
中国科学院微生物研究所、中国微生物学会主办
文章信息
- 仲召鹏, 胡小松, 郑浩, 王小斐
- Zhong Zhaopeng, Hu Xiaosong, Zheng Hao, Wang Xiaofei
- 膳食脂肪、肠道微生物与宿主健康的研究进展
- Crosstalk among dietary lipids, gut microbiome, and host metabolic health
- 生物工程学报, 2021, 37(11): 3836-3852
- Chinese Journal of Biotechnology, 2021, 37(11): 3836-3852
- 10.13345/j.cjb.210442
-
文章历史
- Received: June 9, 2021
- Accepted: September 24, 2021
脂肪是人类膳食的重要组成部分,是人体重要的能量和营养来源。近年来随着经济水平的提高,人们的膳食结构逐渐向高热量饮食转变,膳食脂肪的供能比从1992年的28.4%上升到现在的36.4%[1]。膳食脂肪的摄入增加诱发出包括肥胖、糖尿病、脂肪肝、心血管疾病、肾病等慢性代谢疾病,衍生出一系列健康问题。
人体肠道内的菌群含量数以亿计,密度高达1011个/g[2]。肠道微生物能够影响宿主对膳食的摄取能力,与宿主的消化、营养代谢、免疫、行为等密切相关[3]。膳食脂肪经小肠后,不能被小肠吸收的脂肪将进入结肠,进而被肠道微生物代谢生成短链脂肪酸等次级代谢产物。不同的肠道菌对膳食脂肪的代谢能力不同,进而影响肠道微生物的结构和功能。因此,膳食脂肪通过改变肠道微生物的组成以及代谢功能,进一步对宿主的健康产生影响。肠道微生态与膳食脂肪及其摄入不当引起的慢性疾病成为近年来的研究热点。
尽管人们意识到膳食脂肪摄入不当会破坏肠道微生物的稳态,引起血清、脂肪组织、肝脏和大脑中次级代谢产物的变化[4],但目前对膳食脂肪与肠道微生物的研究还是微乎其微,两者之间的作用机制还不明确。本文将基于膳食脂肪、肠道微生物、宿主代谢以及目前其在动物模型中的研究,阐述膳食脂肪如何调节肠道微生物的结构和功能,进而影响宿主健康,展望基于肠道微生物以膳食脂肪为靶点的预防代谢性疾病的前景。
1 膳食脂肪摄入对宿主健康的影响膳食脂肪中主要存在3种类型的脂肪酸:饱和脂肪酸(Saturated fatty acid,SFA)、单不饱和脂肪酸(Monounsaturated fatty acid,MUFA) 和多不饱和脂肪酸(Polyunsaturated fatty acid,PUFA)。长链多不饱和脂肪酸(Long-chain polyunsaturated fatty acid,LC-PUFA) 可分为n-3和n-6脂肪酸,例如二十碳五烯酸(Eicosapentaenoic acid,EPA) 和二十二碳六烯酸(Docosahexaenoic acid,DHA) 属于n-3脂肪酸[5-6]。
膳食脂肪有动物、植物、微生物等来源。常见的动物来源脂肪有猪油、牛油、乳脂肪等;植物来源脂肪有豆油、玉米油、花生油、橄榄油、菜籽油、棕榈油等;微生物来源脂肪又称作单细胞油脂,是指从某些微生物例如酵母菌、藻类等细胞内提取得到的可食用油脂[7]。
膳食脂肪的不当摄入会驱动机体能量不平衡,引发慢性低度炎症、肥胖症和心血管疾病等代谢性疾病[8]。相较于食物中的蛋白质和碳水化合物的含量,脂肪含量能在更大程度上影响宿主的代谢,不同类型的脂肪摄入会导致不同甚至相反的结果。
1.1 膳食脂肪含量对宿主健康的影响脂肪的过量摄入会因能量摄入增加导致过剩的能量以脂肪的形式积累在体内,常伴有糖、脂肪和水盐代谢异常,进而诱发肥胖、糖尿病、脂肪肝等代谢性疾病。对食用不同水平膳食脂肪的健康人的研究发现,控制热量摄入时,较高的脂肪摄入量会导致较高的粪便脂肪和胆汁酸排泄[9]。高脂饮食(High fat diet,HFD) 的摄入会增加血液中的游离脂肪酸和内毒素如脂多糖(Lipopolysaccharides,LPS) 的含量,进而激活Toll样受体从而导致促炎细胞因子的产生,活化的炎症巨噬细胞经血液到达肝脏、脂肪、肌肉组织和大脑,从而诱发全身性低度炎症[10-12]。与常规饮食相比,饲喂高脂饮食(猪油和植物脂肪混合物,40%摄入脂肪能量) 的小鼠胰岛素受体的酪氨酸磷酸化降低和胰岛素抵抗受体的丝氨酸磷酸化升高,导致胰岛素抵抗;伴随着大脑中β淀粉样蛋白沉积和突触可塑性下降,小鼠认知能力下降[13]。高脂饮食主要是通过参与宿主代谢过程如能量传递、免疫信号因子表达和胰岛素产生等,产生低度炎症、肠道疾病、中枢神经系统疾病和诱发多种代谢疾病等效果。近年来生酮饮食(饮食中脂肪占总营养素约60%) 被认为是一种高脂肪饮食,主要摄入饱和脂肪酸和单不饱和脂肪酸,对减肥和抗癫痫病等有一定作用[14],饮食中高脂肪和低碳水造成三羧酸循环的代谢效率降低,导致脂肪酸氧化产生大量乙酰辅酶A,进而合成酮体,进入血液循环;酮体作为葡萄糖的替代能源,或通过血脑屏障进入脑中参与能量代谢、神经递质、离子通道和氧化应激作用过程;但长期摄入生酮饮食的安全性还有待进一步的探究[15]。
作为膳食中三大宏量营养素之一,脂肪为宿主提供能量维持体温,参与机体代谢活动。膳食脂肪摄入不足可能会降低身体的代谢能力,导致营养不良。对于跑步运动员来讲,脂肪摄入量低会减弱运动能力[16]。尤其是多不饱和脂肪酸的摄入不足会导致心脏相关疾病的增加。针对1990年到2010年涉及186个国家的数据分析发现,心脏相关疾病(高血压、心脑血管疾病、冠心病等) 患者中因脂肪摄入过量而死亡的有25万例,相比之下源于n-6多不饱和脂肪酸摄入不足导致的患者高达71万例[17]。因此,不仅膳食脂肪的含量能影响宿主的健康,膳食脂肪的类型也会因为其饱和程度、双键位置等对宿主的健康造成不同程度的影响。
1.2 膳食脂肪类型对宿主健康的影响不同脂肪酸类型在机体内合成和代谢的途径不同,相互不可替代,协同竞争相互作用,共同调节人体健康。对成人膳食脂肪摄入量和健康结果的系统评价结果显示,用PUFA和MUFA代替SFA可改善血脂和血糖控制,其中PUFA的作用更大,这表明用PUFA和MUFA替代SFA并避免食用工业反式脂肪酸(Trans fatty acid,TFA) 是合理的[18]。
1.2.1 饱和脂肪酸棕榈酸(Palmitic acid,C16:0) 是膳食中主要的饱和脂肪酸,主要存在于肉制品、黄油和棕榈油中。研究发现,过多的饱和脂肪酸摄入与全因死亡风险较高有关,而在能量保持恒定的情况下,饱和脂肪酸会增加死亡率和偶发心血管疾病的风险[19]。膳食饱和脂肪酸进入细胞后,细胞会将其代谢为构成细胞膜的磷脂分子,长而直的饱和脂肪链倾向于紧密规则地排列聚集,使脂质分子“固相”化,因此,高饱和脂肪酸的摄入导致细胞膜的流动性不复存在,产生脂毒性进而造成肥胖、糖尿病等代谢性疾病[20]。此外,摄入高饱和脂肪酸组(来源于红肉和黄油) 的65岁以上女性受试者认知和记忆功能随着时间的推移显著退步[21]。
1.2.2 单不饱和脂肪酸膳食中的单不饱和脂肪酸主要是油酸(Oleic acid,C18:1),主要来源于橄榄油、菜籽油等植物油脂[22]。单不饱和脂肪酸会通过影响与冠心病相关的各种标志物,包括血清脂肪、脂蛋白、血管功能标志物和餐后血管功能等来影响心血管健康[23]。在一项人类饮食干预研究中,与食用富含饱和脂肪酸的组相比,食用富含单不饱和脂肪酸组的人低密度脂蛋白胆固醇和甘油三酸酯水平下降,伴随血浆磷脂和中性脂质油酸水平的增加,这表明摄入富含MUFA的饮食对血脂和凝血因子Ⅶc具有良好的效果,是预防心脏病的有效饮食[24]。此外,一项在西班牙儿童中开展的横截面调查发现MUFA摄入增加与健康的血脂谱相关[25]。
1.2.3 多不饱和脂肪酸多不饱和脂肪酸对心血管疾病、癌症、抗炎和中枢神经系统有益处,大部分是人类饮食中的必需脂肪酸[26-27],主要来源于深海鱼油以及豆油、亚麻籽油等植物油。n-6 PUFA包括亚油酸(Linoleic acid,C18:2, LA)、γ-亚麻酸(γ-linoleinic acid,C18:3,GLA) 和花生四烯酸(Arachidonic acid,C20:4,AA),n-3 PUFA主要有α-亚麻酸(α-linoleinic acid,C18:3,ALA)、二十碳五烯酸(Eicosapentaenoic acid,C20:5,EPA) 和二十二碳六烯酸(Docosahexaenoic acid,C22:6,DHA)。
流行病学和临床试验数据表明,多不饱和脂肪酸的摄入和冠心病等心血管疾病的发病率呈负相关。研究表明,补充n-3 LC-PUFA能让个体冠心病死亡风险和冠心病发生事件下降大约10%[28]。摄入富含n-3的芥花籽油减少血浆中低密度脂蛋白胆固醇受体的浓度,显著降低了甘油三酯的浓度,从而降低了心血管疾病的风险[29]。
