetramethylethy lendiamine) and ammonium persulfate and sample loading, the electrophoresis conditions used were 5 h at 160 V and 60 ℃ in TAE buffer. Gels were dyed with 0.2% AgNO3 and the dominant bands were excised and soaked in 10 μL TE buffer (pH 8.0) at 4 ℃ overnight. PCR was then performed to gain the 16S rRNA gene fragments of each DGGE bands using TE buffers (as above) as the templates. Primers of 16S rRNA gene without GC-rich sequence (forward primer: 5′-ATTACCGCGGCTGCTGG-3′, reverse primer: 5′-CCTACGGGAGGCAGCAG-3′) were used in the PCR. The PCR products were sequenced by the Biosune Biotechnology Company of Shanghai.
Nucleotide sequences were compared with sequences in the National Center for Biotechnology Information (NCBI) GenBank database (http://www.ncbi.nlm.nih.gov/) using the BLASTn program.
1.5 Statistical analysisIn order to detect significant differences in the data, analysis of variance (ANOVA) was performed, followed by the least significant difference test (LSD) at the P=0.05 level.
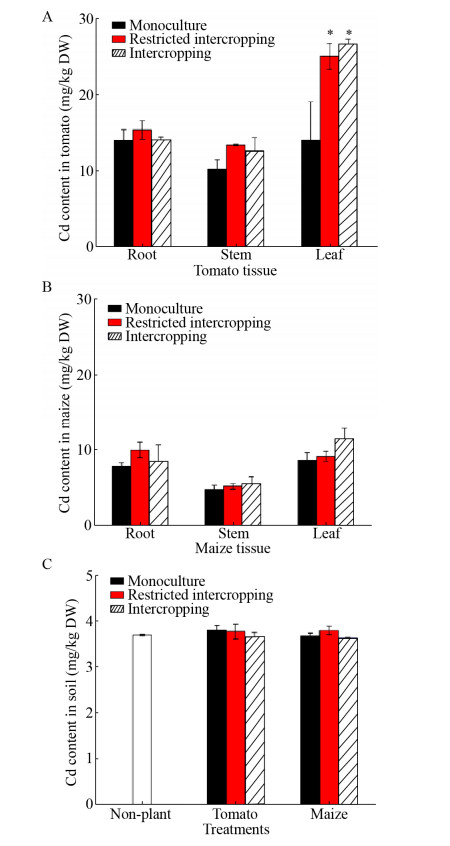
2 Results and discussion 2.1 Effects of intercropping on Cd content in tomato and maizeCompared with the monoculture method, results for restricted intercropping and intercropping recorded increased levels of Cd in tomato stems and leaves, and in the roots, stems and leaves of maize (Fig. 2A and 2B). However, ANOVA results showed that only the Cd content in tomato leaves significantly increased under restricted intercropp-ing and intercropping (P < 0.05).
|
| Fig. 2 Cd content in tomato (A), maize (B) and soil (C) under the three planting patterns. * indicated significant differences at P < 0.05 level. |
| |
Cd concentrations in soil samples (Fig. 2C) were recorded to be 3.70 mg/kg in the control pots. Cd levels in tomato rhizosphere soils in monoculture pots, restricted intercropping pots and intercropping pots were 3.81, 3.77 and 3.63 mg/kg, respectively. Cd levels in rhizosphere soils in monoculture pots, restricted intercropping pots and intercropping pots for maize were 3.76, 3.85 and 3.68 mg/kg, respectively. Although Cd levels in rhizosphere soil for tomato under the intercropping method and maize under the monoculture recorded a decreasing trend, ANOVA indicated that there was no significant alteration in Cd contents of rhizosphere soil between the different planting patterns. This finding was due to the limited phytoextraction capability of Cd by tomato and maize only in one growth-cycle.