多不饱和脂肪酸能明显抑制肿瘤的发生、增殖和转移,可用于肿瘤病人的辅助治疗[30]。给秀丽隐杆线虫饲喂富含二高-γ-亚麻酸(Dihomo-γ- linolenic acid,DGLA) 的食物后,会导致线虫生殖细胞肥大和不育,使细胞死亡,如果将DGLA精确递送至人类癌细胞,同样会诱发细胞肥大症,并导致癌细胞死亡[31]。DHA的代谢产物环氧树脂二十二碳五烯酸可以抑制新的血管生成,通过切断肿瘤氧气和营养物质的供应,抑制肿瘤的生长和蔓延[32]。
多不饱和脂肪酸,尤其DHA是大脑中脂肪酸的主要成分,中枢神经系统细胞中的膜磷脂酰乙醇胺(脑磷脂) 中含量丰富,占总脂肪酸的24%–27%[33]。在神经系统发育和记忆功能方面,n-3脂肪酸在保持血脑屏障完整性中发挥着至关重要的作用,血脑屏障保护中枢神经系统免受血源性细菌、毒素和其他病原体的伤害[34-35]。长期服用n-3脂肪酸使受试者的精神衰弱、精神分裂症等其他精神病的发病几率降低,推迟高危对象精神病性障碍的首次发病[36]。高水平的n-3脂肪酸和大脑前扣带皮层的较大容量相关,增强老年个体的认知灵活性[37]。补充n-3脂肪酸使轻度认知障碍患者单核细胞β-淀粉样蛋白的吞噬作用显著增加,但阿尔兹海默症患者的增加量却不明显[38]。
多不饱和脂肪酸还是炎性介质底物的主要来源。食用富含亚油酸的植物油后,亚油酸在体内会转化为花生四烯酸(AA),同时增加环氧合酶-2的表达,将花生四烯酸转化为促炎性类花生酸进而影响炎症途径,导致低度炎症、氧化应激、内皮功能障碍和动脉粥样硬化[39];与之相反的是,n-3 PUFA在改变血脂谱和膜脂成分并影响类花生酸生物合成、细胞信号级联反应和基因表达中起主要作用,从而减少AA代谢引起的炎症[40]。
1.2.4 反式脂肪酸反式脂肪酸(TFA) 是反式非共轭双键结构的不饱和脂肪酸,大多是在工业食品制造过程中产生的,如植物油和鱼油的氢化[41]。增加TFA的摄入会降低内皮功能,上调血浆细胞因子和低密度脂蛋白胆固醇的水平,从而导致心血管疾病[42]。TFA通过靶向细胞凋亡信号调节激酶1促进细胞外ATP诱导的细胞凋亡,可能是TFA引起动脉粥样硬化的发病机制。冠心病患者摄入反式脂肪酸后红细胞谷胱甘肽过氧化物酶和超氧化物歧化酶等抗氧化指标下降,过氧化氢酶活性、共轭二烯和丙二醛含量上升,并且血浆和红细胞中反式脂肪酸含量上升,这表明高水平的反式脂肪酸摄入与氧化应激和脂质过氧化的诱导高度相关[43]。
2 膳食脂肪摄入对肠道微生物的影响膳食营养是塑造肠道微生物最为重要的因素[44]。改变饮食可以在较短时间内调节肠道微生物的结构和功能,进而影响宿主的代谢情况。肠道微生态失衡与很多疾病发病机制相关,如肥胖、心血管代谢疾病、免疫炎症等,与宿主肠道直接接触的饮食是导致许多新陈代谢紊乱的重要因素。作为人体重要的营养物质之一,膳食脂肪的种类和数量会影响肠道菌群的组成和数量;同时肠道微生物又能参与宿主的代谢调控从而影响肠道屏障功能,进而影响宿主健康[45]。
2.1 膳食脂肪含量对肠道微生物的影响近年来研究发现,高脂饮食会引起宿主肠道微生物的变化,而这种变化与宿主代谢表型的改变存在着密切的关系。膳食脂肪对肠道微生物的影响研究主要集中在高脂与低脂水平的对比上(表 1)。与饲喂对照组小鼠(10%摄入脂肪能量) 相比,饲喂高脂饮食的小鼠(60%摄入脂肪能量) 盲肠和结肠粘膜中拟杆菌门(Bacteroidetes) 和厚壁菌门(Firmicutes) 的比率有显著下降,伴随体重、血糖和肝脏三酰甘油水平的显著升高[58]。长期的高脂饮食导致乳杆菌属(Lactobacillus spp.)、双歧杆菌属(Bifidobacterium spp.)、拟杆菌-普氏菌属(Bacteroides-Prevotella spp.) 等肠屏障保护功能菌属含量下降,反之可以产生硫化氢和内毒素脂多糖(LPS) 的破坏肠屏障功能的硫酸盐还原菌的丰度显著增加。高脂饮食(60%脂肪含量饮食) 会造成小鼠肠道菌多样性和丰度发生变化,并且引起瘤胃菌科(Ruminococcaceae) 的减少和理研菌科(Rikenellaceae) 的增加[4]。在饲喂12周高脂饮食的小鼠肠道中,厚壁菌门(Firmicutes) 和变形菌门(Proteobacteria) 趋于增加,拟杆菌门(Bacteroidetes) 和疣微菌门(Verrucomicrobia) 趋于减少,对宿主代谢有益的艾克曼菌Akkermansia muciniphila数量显著降低[59]。肠道微生态的失衡使肠道通透性增加,血液中肠源性毒素的含量增加,进而造成宿主慢性炎症反应。血液中过量的LPS通常会与Toll样受体4 (Toll like receptor 4,TLR4) 结合,激活免疫细胞释放肿瘤坏死因子α (Tumor necrosis factor α,TNF-α) 等炎性因子,进一步对胰岛素的信号转导通路造成破坏,最终导致胰岛素抵抗、糖尿病等代谢性疾病[60]。总而言之,在动物饲喂高脂饮食后,肠道菌的一个明确的变化是厚壁菌门(Firmicutes) 和拟杆菌门(Bacteroidetes) 的比例增加,即高脂饮食摄入导致厚壁菌门的增加,而肠道微生态的变化进而导致糖尿病、炎症等疾病的发生发展。生酮饮食作为高脂肪饮食也会导致宿主的肠道菌群的变化,小鼠在摄入以氢化植物油和玉米油(70%摄入脂肪能量) 为脂肪组分的生酮饮食后,艾克曼菌Akkermansia muciniphila和狄氏副拟杆菌Parabacteroides distasonis的丰度增加,进一步研究发现这两种菌对生酮饮食的抗癫痫作用是必需的[14]。
| Dietary sources | Dietary fat | Gut microbes | References |
| Animal source | Milk (SFA 65%) Lard (SFA 39%) |
Milk: Bacteroidetes↑, Firmicutes↓, Bilohila wadsworthia↑ Lard: Firmicutes↑, Bacteroidetes↓ |
[46] |
| Lard (45% E) Fish oil (45% E) |
Lard: Bacteroides, Bilophila wadsworthia, Lactobacillus↑ Fish oil: Bifidobacterium, Lactobacillus, Streptococcus, Akkermansia muciniphila↑ |
[47] | |
| Lard (48 wt%); control (12 wt%); Lard (48% E); control (12% E) | Lard (48 wt%): Clostridiales spp.↑, Bacteroides↓ Lard (fat 48% E): Clostridiales↑, Bacteroides↓ |
[48] | |
| Lard (4 wt%) Soybean oil (4 wt%) |
Lard: Firmicutes/Bacteroidetes, Verrucomicrobia, Tenericutes, Akkermansia↑ Soybean oil: Helicobacter↑ |
[8] | |
| Plant source | 45% E: palm oil, olive oil, safflower oil (0.4, 1.1, 7.8) Low fat palm oil (10% E) |
High fat palm oil: Firmicutes/Bacteroidetes, Clostridium↑, Bacteroides↓ High fat olive oil: Bacteroides↑, Clostridium, Fibrobacteres↓ |
[49] |
| Flax oil (n-3 LC-PUFA 50.3%) Soybean oil (n-6 LC-PUFA 54.3%) Mixed soybean oil (SFA-53.2%) | Flax oil: Bacteroides↓10% Soybean oil: Bacteroides↓12%, Porphyromonadaceae↓ Mixed soybean oil: Bacteroides↓28%, Lachnospiraceae↓ |
[50] | |
| Low fat (12% E) High fat (45% E): palm oil (SFA); olive oil (MUFA); safflower oil (n-6 PUFA); rapeseed oil/fish oil (n-3 PUFA) |
High fat palm oil vs Low fat: Bacteroides↓, Lachnospiraceae↑ High fat olive oil vs Low fat: Bacteroides↑, Erysipelotrichaceae, Allobaculum↑ High fat safflower oil vs Low fat: Lachnospiraceae↓ High fat rapeseed oil/fish oil: Allobaculum, Erysipelotrichaceae, Bifidobacterium↑ |
[51] | |
| Plant source | Corn oil (6 mL/kg) Fish oil (6 mL/kg) Control (PBS) Ketogenic diet (80 E%) |
Corn oil: Bacteroides, Clostridium↑; Fish oil: Bacteroides spp., Clostridium↓, Lactobacillales spp.↑; Vegetable shortening, Corn oil: Akkermansia muciniphila, Parabacteroides distasonis↑ |
[52] [14] |
| PUFA | High fat diet (fat 50% E) High fat diet+n-3 LC-PUFA Control (fat 12.3% E) Control+n-3 LC-PUFA |
High fat diet: Bacteroidetes↓, Firmicutes↑ High fat diet+ n-3 LC-PUFA vs high fat diet: Bacteroidetes↑, Firmicutes↓ Control+ n-3 LC-PUFA vs control: Bacteroidetes↑ |
[53] |
| n-3 LC-PUFA (EPA, DHA 1 g/100 g) n-3 LC-PUFA deficiency | n-3 LC-PUFA: Actinobacteria↑, Tenericutes↓, Bifidobacterium, Lactobacillus↑ n-3 LC-PUFA deficiency: Bacteroidetes↓ |
[54] | |
| n-3 LC-PUFA (2.6 LA, 0.4 ALA, 0.35 EPA, 0.66 DHA, 2.6 n-6 LC-PUFA, 1.53 n-3 LC-PUFA) n-3 LC-PUFA deficiency (2.6 wt% LA, 2.6 n-6 LC-PUFA) |
n-3 LC-PUFA: Bacteroidetes↑, Firmicutes/Bacteroidetes↓ n-3 LC-PUFA deficiency: Tenericutes, Anaeroplasma, Gelria, Cyanobacteria, Coriobacteriaceae, Thermoanaerobacter Acaea, Anaeroplasmataceae↑ |
[55] | |
| 80% EPA, 20% DHA, 1 g/(kg·d) Control |
80% EPA, 20% DHA, 1 g/(kg·d): Butyrivibrio, Actinobacteria↑, Proteobacteria↓ | [56] | |
| 0.16% AA, 0.16% DHA Control |
0.16% AA, 0.16% DHA: Bifidobacteria↑, Clostridia, Enterobacteriaceae↓ | [57] |
除了高脂饮食之外,膳食脂肪中饱和脂肪酸、单不饱和脂肪酸和多不饱和脂肪酸对肠道菌群结构和功能的影响显著不同(表 1)。与鱼油或大豆油相比,饲喂猪油的大鼠粪便中富含疣微菌门(Verrucomicrobia)、软壁菌门(Tenericutes) 和艾克曼菌Akkermansia muciniphila[61]。类似地,高饱和脂肪酸(猪油来源)饮食喂养的小鼠肠道微生物多样性和丰富度降低,拟杆菌门(Bacteroidetes) 的丰度降低,厚壁菌门(Firmicutes) 丰度提高,从而改变宿主脂肪组织炎症和脂肪生成。反之,摄入MUFA或者来源于鱼油的n-3 PUFA可以增加肠道微生物的多样性,降低了厚壁菌门(Firmicutes) 与拟杆菌门(Bacteroidetes) 的比率,并产生短链脂肪酸,缓解肥胖、肠炎等疾病[46, 62]。多不饱和脂肪酸中n-3和n-6被认为对肠道微生物影响有益,在野生型小鼠(C57BL/6) 研究中,将饮食从富含n-6 PUFA向富含n-3 PUFA切换2个月后,产脂多糖(LPS) 和促炎性的肠道细菌丰度下降,抑制LPS和消炎性细菌如变形杆菌门(Proteobacteria) 的数量增加[63],可以推测n-3 PUFA可能会抑制引起肥胖和炎症相关的肠道微生物的生长,促进有益菌的生长。
Caesar等将猪油、豆油和鱼油按照脂肪功能比(45%) 添加到小鼠饮食中,发现猪油组中拟杆菌门(Bacteroidetes)、沃氏嗜胆菌Bilophila wadsworthia丰度较高,而鱼油组中双歧杆菌Bifidobacterium、乳杆菌Lactobacillus、链球菌Streptococcus和艾克曼菌Akkermansia muciniphila的相对丰度较高[47]。艾克曼菌Akkermansia muciniphila的丰度与体内甘油三酯的含量呈负相关,被认为在控制肥胖等代谢疾病中起着重要作用[64]。坚持摄入富含n-3 PUFA的沙丁鱼6个月使初发糖尿病患者体内厚壁菌门(Firmicutes) 与拟杆菌门(Bacteroidetes) 比例下降,拟杆菌门普雷沃氏菌属Bacteroides Prevotella丰度增加,抗炎因子脂联素的生产提高,改善了糖尿病的症状。
3 膳食脂肪与肠道微生物影响宿主健康的可能机制肠道微生物作为膳食脂肪和宿主之间的“桥梁”,通过微生物-宿主共代谢网络作用于膳食脂肪,影响着膳食脂肪的消化与吸收;膳食脂肪的摄入同样也会对肠道微生态产生影响,影响肠道微生物的结构并调节其代谢产物,导致其产生的部分代谢产物穿过肠屏障进入血液循环系统,对宿主多种信号通路和代谢循环进行调控,进而影响宿主代谢和机体的健康状态(图 1)[58, 65]。此外,膳食脂肪的摄入也会改变肠道菌群对宿主肠道上皮细胞的粘附性,影响其渗透性,参与调节肠上皮的完整性[48],但不同种类的膳食脂肪是如何作用于肠道微生态及其分子机制仍是亟待探究和解决的问题。
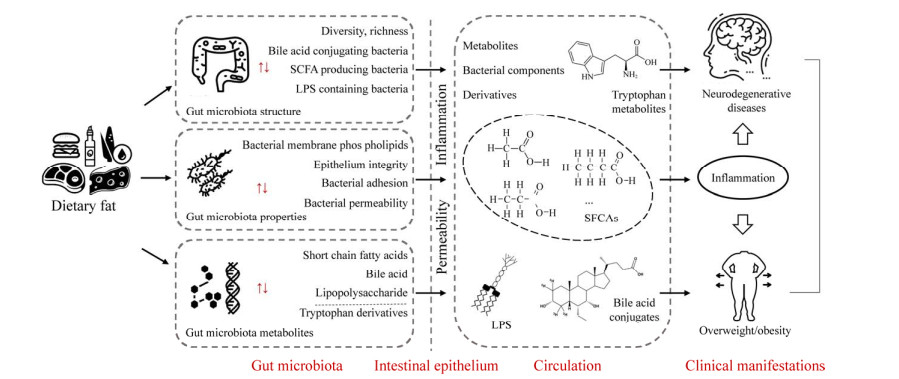
|
| 图 1 膳食脂肪与肠道微生物影响宿主健康的可能机制 Fig. 1 Possible crosstalk mechanisms among dietary lipids-gut microbiota-host metabolic health. |
| |
膳食脂肪可以调节肠道微生物的细菌特性,例如改变其对宿主肠道上皮细胞的粘附性,影响肠道通透性[60]。微生物在肠道表面的粘附和定植直接影响肠道微生物群与宿主的相互作用。大多数肠道微生物能够利用宿主饮食中提供的外源脂肪酸,并将这些脂肪酸整合到细胞膜的磷脂分子中,其中SFA的饱和脂肪链紧密而规则地聚集在一起,混入不饱和脂肪酸之后,其灵活性甚至可以打破SFA之间的紧密排列,因此饱和脂肪酸与不饱和脂肪酸的比例以及PUFA对膜的流动性具有一定的调节作用[66]。与仅酪蛋白微囊化相比,干酪乳杆菌在添加了富含n-3 PUFA的金枪鱼油的微囊化后,其存活率和细菌表面疏水性增加,表明n-3 PUFA能够改善干酪乳杆菌对肠壁的粘附,这也解释了PUFA对肠道微生物具有有益影响的可能机制[67]。肠道微生物影响宿主健康的另一个途径是增加肠道的通透性。富含SFA的高脂饮食能增加产LPS的肠道菌的丰度,改变肠道上皮细胞的通透性,损害肠道的完整性,使盲肠中LPS的含量增加,LPS进入血液循环,对宿主产生危害[68]。这些结果表明,膳食脂肪会改变肠道菌的特性如粘附性,从而影响肠道菌群与宿主之间的互作。