The change of the available Cd showed that the tomato monoculture group, the maize monoculture group and the intercropping group of the two crops (including the restricted intercropping) showed a similar downward trend. Compared with the soil available Cd content of non-planted group (1.39 mg/kg), the available Cd contents in the tomato monoculture group and the maize monoculture group decreased to 1.01 mg/kg and 1.27 mg/kg, respectively, while the contents were decreased to 1.07 mg/kg and 1.16 mg/kg in the intercropping and restricted intercropping groups, respectively. However, the difference in the available Cd content between the various planting methods did not reach a significant level. Moreover, compared with the biomass per plant of tomato and maize grown in non- contaminated soil, the biomass per plant did not change significantly in each treatment group. It showed that the effect of maize on the production of available Cd was negligible, indicating that the effect of the intercropping group on the production of various forms of Cd is mainly due to the role of tomato plants. The tomato intercropping group and the monoculture group had similar effects on the available Cd, indicating that the intercropping improved the tomato's ability to affect the effective Cd, and to some extent improved the remediation efficiency of the tomato Cd pollution.
Previous studies have also shown that the use of Cd high-accumulation herb crops can effectively reduce the uptake of heavy metals in the soil by intercropping with water spinach; this method can also reduce soil Cd content to a certain extent[17]. It can be seen that when plant species with different heavy metal uptake capacities are interplanted, the uptake capacity of Cd by relatively high accumulation plants will be improved in this study. Therefore, plants with high and low heavy metal accumulation properties can be used in intercropping to improve the phytoremediation efficiency of heavy metal contaminated soil. However, results in our study indicated that there was no significant difference in the uptake of Cd by maize under different planting patterns. This finding may be due to limitations on maize growth associated with the pot experiment, the physical and chemical properties of the soil on maize seeds, or to the difference in planting time. In addition, under the conditions of intercropping and limiting intercropping, the uptake trend of Cd in tomato plants was similar, indicating that Cd uptake by tomato plants was through the nylon mesh rather than by direct contact with roots. It can be seen that the process of Cd uptake by crops is not affected by the direct connection of roots, but it may be affected by the transport of microorganisms, soil pH, enzyme activities and other substances in the nylon mesh[18-19].
2.2 Effects of intercropping on crop root pH and soil enzyme activitySoil pH was recorded as 5.28 in the control sample (Fig. 3A). pH levels (monoculture, restricted intercropping and intercropping) were recorded to decrease (5.28, 5.15 and 5.11, respectively) in the tomato rhizosphere soil and increase (5.34, 5.45 and 5.42, respectively) in the maize rhizosphere soil. However, statistical analysis showed that there was no significant change in soil pH for either tomato or maize.

|
| Fig. 3 Soil pH (A), urease (B) and acid phosphatase (C) under the three planting patterns. Different characters indicate significant differences at P < 0.05 level. |
| |
pH is critical to the chemical form and adsorption efficiency of heavy metals in soils. Under different planting patterns, changes in soil pH may be one of the most important factors for crops to absorb Cd[15]. Previous studies have shown that after intercropping of legumes and maize, pH of the roots of leguminous plants significantly decreased, thereby increasing the bioavailability and mobility of heavy metals in the soil[6]. It is speculated that intercropping decreased pH levels in the tomato rhizosphere soil, a change which may contribute to enhanced Cd uptake by tomato plants. Although the pH of maize roots slightly increased, it may not be obvious because the uptake capacity of Cd was limited.
Compared with the soil of uncultivated crops (Fig. 3B, white column), urease activity in tomato and maize rhizosphere soil decreased with monoculture and restricted intercropping; urease activity in tomato and maize soil under intercropping had an increasing trend. However, ANOVA results indicated that there was no significant difference (Fig. 3B).
In contrast to urease, the activity of acid phosphatase in tomato and maize roots increased under the three planting patterns (Fig. 3C). The highest activity was recorded in the tomato intercropping treatment group and the acid phosphatase activity of this group was higher than that of unplanted group; The tomato intercropping treatment group increased by 32.2%, followed by the restricted maize treatment group (16.4%). Our results suggest that intercropping can increase the acid phosphatase activity of crop rhizosphere soil.
There are significant differences in the sensitivity of different soil enzymes to heavy metals, but heavy metal pollution generally has a certain inhibitory effect on soil enzyme activities[16, 20]. Changes in soil enzyme activity also affect crop heavy metal uptake, and changes in soil enzyme activity caused by intercropping may be another important factor affecting the ability of crops to absorb Cd. Results by Yan et al. showed that intercropping for five years using garlic and tomato resulted in increased soil enzyme activity (catalase, sucrase, urease and alkaline phosphatase activity), and that intercropped soil available potassium content was higher than that recorded under monoculture[21]. It showed that group soil can greatly improve the effectiveness of soil heavy metals. In our study, compared with monoculture, the urease and acid phosphatase activities of tomato roots increased under intercropping (including restrictive intercropping), especially the acid phosphatase content increased significantly and higher than the no-plant treatment group, which may be important for increasing the bioavailability of Cd in the soil around tomato roots and increasing Cd uptake.