3.2 膳食脂肪影响肠道微生物的组成不同种类的膳食脂肪对宿主肠道微生物的组成和数量有着明显的差异。富含饱和脂肪酸的脂质如猪油、黄油、棕榈油等,造成肠道中厚壁菌门(Firmicutes)、变形菌门(Proteobacteria)、沃氏嗜胆菌Bilophila wadsworthia和梭菌属Clostridium的相对丰度增加,而双歧杆菌Bifidobacterium、乳杆菌Lactobacillus、艾克曼菌Akkermansia muciniphila的数量减少[58-59]。富含n-3 PUFA的膳食脂肪如亚麻籽油、核桃油、红花油、鱼油等,引起乳杆菌Lactobacillus、双歧杆菌Bifidobacterium、艾克曼菌Akkermansia muciniphila等的丰度增加,梭菌Clostridium、脱硫弧菌Desulfovibrio等的数量相对减少。富含n-6 PUFA的脂质如豆油、玉米油等,使肠道中拟杆菌门(Bacteroidetes)、粪球菌Enterococcus faecalis、肠杆菌(Enterobacteriaceae) 等增加[47, 61]。但因实验环境、研究对象、测序方法等的不同造成了不同的研究结果有所差异,导致并非所有结果中肠道菌群的增加或减少都保持一致。
3.3 膳食脂肪影响肠道微生物的代谢产物及其衍生物肠道微生物可以产生大量的代谢产物及衍生物,进入血液循环,进而对体内其他器官造成影响,比如脂多糖作用于脂肪组织,胆汁酸、丁酸、胆碱等作用于肝脏[69]。膳食脂肪可能通过改变肠道微生物的代谢物及其衍生物,进而对宿主造成影响。
3.3.1 短链脂肪酸(SCFA)肠道微生物能够将膳食中的营养物质在内源性肠粘液的作用下发酵,生产短链脂肪酸(Short chain fatty acid,SCFA),短链脂肪酸是具有1–6个碳原子的有机脂肪酸,而其中乙酸、丙酸和丁酸占肠道内短链脂肪酸的90%–95%[70]。高脂饮食可降低产生丙酸的肠道微生物艾克曼菌Akkermansia muciniphila的水平,但n-3 LC-PUFA饮食组处理可增加该物种的丰度[71]。高脂饮食的摄入减少了大鼠中SCFA的生成,并且随着喂养时间的延长其浓度逐渐升高。与瘦的人相比,在超重和肥胖者的粪便样本中检出的SCFA含量更高[72-73]。SCFA不仅能作为宿主的供能物质,还显示出抗炎和免疫信号转导特性。丁酸和丙酸可以抑制结肠上皮细胞和免疫细胞内组蛋白去乙酰化酶的活性,使促炎因子(Interleukin-6,IL-6和Interleukin-12,IL-12) 的表达下调,抑制炎症的产生。短链脂肪酸对Toll样受体4以及对细胞因子产生的抑制作用使其具备充当抗炎药的能力[74]。此外,短链脂肪酸还能够减少血浆中脂肪酸和甘油的含量,调节参与葡萄糖稳态的介质胰高血糖素样肽1的产生,从而达到改善糖尿病和肥胖的效果[75]。
3.3.2 胆汁酸(Bile acid)胆汁酸主要是由胆固醇合成的,胆固醇在肝脏中通过多级酶促反应转化为初级游离型胆汁酸,如胆酸、鹅脱氧胆酸[76]。大部分游离型胆汁酸在肝脏中与牛磺酸或甘氨酸结合,形成结合型胆汁酸,被排到胆汁中储存在胆囊,排入十二指肠,帮助饮食脂肪的乳化和吸收,进而参与葡萄糖和脂肪等的代谢调节以及炎症过程。小部分的初级游离型胆汁酸在结肠微生物的作用下发生脱氢、脱羟基和差向异构化作用,进一步代谢为次级胆汁酸,如脱氧胆酸(Deoxycholic acid,DCA) 和石胆酸(Lithocholic acid,LCA)[68]。除了参与脂肪消化作用外,胆汁酸还可以通过与类法尼醇X受体(Farnesoid X receptor,FXR) 和G蛋白偶联受体(Takeda G-protein-coupled receptor 5,TGR5) 结合来调节胆汁酸的合成和宿主代谢。FXR参与脂肪代谢的调节,特别是甘油三酯的运输、合成和利用;因此,胆汁酸的微生物加工可能会通过与FXR相互作用来影响脂肪代谢。高脂饮食饲养野生型和FXR基因敲除小鼠,FXR改变了肠道微生物的组成,引起体重增加、脂肪变性和炎症的发生,表明FXR可能通过改变肠道微生物的组成来导致肥胖[76, 78-80]。TGR5不仅能够激活棕色脂肪组织和肌肉组织,增加能量的消耗率;还可以诱导肠内分泌细胞释放胰高血糖素样肽-1,改善肥胖小鼠的肝脏和胰腺功能;此外,作为调节胆汁酸运动性能的重要受体,TGR5易受到微生物产生的胆汁酸LCA和DCA的激活,直接作用于神经元并间接刺激血清素的释放[81–83]。
3.3.3 脂多糖(LPS)脂多糖(Lipopolysaccharides,LPS),也称为内毒素,是革兰氏阴性菌细胞壁的组成成分,通过激活TLR4诱导宿主的炎症反应,在肥胖症和糖尿病等代谢性疾病中均检测到高LPS浓度[84]。高脂饮食会影响肠道微生物的种类和数量,使小鼠肠道中拟杆菌(Bacteroidetes) 的数量减少,厚壁菌门(Firmicutes) 的数量增加,产生LPS的菌如变形菌(Proteobacteria) 的数量增加,抑制LPS的菌如双歧杆菌Bifidobacterium的数量减少,破坏了肠屏障功能,改变肠道的通透性[85]。除了调节肠道中LPS的含量并影响肠道上皮的完整性外,高脂饮食也会影响LPS的运输[86]。肠道中的LPS以乳糜微粒的形式运输,过多的脂肪摄入导致肠道内乳糜微粒增加,使LPS易位穿过肠道黏膜进入血液循环,增加血液中LPS的水平,进而导致代谢性内毒素血症的发生。过量的LPS通过外周循环系统进入肝脏和脂肪组织,TLR4在免疫细胞(例如巨噬细胞) 以及许多其他类型细胞(包括肝细胞和脂肪细胞) 中表达,导致脂肪细胞中的肿瘤坏死因子α (Tumor necrosis factor α,TNF-α) 和白细胞介素-6 (IL-6) 水平升高,进而诱发炎症反应、血脂异常、非酒精性脂肪肝和心血管等疾病[87-88]。
3.3.4 色氨酸衍生物(Tryptophan metabolites)色氨酸是一种必需氨基酸,需要从饮食或微生物来源中获取,是神经递质5-羟色胺(5-HT) 的氨基酸前体[89]。肠道菌群在调节5-HT的合成中起着关键作用,5-HT是调节情绪和认知的肠脑轴中关键的激素/神经递质。高脂饮食会使吲哚-3-醛和色胺两种色氨酸代谢物的含量降低,它们都是芳烃受体的配体,表明膳食脂肪通过肠道微生物调节色氨酸代谢物的含量,进而影响肠道和中枢神经系统的功能[90]。色氨酸主要与血浆白蛋白结合,使色氨酸优先提供给血脑屏障中的大型中性氨基酸转运蛋白,从而转化为5-HT。长期高糖/低脂饮食导致海马体神经炎症的增加,造成海马体相关的记忆力下降;相反从蔬菜、水果豆类和全谷物中摄入膳食纤维等复杂碳水化合物,对神经健康产生有益作用[91]。
4 利用模式动物研究膳食脂肪、肠道微生物与宿主健康之间关系人类肠道菌群是个极其复杂的生态体系[92],大部分菌株无法体外培养,因此人们通常利用模式动物体系进行研究。目前研究的动物模型(表 2) 主要是啮齿动物如小鼠,高脂饮食会导致小鼠肥胖和代谢紊乱,增加疾病风险,并且引起厚壁菌门(Firmicutes) 和拟杆菌门(Bacteroidetes) 的比率增加[58]。除了高脂饮食,小鼠还被用来探究不同类型脂肪酸对肠道菌和健康的影响,与摄入饱和脂肪酸的小鼠相比,摄入不饱和脂肪酸会产生相反的效果,包括增加肠道菌的多样性以及增加厚壁菌门(Firmicutes) 和拟杆菌门(Bacteroidetes) 的比率[105];但其成本高、研究周期长且无菌个体难实现。哺乳动物如猪和猴子等由于肠道菌组成复杂,且实验操作较困难,无法大量使用[106]。无脊椎模式动物(果蝇、线虫等) 因寿命短、维护成本低、高通量等优点而逐渐受到广泛利用[107];但它们的肠道菌群组成不稳定,大部分为环境菌种,易受到外界因素、实验条件等影响,例如即使采用相同的饲养条件,黑腹果蝇肠道中的某些菌属在不同性别、不同年龄的宿主中仍存在偏向性[105]。因此,亟需新型的高通量优良模式动物来解析肠道微生物对脂肪代谢的调节机制。
| Animal models | Dietary | References |
| Pig | Tallow (SFA 5% W/V) Coconut oil (SFA 5% W/V) Corn oil (PUFA 5% W/V) Fish oil (PUFA 5% W/V) |
[93] |
| Anhydrous milk (SFA 38% E) Canola, soybean and corn oils (MUFA PUFA 38% E) |
[94] | |
| Monkey | SFA and cholesterol (31.8% E) | [95] |
| SFA (34.4% W/W); MUFA (35.1% W/W) n-6 PUFA (27.9% W/W); n-3 PUFA (2.6% W/W) |
[96] | |
| Mouse | Milk (SFA 65%); Lard (SFA 39%) | [46] |
| Lard (SFA 48% E) | [48] | |
| Lard (SFA fat 45% E) Fish oil (PUFA fat 45% E) |
[47] | |
| SFA (45% E); MUFA (45% E) | [97] | |
| Rat | Camelina oil (MUFA 11% W/W) Fish oil (PUFA 11% W/W) |
[98] |
| Linseed oil (PUFA 0.04 g/kg weight) Fish oil (PUFA 0.04 g/kg weight) |
[99] | |
| Nematode | EPA (PUFA) | [100] |
| Drosophila | Coconut oil (SFA 20% W/V) | [101] |
| Lard (SFA 3% W/V) Lard (SFA 7% W/V) Lard (SFA 10% W/V) |
[102] | |
| Palm oil (SFA 20% W/V) | [103] | |
| Honey bee | Palm oil (SFA 5% W/V) Soybean oil (PUFA 5% W/V) |
[103] |
| Corn oil (PUFA 1%–8% W/W) Flax oil (PUFA 1%–8% W/W) Corn oil, sesame oil, sage oil and flax oil (PUFA 17% W/W) |
[104] [104] |
蜜蜂Apis mellifera具有高度进化的社会结构和丰富复杂的行为活动。虽然大脑只有96万个神经元[108],但蜜蜂能够执行复杂的认知任务,并且在蜂巢内具有严密的社会分工,蜜蜂已成为生命科学,尤其是认知神经科学中良好的模式动物[109]。除此之外,近年来研究发现,蜜蜂是研究肠道微生物与宿主相互作用理想的动物模型[107]。蜜蜂的肠道菌群组成简单且稳定,仅由少数的核心菌种组成,均可以进行体外培养[110],且在肠道内的分布具有一定的规律,与外界环境菌种无关[111]。此外,有研究发现,肠道菌可以帮助蜜蜂代谢食物组分,产生短链脂肪酸和氨基酸等,促进胰岛素样肽等激素信号相关基因的表达,进一步证明了蜜蜂与人类等其他动物的肠道菌群具有相似的功能[112]。
蜜蜂的脂肪体是参与脂质代谢和免疫功能的重要器官,被拟为哺乳动物的肝脏。花粉是蜜蜂脂肪的主要来源,其中脂类含量因地区和植物品种差异而不同,但脂肪酸组成结构基本一致,包括亚油酸(C18:2)、棕榈酸(C16:0)、亚麻酸(C18:3) 和油酸(C18:1)[113] (表 3)。笔者实验团队调研发现,蜜蜂有370个脂肪代谢相关基因、17条脂肪代谢通路(脂肪酸的合成、降解和代谢等) 与人类脂肪代谢具有保守性。饲喂添加亚麻酸的蜂粮的蜜蜂,体内脂肪酸合成酶、乙酰辅酶A羧化酶、围脂滴蛋白的表达水平受到抑制,脂解脂肪酶的表达上升,进而显著影响蜜蜂甘油三酯、胆固醇、低密度脂蛋白和高密度脂蛋白的含量[114]。除此之外,我们发现蜜蜂饲喂高脂饮食(棕榈油和大豆油) 会导致肥胖和代谢紊乱,过量的膳食脂肪,尤其是棕榈油,会导致蜜蜂体重增加、存活率下降、血糖升高和脂肪堆积,然而在无肠道菌蜜蜂中高脂饮食并未改变以上表型;值得注意的是,高脂饮食增加了蜜蜂核心肠道菌Gilliamella的相对丰度,并且摄入不同膳食脂肪的蜜蜂在转录层面上包括转录因子、胰岛素分泌以及Toll和Imd信号通路在内的生物学过程存在显著差异[115]。另外,缺乏n-3多不饱和脂肪酸的花粉改变了蜜蜂体内的脂肪酸含量和组成,并使蜜蜂的学习能力和对蔗糖的敏感性显著降低[116]。基于肠道微生物的优势和脂肪代谢的同源保守性,蜜蜂成为研究肠道微生物对脂肪代谢调节机制的优良模式动物。
| Species FA/ingredient (%) |
Myristic C14:0 |
Palmitic C16:0 |
Stearic C18:0 |
Oleic C18:1 |
Linoleic C18:2 |
Linolenic C18:3 |
| Pollen | ||||||
| Brassicaceae spp. | 1.70 | 29.60 | 4.42 | 12.50 | 7.00 | 39.90 |
| Trifolium pratense | 1.21 | 38.30 | 2.29 | 10.50 | 5.01 | 41.40 |
| Onobrychis viciifolia | 1.84 | 35.60 | 4.13 | 9.33 | 14.30 | 32.80 |
| Vicia faba | 11.90 | 25.90 | 3.79 | 14.80 | 12.80 | 23.60 |
| Linum usitatissimum | 11.00 | 21.60 | 2.30 | 14.20 | 3.44 | 42.90 |
| Brassica napus | 13.30 | 16.20 | 11.00 | 5.13 | 7.70 | 24.80 |
| Edible oil | ||||||
| Soybean oil | – | 15.32 | 5.16 | 25.44 | 45.15 | 7.95 |
| Peanut oil | – | 12.11 | 3.97 | 41.07 | 32.88 | 1.08 |
| Palm oil | 2.59 | 47.50 | 0.50 | 33.30 | 14.50 | 0.57 |
| Olive oil | – | 11.48 | 4.37 | 75.21 | 5.99 | 0.73 |
| Corn oil | – | 14.12 | 2.31 | 32.48 | 48.64 | 1.51 |
| Canola oil | – | 5.03 | 2.32 | 59.91 | 20.10 | 11.00 |
综上所述,膳食脂肪对宿主的健康至关重要,摄入不当会造成代谢紊乱,引起肥胖、心血管疾病和炎症等代谢性疾病。膳食脂肪和肠道微生物相互影响,主要体现在肠道微生物的组成、特性和代谢的变化,进而引起宿主的代谢改变。高饱和脂肪酸饮食增加厚壁菌和拟杆菌的比例并降低双歧杆菌的丰度、引起肠道微生物的失调、引起炎症水平的提高和抗炎因子水平的下降,导致胰岛素抵抗,进而引发肥胖、糖尿病等代谢性疾病。而摄入单不饱和脂肪酸和多不饱和脂肪酸可以改善肠道微生物的“营养健康”,改善菌群失调和代谢失衡。蜜蜂作为一种模式生物,简单特异的肠道微生物组成对研究膳食脂质和肠道微生物之间的互作提供了便利,且蜜蜂与人类脂代谢的同源性也为两者之间互作后对宿主代谢影响的探究提供基础。目前越来越多的研究表明,肠道微生物在调节宿主代谢中存在着因果关系。然而膳食脂肪除了为机体提供能量和营养以外,到底在多大程度上通过肠道菌群影响宿主代谢,在膳食脂肪-肠道微生物-宿主代谢这条通路上到底存在着一种怎样的机制,尚需要更多更深入的研究。
| [1] |
中国营养学会编著. 中国居民膳食指南科学研究报告(2021). 2021. Chinese Nutrition Society. Scientific research report on dietary guidelines for Chinese residents (2021). 2021 (in Chinese). |
| [2] |
Sender R, Fuchs S, Milo R. Revised estimates for the number of human and bacteria cells in the body. PLoS Biol, 2016, 14(8): e1002533. DOI:10.1371/journal.pbio.1002533
|
| [3] |
Bäckhed F, Ding H, Wang T, et al. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci USA, 2004, 101(44): 15718-15723. DOI:10.1073/pnas.0407076101
|
| [4] |
Daniel H, Gholami AM, Berry D, et al. High-fat diet alters gut microbiota physiology in mice. Isme J, 2014, 8(2): 295-308. DOI:10.1038/ismej.2013.155
|
| [5] |
Grossman MR. FDA issues order to ban artificial trans fat by 2018. Eur Food Feed Law Rev, 2015, 10: 317.