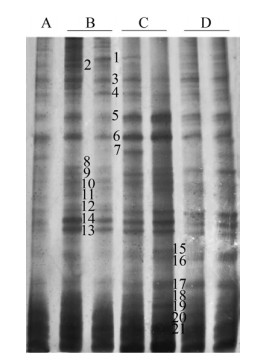
2.3 Bacterial population in rhizosphere soilSequencing of soil DGGE bands under different planting patterns (Fig. 4) recorded many bands on the PCR-DGGE gel, indicating that there are different types of microbial populations in the soil[22]. The DGGE strips of the three different planting methods were significantly different from the soils of the non-planted pot, and the number of control strips of the non-planted pot was the least (Fig. 4A), indicating that the microbial population had the lowest abundance. Previous studies have also shown that the abundance of microbial populations in plant roots is often ten to one hundred times farther away from the soil of plants [23-24].
|
| Fig. 4 DGGE banding pattern of the 16S rRNA in soil under different cropping patterns. (A) Non-planted soil. (B) Monoculture soil. (C) Restricted intercropping soil. (D) Intercropping soil. The left column of B, C, and D is the root soil of tomato, and the right column is the soil of maize root. |
| |
DNA sequence analysis of the dominant 21 bands was shown in Table 2. Nucleotide sequence alignment showed that the 21 microbial populations belonged to four Phyla: Acidobacteria, Actinomycetes, Proteobacteria and Bacteroidetes. Most microbial populations are associated with heavy metal antagonism, such as strips 2 and 9: Betaproteo; strips 3 and 6: Acidobacteria; strips 5, 12 and 16: Bacteroidetes; strip 15: Proteobacterium; and strip 17: Xanthomonadaceae[25-29].
| DGGE band | Accession number | Closest match by BLASTn search | Sequence identity (%) | Phylogenetic affiliation |
| 1 | FM209083.1 | Uncultured Acidimicrobiales bacterium | 100 | Actinobacteria |
| 2 | EU169048.1 | Uncultured beta Proteobacterium | 99 | β-proteobacteria |
| 3 | AY921847.1 | Uncultured Acidobacteria bacterium | 100 | Acidobacteria |
| 4 | JF833700.1 | Uncultured Arthrobacter sp. clone | 98 | Actinobacteria |
| 5 | JN408944.1 | Uncultured Bacteroidetes bacterium | 99 | Bacteroidetes |
| 6 | JN409253.1 | Uncultured Acidobacteria bacterium | 98 | Acidobacteria |
| 7 | GU257882.1 | Uncultured Sphingobacteriaceae bacterium | 99 | Sphingobacteria |
| 8 | GQ225156.1 | Uncultured fungus clone | 98 | |
| 9 | EF173332.1 | Uncultured beta Proteobacterium | 97 | β-proteobacteria |
| 10 | HQ821468.1 | Uncultured gamma Proteobacterium clone | 95 | γ-proteobacteria |
| 11 | HM724466.1 | Uncultured bacterium clone | 99 | |
| 12 | JN409002.1 | Uncultured Bacteroidetes bacterium | 98 | Bacteroidetes |
| 13 | HM658876.1 | Uncultured bacterium clone | 100 | |
| 14 | GQ502268.1 | Uncultured bacterium clone | 99 | |
| 15 | GQ242694.1 | Uncultured Proteobacterium | 96 | Proteobacteria |
| 16 | FJ569360.1 | Uncultured Bacteroidetes bacterium | 100 | Bacteroidetes |
| 17 | HM438443.1 | Uncultured Xanthomonadaceae bacterium | 99 | γ-proteobacteria |
| 18 | AM936464.1 | Uncultured Sphingobacteriales bacterium | 98 | Sphingobacteria |
| 19 | GU219915.1 | Uncultured Sphingomonadaceae bacterium | 99 | α-proteobacteria |
| 20 | JN002873.1 | Uncultured Actinobacterium | 98 | Actinobacteria |
| 21 | AM936152.1 | Uncultured Acidimicrobidae bacterium | 96 | Actinobacteria |
| Note: phylogenetic affiliation of nucleotide sequences derived based on BLAST comparisons to the GenBank database. | ||||
Compared with the control group, the strips of the three different planting treatment groups (B-D in Fig. 4) were brighter. Under the monoculture, the brightness of the genus Arthrobacter (strip 4) was brighter than that of the intercropping treatment. More brighter strips indicating that the abundance of this type of microorganisms is higher in monoculture. Bacteroides (stripe 12 and 16), Sphingidae (strips 7 and 18), Proteobacteria (strip 15) and yellow single cells family (strip 17), treated with intercropping and restriction intercropping, compared to monocropping is relatively bright, indicating that the abundance of these micro-organisms is relatively high during intercropping.
Metabolites secreted by soil microbes can increase the ability of heavy metals to move, thereby changing the ability of plants to absorb heavy metals, and they may also play an important role in Cd uptake under intercropping[30-31]. Results from our study show that different planting methods have an impact on microbial diversity in Cd-contaminated soils, and the effects of different intercropping methods on soil microbial environment differ.
Although results by Zhou et al[32] showed that continuous monoculture resulted in a decrease in the diversity of microbial communities in the soil, intercropping can result in a stable microbial population diversity. The results also explain that intercropping can indeed increase soil species abundance and keep microbial population diversity stable. Additionally, soil biodiversity and microbial population structure also have an impact on the ability of crops to absorb heavy metals. It has been reported by Moreels[33] that soil microorganisms play an important role in soil heavy metal repair. His treatment group without added Ni was more abundant than the target microbial population of S. sphaericus in the Ni treatment group, and the bioavailability of Ni in the soil was also stronger. The study by Liu et al examining water, sediments and eight dominantfamilies in a copper tailings pond recorded that only the relative abundance of the Sphingomonas family had a significant positive correlation with heavy metal content such as Cd. This finding indicates that the majority of bacteria are inhibited by heavy metals, and Sphingomonas bacteria are highly tolerant to heavy metals, thereby having the potential to repair heavy metal contamination[34]. These findings indicate that microorganisms of Sclerotium and Pseudobacteria can increase the ability of plants to absorb heavy metals.
In our study, the majority of microbial populations of bacteria isolated from crop root soil were associated with heavy metal antagonism, including β-proteobacteria, acid bacillus, Bacteroides, Proteobacteria and Xanthomonas. On the one hand, compared with the monoculture treatment, the microbial population abundance of S. sphaericus, Bacteroides, Proteobacteria and Xanthomonas increased during intercropping, and the abundance of these two microbial populations in intercropped tomato root soil was high. In the monocultrue group, this may be an important factor in promoting the uptake of Cd in tomato during intercropping. On the other hand, the abundance of microbial populations of the genus Arthrobacter decreased. According to Yohey et al[35], microorganisms of this genus can accumulate heavy metals in their own cells, thus being considered to be one of the most fixed heavy metal potential microorganisms. Recent findings by Li et al[36] show that Arthrobacter bacteria are widely distributed in various environments, especially soils and water bodies. This bacterium has a strong environmental adaptability and resistance, including efficient degradation of organic pollutants in the environment and adsorption of heavy metals, therefore having the potential to be used for bioremediation of contaminated water and soil. In our experiment, the microorganisms of Arthrobacter were highly abundant in the tomato root soil of the single treatment group, and it is possible to accumulate Cd in their cells to achieve more Cd fixation. The decrease in bioavailability due to the decrease in the ability of tomato plants to absorb Cd. Therefore, microorganisms of the Arthrobacter genus play an important role in reducing the uptake of Cd by tomatoes.
In summary, soil pH, soil enzyme activity and soil root microbes may play an important role in crop Cd uptake. These effects are not independent, and their interactions affect each other and affect the ability of plants to absorb Cd. Although a substantial amount of research has been undertaken on microorganisms related to heavy metal resistance, little is known about the mechanism of heavy metal tolerance and the effect of microorganisms on the uptake of heavy metals. Further research is therefore needed to examine these problems. In this study, changes of soil pH, soil enzyme activity and bacterial community in crop roots under different planting patterns were studied, and the mechanism of plant uptake of Cd was explored. Previous findings have shown that cash crops can change their own uptake of Cd through intercropping, and to some extent, the use of super-accumulated crops such as Sedum alfredii Hance, Solanum nigrum L, and Lonicera japonica, which can absorb more Cd than normal crop[37-39]. Using intercropping with phytoremediation techniques may result in long-term defects, such as prolonging the repair period and having low economic benefit. The results, however, provide new ideas for phytoremediation of heavy metals in soil.