|
| [6] |
Merrill AL, Watt BK. Energy Value of Foods: Basis and Derivation. Washington, DC: Agriculture Handbook 74, U.S. Government Printing Office, 1973: 105.
|
| [7] |
谢佳琦, 王远亮, 李宗军. 膳食脂肪对肠道微生物的影响及宿主代谢调控的研究进展. 中国微生态学杂志, 2018, 30(9): 1102-1109. Xie JQ, Wang YL, Li ZJ. Effects of dietary fats on intestinal microorganisms and metabolism of host. Chin J Microecol, 2018, 30(9): 1102-1109 (in Chinese). |
| [8] |
Nettleton JA, Villalpando S, Cassani RS, et al. Health significance of fat quality in the diet. Ann Nutr Metab, 2013, 63(1/2): 96-102.
|
| [9] |
Cummings JH, Wiggins HS, Jenkins DJ, et al. Influence of diets high and low in animal fat on bowel habit, gastrointestinal transit time, fecal microflora, bile acid, and fat excretion. J Clin Invest, 1978, 61(4): 953-963. DOI:10.1172/JCI109020
|
| [10] |
Lumeng CN, Saltiel AR. Inflammatory links between obesity and metabolic disease. J Clin Invest, 2011, 121(6): 2111-2117. DOI:10.1172/JCI57132
|
| [11] |
Bleau C, Karelis AD, St-Pierre DH, et al. Crosstalk between intestinal microbiota, adipose tissue and skeletal muscle as an early event in systemic low-grade inflammation and the development of obesity and diabetes. Diabetes Metab Res Rev, 2015, 31(6): 545-561. DOI:10.1002/dmrr.2617
|
| [12] |
Kim KA, Gu W, Lee IA, et al. High fat diet-induced gut microbiota exacerbates inflammation and obesity in mice via the TLR4 signaling pathway. PLoS ONE, 2012, 7(10): e47713. DOI:10.1371/journal.pone.0047713
|
| [13] |
Kothari V, Luo Y, Tornabene T, et al. High fat diet induces brain insulin resistance and cognitive impairment in mice. Biochim Biophys Acta Mol Basis Dis, 2017, 1863(2): 499-508. DOI:10.1016/j.bbadis.2016.10.006
|
| [14] |
Olson CA, Vuong HE, Yano JM, et al. The gut microbiota mediates the anti-seizure effects of the ketogenic diet. Cell, 2018, 173(7): 1728-1741. e13. DOI:10.1016/j.cell.2018.04.027
|
| [15] |
Barzegar M, Afghan M, Tarmahi V, et al. Ketogenic diet: overview, types, and possible anti-seizure mechanisms. Nutr Neurosci, 2021, 24(4): 307-316. DOI:10.1080/1028415X.2019.1627769
|
| [16] |
Mozaffarian D. Dietary and policy priorities for cardiovascular disease, diabetes, and obesity: a comprehensive review. Circulation, 2016, 133(2): 187-225. DOI:10.1161/CIRCULATIONAHA.115.018585
|
| [17] |
Mozaffarian D, Appel LJ, Van Horn L. Components of a cardioprotective diet: new insights. Circulation, 2011, 123(24): 2870-2891. DOI:10.1161/CIRCULATIONAHA.110.968735
|
| [18] |
Schwingshackl L, Zähringer J, Beyerbach J, et al. Total dietary fat intake, fat quality, and health outcomes: a scoping review of systematic reviews of prospective studies. Ann Nutr Metab, 2021, 77(1): 4-15. DOI:10.1159/000515058
|
| [19] |
Ho FK, Gray SR, Welsh P, et al. Associations of fat and carbohydrate intake with cardiovascular disease and mortality: prospective cohort study of UK Biobank participants. BMJ, 2020, 368: m688.
|
| [20] |
Shen Y, Zhao Z, Zhang L, et al. Metabolic activity induces membrane phase separation in endoplasmic Reticulum. Proc Natl Acad Sci USA, 2017, 114(51): 13394-13399. DOI:10.1073/pnas.1712555114
|
| [21] |
Okereke OI, Rosner BA, Kim DH, et al. Dietary fat types and 4-year cognitive change in community-dwelling older women. Ann Neurol, 2012, 72(1): 124-134. DOI:10.1002/ana.23593
|
| [22] |
Siriwardhana N, Kalupahana NS, Cekanova M, et al. Modulation of adipose tissue inflammation by bioactive food compounds. J Nutr Biochem, 2013, 24(4): 613-623. DOI:10.1016/j.jnutbio.2012.12.013
|
| [23] |
Hammad S, Pu S, Jones PJ. Current evidence supporting the link between dietary fatty acids and cardiovascular disease. Lipids, 2016, 51(5): 507-517. DOI:10.1007/s11745-015-4113-x
|
| [24] |
Allman-Farinelli MA, Gomes K, Favaloro EJ, et al. A diet rich in high-oleic-acid sunflower oil favorably alters low-density lipoprotein cholesterol, triglycerides, and factor Ⅶ coagulant activity. J Am Diet Assoc, 2005, 105(7): 1071-1079. DOI:10.1016/j.jada.2005.04.008
|
| [25] |
Sanchez-Bayle M, Gonzalez-Requejo A, Pelaez MJ, et al. A cross-sectional study of dietary habits and lipid profiles. The rivas-vaciamadrid study. Eur J Pediatr, 2008, 167(2): 149-154. DOI:10.1007/s00431-007-0439-6
|
| [26] |
Elagizi A, Lavie CJ, Marshall K, et al. Omega-3 polyunsaturated fatty acids and cardiovascular health: a comprehensive review. Prog Cardiovasc Dis, 2018, 61(1): 76-85. DOI:10.1016/j.pcad.2018.03.006
|
| [27] |
Shahidi F, Ambigaipalan P. Omega-3 polyunsaturated fatty acids and their health benefits. Annu Rev Food Sci Technol, 2018, 9: 345-381. DOI:10.1146/annurev-food-111317-095850
|
| [28] |
Schulze MB, Minihane AM, Saleh RNM, et al. Intake and metabolism of Omega-3 and Omega-6 polyunsaturated fatty acids: nutritional implications for cardiometabolic diseases. Lancet Diabetes Endocrinol, 2020, 8(11): 915-930. DOI:10.1016/S2213-8587(20)30148-0
|
| [29] |
Pu S, Rodríguez-Pérez C, Ramprasath VR, et al. Dietary high oleic canola oil supplemented with docosahexaenoic acid attenuates plasma proprotein convertase subtilisin kexin type 9 (PCSK9) levels in participants with cardiovascular disease risk: a randomized control trial. Vascul Pharmacol, 2016, 87: 60-65. DOI:10.1016/j.vph.2016.06.007
|
| [30] |
Ma Y, Wang J, Li Q, et al. The effect of Omega-3 polyunsaturated fatty acid supplementations on anti-tumor drugs in triple negative breast cancer. Nutr Cancer, 2021, 73(2): 196-205. DOI:10.1080/01635581.2020.1743873
|
| [31] |
Perez MA, Magtanong L, Dixon SJ, et al. Dietary lipids induce ferroptosis in caenorhabditiselegans and human cancer cells. Dev Cell, 2020, 54(4): 447-454. e4. DOI:10.1016/j.devcel.2020.06.019
|
| [32] |
Zhang G, Panigrahy D, Mahakian LM, et al. Epoxy metabolites of docosahexaenoic acid (DHA) inhibit angiogenesis, tumor growth, and metastasis. Proc Natl Acad Sci USA, 2013, 110(16): 6530-6535. DOI:10.1073/pnas.1304321110
|
| [33] |
蔡双莲, 李敏. 多不饱和脂肪酸的研究进展. 生命科学研究, 2003, 7(4): 289-292, 304. Cai SL, Li M. Advances in polyunsaturated fatty acids. Life Sci Res, 2003, 7(4): 289-292, 304 (in Chinese). DOI:10.3969/j.issn.1007-7847.2003.04.002 |
| [34] |
Andreone BJ, Chow BW, Tata A, et al. Blood-brain barrier permeability is regulated by lipid transport-dependent suppression of caveolae-mediated transcytosis. Neuron, 2017, 94(3): 581-594. e5. DOI:10.1016/j.neuron.2017.03.043
|
| [35] |
Ben-Zvi A, Lacoste B, Kur E, et al. Mfsd2a is critical for the formation and function of the blood-brain barrier. Nature, 2014, 509(7501): 507-511. DOI:10.1038/nature13324
|
| [36] |
Amminger GP, Schäfer MR, Schlögelhofer M, et al. Longer-term outcome in the prevention of psychotic disorders by the Vienna Omega-3 study. Nat Commun, 2015, 6: 7934. DOI:10.1038/ncomms8934
|
| [37] |
Zamroziewicz MK, Paul EJ, Rubin RD, et al. Anterior cingulate cortex mediates the relationship between O3PUFAs and executive functions in APOE e4 carriers. Front Aging Neurosci, 2015, 7: 87.