3 ConclusionIn this study, in order to examine the effects of intercropping on the uptake of Cd by crops and their possible mechanisms, three cropping methods (monoculture, intercropping and restriction intercropping) were investigated. Heavy metal uptake by the different planting methods was studied using a high and a low heavy-metal accumulating plant species (tomato and maize, respectively). Results from our study indicated that: (1) intercropping (including restrictive intercropping) can improve the ability of tomato to absorb and accumulate Cd, especially in its leaves, while this cropping method has little effect on maize; (2) intercropping will change soil pH and restore soil enzyme activity, thus affecting the bioavailability of soil Cd and its crop uptake characteristics; (3) intercropping can increase the abundance of the soil microbial population, especially the microbial abundance that affects the uptake or inhibition of heavy metals in crops, thus affecting the ability of crops to absorb heavy metals. Findings from this study provide a perspective for using the intercropping model to control soil heavy metal pollution and to ensure food safety. However, in actual agricultural production, it is necessary to pay attention to the high-accumulation crops and the food safety problems caused by these high accumulators.
| [1] |
Hu WY, Huang B, Tian K, et al. Heavy metals in intensive greenhouse vegetable production systems along Yellow Sea of China: levels, transfer and health risk. Chemosphere, 2017, 167: 82-90. DOI:10.1016/j.chemosphere.2016.09.122 |
| [2] |
Han SH. A comparative study on removal performance of mercury and cadmium in agricultural soils for several plants[D]. Shanghai: Donghua University, 2012 (in Chinese). 韩少华.几种植物对Hg、Cd污染农田土壤修复效果的比较研究[D].上海: 东华大学, 2012. |
| [3] |
Xiao WD, Ye XZ, Zhang Q, et al. Evaluation of cadmium transfer from soil to leafy vegetables: Influencing factors, transfer models, and indication of soil threshold contents. Ecotoxicol Environ Safe, 2018, 164: 355-362. DOI:10.1016/j.ecoenv.2018.08.041 |
| [4] |
Agegnehu G, Ghizaw A, Sinebo W. Yield performance and land-use efficiency of barley and faba bean mixed cropping in Ethiopian highlands. Eur J Agron, 2006, 25(3): 202-207. DOI:10.1016/j.eja.2006.05.002 |
| [5] |
Dai CC, Hui X, Wang XX, et al. Intercropping peanut with traditional Chinese medicinal plants improves soil microcosm environment and peanut production in subtropical China. Afr J Biotechnol, 2009, 8(16): 3739-3746. |
| [6] |
Antoniadis V, Robinson JS, Alloway BJ. Effects of short-term pH fluctuations on cadmium, nickel, lead, and zinc availability to ryegrass in a sewage sludge- amended field. Chemosphere, 2008, 71(4): 759-764. DOI:10.1016/j.chemosphere.2007.10.015 |
| [7] |
Li NY, Li ZA, Zhuang P, et al. Cadmium uptake from soil by maize with intercrops. Water Air Soil Poll, 2009, 199(1/4): 45-56. |
| [8] |
Azeez JO, Hassan OA, Adesodun JK, et al. Soil metal sorption characteristics and its influence on the comparative effectiveness of EDTA and legume intercrop on the phytoremediative abilities of maize (Zea mays), Mucuna (Mucuna pruriens), Okra (Abelmoschus esculentus), and Kenaf (Hibiscus cannabinus). J Soil Contam, 2013, 22(8): 930-957. DOI:10.1080/15320383.2013.770442 |
| [9] |
Yu BG, Qin L, Zhan FD, et al. Effects of intercropping on Pb, Cd, and Zn compounds in Cyperus glomeratus and Vicia faba. J Agro-Environ Sci, 2018, 37(4): 621-631. 于保港, 秦丽, 湛方栋, 等. 间作对莎草与蚕豆体内铅镉锌化学形态分布的影响. 农业环境科学学报, 2018, 37(4): 621-631. |
| [10] |
Chen YF, Cheng WZ, Jiang ZL, et al. Effect of Different intercropping methods on the reduction of heavy metal content in flue-cured tobacco. Meteorol Environ Res, 2018, 9(2): 88-90. |
| [11] |
Husson O. Redox potential (Eh) and pH as drivers of soil/plant/microorganism systems: a transdisciplinary overview pointing to integrative opportunities for agronomy. Plant Soil, 2013, 362(1/2): 389-417. |
| [12] |
Altundag H, Albayrak S, Dundar MS, et al. Investigation of the influence of selected soil and plant properties from sakarya, turkey, on the bioavailability of trace elements by applying an in vitro digestion model. Biol Trace Elem Res, 2015, 168(1): 276-285. DOI:10.1007/s12011-015-0330-7 |
| [13] |
Nannipieri P, Pedrazzini F, Arcara PG, et al. Changes in amino acids, enzyme activities and biomass during soil microbial growth. Soil Sci, 1979, 127(1): 26-34. |
| [14] |
Tabatabai MA, Bremner JM. Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol Biochem, 1969, 1(4): 301-307. DOI:10.1016/0038-0717(69)90012-1 |
| [15] |
Zhou J, Bruns MA, Tiedje JM. DNA recovery from soils of diverse composition. Appl Environ Microbiol, 1996, 62(2): 316-322. DOI:10.1128/AEM.62.2.316-322.1996 |
| [16] |
Muyzer G, de Waal EC, Uitterlinden AG. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl Environ Microbiol, 1993, 59(3): 695-700. DOI:10.1128/AEM.59.3.695-700.1993 |
| [17] |
Meng N, Wang M, Chen L, et al. Remediation efficiency of Cd polluted soil by intercropping with herbaceous plants. China Environ Sci, 2018, 38(7): 2618-2624. 孟楠, 王萌, 陈莉, 等. 不同草本植物间作对Cd污染土壤的修复效果. 中国环境科学, 2018, 38(7): 2618-2624. |
| [18] |
Dhima KV, Lithourgidis AS, Vasilakoglou IB, et al. Competition indices of common vetch and cereal intercrops in two seeding ratio. Field Crop Res, 2007, 100(2/3): 249-256. |
| [19] |
Mao LL, Zhang LZ, Li WQ, et al. Yield advantage and water saving in maize/pea intercrop. Field Crop Res, 2012, 138: 11-20. DOI:10.1016/j.fcr.2012.09.019 |
| [20] |
Rungruang N, Babel S. Cadmium removal potential from contaminated soil by Tagetes erecta L. and Panicum maximum: influence of soil pH. J Soil Contam, 2016, 25(2): 133-150. DOI:10.1080/15320383.2016.1111859 |
| [21] |
Yan WM, Cheng ZH, Yuan PL, et al. Effects of different garlic intercropping patterns on enzyme activities and available nutrients of tomato field under plastic tunnel. J Northwest A & F Univ Nat Sci Ed, 2016, 44(1): 125-132. 闫伟明, 程智慧, 袁鹏丽, 等. 不同套蒜模式对大棚番茄土壤酶活性和速效养分的影响. 西北农林科技大学学报:自然科学版, 2016, 44(1): 125-132. |
| [22] |
Tan Y, Chen Q, Liu HJ, et al. Bacteria community in different aged Coptis chinensis planting soil revealed by PCR-DGGE analysis. China J Chin Mat Med, 2015, 40(16): 3147-3151. 谭渊, 陈强, 刘汉军, 等. 不同种植年限黄连根系土壤细菌PCR-DGGE分析. 中国中药杂志, 2015, 40(16): 3147-3151. |
| [23] |
Roy S, Labelle S, Mehta P, et al. Phytoremediation of heavy metal and PAH-contaminated brownfield sites. Plant Soil, 2005, 272(1/2): 277-290. |
| [24] |
Courtney R, Harris JA, Pawlett M. Microbial community composition in a rehabilitated bauxite residue disposal area: a case study for improving microbial community composition. Restor Ecol, 2015, 22(6): 798-805. |
| [25] |