|
| [38] |
Fiala M, Halder RC, Sagong BE, et al. Ω-3 supplementation increases amyloid-β phagocytosis and resolvin D1 in patients with minor cognitive impairment. FASEB J, 2015, 29(7): 2681-2689. DOI:10.1096/fj.14-264218
|
| [39] |
DiNicolantonio JJ, O'Keefe JH. Importance of maintaining a low Omega-6/Omega-3 ratio for reducing inflammation. Open Heart, 2018, 5(2): e000946. DOI:10.1136/openhrt-2018-000946
|
| [40] |
Shahidi F, Ambigaipalan P. Novel functional food ingredients from marine sources. Curr Opin Food Sci, 2015, 2: 123-129. DOI:10.1016/j.cofs.2014.12.009
|
| [41] |
Gebauer SK, Psota TL, Kris-Etherton PM. The diversity of health effects of individual trans fatty acid isomers. Lipids, 2007, 42(9): 787-799. DOI:10.1007/s11745-007-3095-8
|
| [42] |
Michas G, Micha R, Zampelas A. Dietary fats and cardiovascular disease: putting together the pieces of a complicated puzzle. Atherosclerosis, 2014, 234(2): 320-328. DOI:10.1016/j.atherosclerosis.2014.03.013
|
| [43] |
Hadj Ahmed S, Kharroubi W, Kaoubaa N, et al. Correlation of trans fatty acids with the severity of coronary artery disease lesions. Lipids Health Dis, 2018, 17(1): 52. DOI:10.1186/s12944-018-0699-3
|
| [44] |
Scott KP, Gratz SW, Sheridan PO, et al. The influence of diet on the gut microbiota. Pharmacol Res, 2013, 69(1): 52-60. DOI:10.1016/j.phrs.2012.10.020
|
| [45] |
Schroeder BO, Bäckhed F. Signals from the gut microbiota to distant organs in physiology and disease. Nat Med, 2016, 22(10): 1079-1089. DOI:10.1038/nm.4185
|
| [46] |
Huang EY, Leone VA, Devkota S, et al. Composition of dietary fat source shapes gut microbiota architecture and alters host inflammatory mediators in mouse adipose tissue. JPEN J Parenter Enteral Nutr, 2013, 37(6): 746-754. DOI:10.1177/0148607113486931
|
| [47] |
Caesar R, Tremaroli V, Kovatcheva-Datchary P, et al. Crosstalk between gut microbiota and dietary lipids aggravates WAT inflammation through TLR signaling. Cell Metab, 2015, 22(4): 658-668. DOI:10.1016/j.cmet.2015.07.026
|
| [48] |
Kübeck R, Bonet-Ripoll C, Hoffmann C, et al. Dietary fat and gut microbiota interactions determine diet-induced obesity in mice. Mol Metab, 2016, 5(12): 1162-1174. DOI:10.1016/j.molmet.2016.10.001
|
| [49] |
de Wit N, Derrien M, Bosch-Vermeulen H, et al. Saturated fat stimulates obesity and hepatic steatosis and affects gut microbiota composition by an enhanced overflow of dietary fat to the distal intestine. Am J Physiol - Gastrointest Liver Physiol, 2012, 303(5): G589-G599. DOI:10.1152/ajpgi.00488.2011
|
| [50] |
Liu T, Hougen H, Vollmer AC, et al. Gut bacteria profiles of Mus musculus at the Phylum and family levels are influenced by saturation of dietary fatty acids. Anaerobe, 2012, 18(3): 331-337. DOI:10.1016/j.anaerobe.2012.02.004
|
| [51] |
Patterson E, O'Doherty RM, Murphy EF, et al. Impact of dietary fatty acids on metabolic activity and host intestinal microbiota composition in C57BL/6J mice. Br J Nutr, 2014, 111(11): 1905-1917. DOI:10.1017/S0007114514000117
|
| [52] |
Li Q, Zhang Q, Wang C, et al. Fish oil enhances recovery of intestinal microbiota and epithelial integrity in chronic rejection of intestinal transplant. PLoS ONE, 2011, 6(6): e20460. DOI:10.1371/journal.pone.0020460
|
| [53] |
曹战江, 于健春, 康维明, 等. 补充n-3多不饱和脂肪酸对高脂饮食大江, 于鼠肠道菌群及门静脉血内毒素的影响. 中国医学科学院学报, 2014, 36(5): 496-500. Cao ZJ, Yu JC, Kang WM, et al. Effect of n-3 polyunsaturated fatty acids on gut microbiota and endotoxin levels in portal vein of rats fed with high-fat diet. Acta Acad Med Sin, 2014, 36(5): 496-500 (in Chinese). DOI:10.3881/j.issn.1000-503X.2014.05.007 |
| [54] |
Robertson RC, Seira Oriach C, Murphy K, et al. Omega-3 polyunsaturated fatty acids critically regulate behaviour and gut microbiota development in adolescence and adulthood. Brain Behav Immun, 2017, 59: 21-37. DOI:10.1016/j.bbi.2016.07.145
|
| [55] |
Robertson RC, Kaliannan K, Strain CR, et al. Maternal Omega-3 fatty acids regulate offspring obesity through persistent modulation of gut microbiota. Microbiome, 2018, 6(1): 95. DOI:10.1186/s40168-018-0476-6
|
| [56] |
Pusceddu MM, El Aidy S, Crispie F, et al. N-3 polyunsaturated fatty acids (PUFAs) reverse the impact of early-life stress on the gut microbiota. PLoS ONE, 2015, 10(10): e0139721. DOI:10.1371/journal.pone.0139721
|
| [57] |
Vivatvakin B, Mahayosnond A, Theamboonlers A, et al. Effect of a whey-predominant starter formula containing LCPUFAs and oligosaccharides (FOS/GOS) on gastrointestinal comfort in infants. Asia Pac J Clin Nutr, 2010, 19(4): 473-480.
|
| [58] |
Shang Y, Khafipour E, Derakhshani H, et al. Short term high fat diet induces obesity-enhancing changes in mouse gut microbiota that are partially reversed by cessation of the high fat diet. Lipids, 2017, 52(6): 499-511. DOI:10.1007/s11745-017-4253-2
|
| [59] |
He C, Cheng D, Peng C, et al. High-fat diet induces dysbiosis of gastric microbiota prior to gut microbiota in association with metabolic disorders in mice. Front Microbiol, 2018, 9: 639. DOI:10.3389/fmicb.2018.00639
|
| [60] |
Mokkala K, Houttu N, Cansev T, et al. Interactions of dietary fat with the gut microbiota: evaluation of mechanisms and metabolic consequences. Clin Nutr, 2020, 39(4): 994-1018. DOI:10.1016/j.clnu.2019.05.003
|
| [61] |
Li H, Zhu Y, Zhao F, et al. Erratum: fish oil, lard and soybean oil differentially shape gut microbiota of middle-aged rats. Sci Rep, 2017, 7(1): 7738. DOI:10.1038/s41598-017-04862-8
|
| [62] |
Gibson DL, Gill SK, Brown K, et al. Maternal exposure to fish oil primes offspring to harbor intestinal pathobionts associated with altered immune cell balance. Gut Microbes, 2015, 6(1): 24-32. DOI:10.1080/19490976.2014.997610
|
| [63] |
Devkota S, Wang YW, Musch MW, et al. Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in Il10−/− mice. Nature, 2012, 487(7405): 104-108. DOI:10.1038/nature11225
|
| [64] |
Ghazalpour A, Cespedes I, Bennett BJ, et al. Expanding role of gut microbiota in lipid metabolism. Curr Opin Lipidol, 2016, 27(2): 141-147. DOI:10.1097/MOL.0000000000000278
|
| [65] |
Shen W, Gaskins HR, McIntosh MK. Influence of dietary fat on intestinal microbes, inflammation, barrier function and metabolic outcomes. J Nutr Biochem, 2014, 25(3): 270-280. DOI:10.1016/j.jnutbio.2013.09.009
|
| [66] |
Yao J, Rock CO. How bacterial pathogens eat host lipids: implications for the development of fatty acid synthesis therapeutics. J Biol Chem, 2015, 290(10): 5940-5946. DOI:10.1074/jbc.R114.636241
|
| [67] |
Eratte D, Dowling K, Barrow CJ, et al. In-vitro digestion of probiotic bacteria and Omega-3 oil co-microencapsulated in whey protein isolate-gum Arabic complex coacervates. Food Chem, 2017, 227: 129-136. DOI:10.1016/j.foodchem.2017.01.080
|
| [68] |
Coelho OGL, Cândido FG, Alfenas RCG. Dietary fat and gut microbiota: mechanisms involved in obesity control. Crit Rev Food Sci Nutr, 2019, 59(19): 3045-3053. DOI:10.1080/10408398.2018.1481821
|
| [69] |
Sun L, Ma L, Ma Y, et al. Insights into the role of gut microbiota in obesity: pathogenesis, mechanisms, and therapeutic perspectives. Protein Cell, 2018, 9(5): 397-403. DOI:10.1007/s13238-018-0546-3
|
| [70] |
Zhang YJ, Li S, Gan RY, et al. Impacts of gut bacteria on human health and diseases. Int J Mol Sci, 2015, 16(4): 7493-7519.