Bollmann A, Palumbo AV, Lewis K, et al. Isolation and physiology of bacteria from contaminated subsurface sediments. Appl Environ Microbiol, 2010, 76(22): 7413-7419. DOI:10.1128/AEM.00376-10 |
| [26] |
Barns SM, Cain EC, Sommerville L, et al. Acidobacteria phylum sequences in uranium-contaminated subsurface sediments greatly expand the known diversity within the phylum. Appl Environ Microbiol, 2007, 73(9): 3113-3116. DOI:10.1128/AEM.02012-06 |
| [27] |
Kielak A, Pijl AS, Veen JAV, et al. Phylogenetic diversity of Acidobacteria in a former agricultural soil. ISME J, 2009, 3(3): 378-382. DOI:10.1038/ismej.2008.113 |
| [28] |
Akob DM, Mills HJ, Kostka JE. Metabolically active microbial communities in uranium-contaminated subsurface sediments. FEMS Microbiol Ecol, 2010, 59(1): 95-107. |
| [29] |
Azarbad H, Niklińska M, Laskowski R, et al. Microbial community composition and functions are resilient to metal pollution along two forest soil gradients. FEMS Microbiol Ecol, 2015, 91(1): 1-11. |
| [30] |
Li L, Li SM, Sun JH, et al. Diversity enhances agricultural productivity via rhizosphere phosphorus facilitation on phosphorus-deficient soils. Proc Natl Acad Sci USA, 2007, 104(27): 11192-11196. DOI:10.1073/pnas.0704591104 |
| [31] |
Kuffner M, Puschenreiter M, Wieshammer G, et al. Rhizosphere bacteria affect growth and metal uptake of heavy metal accumulating willows. Plant Soil, 2008, 304(1/2): 35-44. |
| [32] |
Zhou XG, Yu GB, Wu FZ. Effects of intercropping cucumber with onion or garlic on soil enzyme activities, microbial communities and cucumber yield. Eur J Soil Biol, 2011, 47(5): 279-287. DOI:10.1016/j.ejsobi.2011.07.001 |
| [33] |
Moreels D, Crosson C, Garafola C, et al. Microbial community dynamics in uranium contaminated subsurface sediments under biostimulated conditions with high nitrate and nickel pressure. Environ Sci Poll Res, 2008, 15(6): 481-491. DOI:10.1007/s11356-008-0034-z |
| [34] |
Liu JX, Li J, Jing JH, et al. Composition and environmental adaptation of microbial community in Shibahe copper tailing in Zhongtiao mountain in Shanxi. Environ Sci, 2017, 38(1): 318-326. 刘晋仙, 李毳, 景炬辉, 等. 中条山十八河铜尾矿库微生物群落组成与环境适应性. 环境科学, 2017, 38(1): 318-326. |
| [35] |
Suzuki Y, Banfield JF. Resistance to, and accumulation of, uranium by bacteria from a uranium-contaminated site. Geomicrobiol J, 2004, 21(2): 113-121. DOI:10.1080/01490450490266361 |
| [36] |
Li J, Uwaremwe C, Leng Y, et al. Progress on the study of biodegradation of organic pollutants and adsorption of heavy metals with arthrobacter strains. Environ Sci Technol, 2017, 40(10): 89-97. 李娟, UwaremweC, 冷艳, 等. 节杆菌属细菌处理有机物和重金属污染物的研究进展. 环境科学与技术, 2017, 40(10): 89-97. |
| [37] |
Shahid M, Arshad M, Kaemmerer M, et al. Long-term field metal extraction by pelargonium: phytoextraction efficiency in relation to plant maturity. Int J Phytoremediat, 2012, 14(5): 493-505. DOI:10.1080/15226514.2011.604689 |
| [38] |
Fidalgo F, Freitas R, Ferreira R, et al. Solanum nigrum L. antioxidant defence system isozymes are regulated transcriptionally and posttranslationally in Cd-induced stress. Environ Exp Bot, 2011, 72(2): 312-319. DOI:10.1016/j.envexpbot.2011.04.007 |
| [39] |
Ren J, Wang FH, Zhai YB, et al. Effect of sewage sludge hydrochar on soil properties and Cd immobilization in a contaminated soil. Chemosphere, 2017, 18: 627-633. |
 2020, Vol. 36
2020, Vol. 36