|
| [71] |
Liu W, Crott JW, Lyu L, et al. Diet-and genetically-induced obesity produces alterations in the microbiome, inflammation and Wnt pathway in the intestine of apc+/1638N mice: comparisons and contrasts. J Cancer, 2016, 7(13): 1780-1790. DOI:10.7150/jca.15792
|
| [72] |
Fernandes J, Su W, Rahat-Rozenbloom S, et al. Adiposity, gut microbiota and faecal short chain fatty acids are linked in adult humans. Nutr Diabetes, 2014, 4: e121. DOI:10.1038/nutd.2014.23
|
| [73] |
Schwiertz A, Taras D, Schäfer K, et al. Microbiota and SCFA in lean and overweight healthy subjects. Obesity (Silver Spring), 2010, 18(1): 190-195. DOI:10.1038/oby.2009.167
|
| [74] |
Puddu A, Sanguineti R, Montecucco F, et al. Evidence for the gut microbiota short-chain fatty acids as key pathophysiological molecules improving diabetes. Mediat Inflamm, 2014, 2014: 162021.
|
| [75] |
Freeland KR, Wolever TMS. Acute effects of intravenous and rectal acetate on glucagon-like peptide-1, peptide YY, ghrelin, adiponectin and tumour necrosis factor-α. Br J Nutr, 2010, 103(3): 460-466. DOI:10.1017/S0007114509991863
|
| [76] |
Parks DJ, Blanchard SG, Bledsoe RK, et al. Bile acids: natural ligands for an orphan nuclear receptor. Science, 1999, 284(5418): 1365-1368. DOI:10.1126/science.284.5418.1365
|
| [77] |
Wahlström A, Sayin SI, Marschall HU, et al. Intestinal crosstalk between bile acids and microbiota and its impact on host metabolism. Cell Metab, 2016, 24(1): 41-50. DOI:10.1016/j.cmet.2016.05.005
|
| [78] |
Fang S, Suh JM, Reilly SM, et al. Intestinal FXR agonism promotes adipose tissue browning and reduces obesity and insulin resistance. Nat Med, 2015, 21(2): 159-165. DOI:10.1038/nm.3760
|
| [79] |
Jiang C, Xie C, Lv Y, et al. Intestine-selective farnesoid X receptor inhibition improves obesity-related metabolic dysfunction. Nat Commun, 2015, 6: 10166. DOI:10.1038/ncomms10166
|
| [80] |
Li F, Jiang C, Krausz KW, et al. Microbiome remodelling leads to inhibition of intestinal farnesoid X receptor signalling and decreased obesity. Nat Commun, 2013, 4: 2384. DOI:10.1038/ncomms3384
|
| [81] |
Broeders EPM, Nascimento EBM, Havekes B, et al. The bile acid chenodeoxycholic acid increases human brown adipose tissue activity. Cell Metab, 2015, 22(3): 418-426. DOI:10.1016/j.cmet.2015.07.002
|
| [82] |
Thomas C, Gioiello A, Noriega L, et al. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab, 2009, 10(3): 167-177. DOI:10.1016/j.cmet.2009.08.001
|
| [83] |
Watanabe M, Houten SM, Mataki C, et al. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature, 2006, 439(7075): 484-489. DOI:10.1038/nature04330
|
| [84] |
Schoeler M, Caesar R. Dietary lipids, gut microbiota and lipid metabolism. Rev Endocr Metab Disord, 2019, 20(4): 461-472. DOI:10.1007/s11154-019-09512-0
|
| [85] |
Erridge C, Attina T, Spickett CM, et al. A high-fat meal induces low-grade endotoxemia: evidence of a novel mechanism of postprandial inflammation. Am J Clin Nutr, 2007, 86(5): 1286-1292. DOI:10.1093/ajcn/86.5.1286
|
| [86] |
Cani PD, Amar J, Iglesias MA, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes, 2007, 56(7): 1761-1772. DOI:10.2337/db06-1491
|
| [87] |
Ameziane N, Beillat T, Verpillat P, et al. Association of the Toll-like receptor 4 gene Asp299Gly polymorphism with acute coronary events. Arterioscler Thromb Vasc Biol, 2003, 23(12): e61-e64.
|
| [88] |
Arbour NC, Lorenz E, Schutte BC, et al. TLR4 mutations are associated with endotoxin hyporesponsiveness in humans. Nat Genet, 2000, 25(2): 187-191. DOI:10.1038/76048
|
| [89] |
Liu Z, Dai X, Zhang H, et al. Gut microbiota mediates intermittent-fasting alleviation of diabetes-induced cognitive impairment. Nat Commun, 2020, 11(1): 855. DOI:10.1038/s41467-020-14676-4
|
| [90] |
Krishnan S, Ding Y, Saedi N, et al. Gut microbiota-derived tryptophan metabolites modulate inflammatory response in hepatocytes and macrophages. Cell Rep, 2018, 23(4): 1099-1111. DOI:10.1016/j.celrep.2018.03.109
|
| [91] |
Godos J, Currenti W, Angelino D, et al. Diet and mental health: review of the recent updates on molecular mechanisms. Antioxidants, 2020, 9(4): 346. DOI:10.3390/antiox9040346
|
| [92] |
Lozupone CA, Stombaugh JI, Gordon JI, et al. Diversity, stability and resilience of the human gut microbiota. Nature, 2012, 489(7415): 220-230. DOI:10.1038/nature11550
|
| [93] |
Kellner TA, Gabler NK, Patience JF. The composition of dietary fat alters the transcriptional profile of pathways associated with lipid metabolism in the liver and adipose tissue in the pig. J Anim Sci, 2017, 95(8): 3609-3619.
|
| [94] |
Walker ME, Matthan NR, Goldbaum A, et al. Dietary patterns influence epicardial adipose tissue fatty acid composition and inflammatory gene expression in the Ossabaw pig. J Nutr Biochem, 2019, 70: 138-146. DOI:10.1016/j.jnutbio.2019.04.013
|
| [95] |
He T, Xu C, Krampe N, et al. High-fat diet exacerbates SIV pathogenesis and accelerates disease progression. J Clin Invest, 2019, 129(12): 5474-5488. DOI:10.1172/JCI121208
|
| [96] |
Laugero KD, Smilowitz JT, German JB, et al. Plasma Omega 3 polyunsaturated fatty acid status and monounsaturated fatty acids are altered by chronic social stress and predict endocrine responses to acute stress in titi monkeys. Prostaglandins Leukot Essent Fatty Acids, 2011, 84(3/4): 71-78.
|
| [97] |
Ralston JC, Nguyen-Tu MS, Lyons CL, et al. Dietary substitution of SFA with MUFA within high-fat diets attenuates hyperinsulinaemia and pancreatic islet dysfunction. Br J Nutr, 2020, 124(3): 247-255. DOI:10.1017/S0007114520000859
|
| [98] |
Torrissen M, Svensen H, Stoknes I, et al. Deposition and metabolism of dietary n-3 very-long-chain PUFA in different organs of rat, mouse and Atlantic salmon. Br J Nutr, 2021, 1-20.
|
| [99] |
Czyż K, Sokoła-Wysoczańska E, Bodkowski R, et al. Dietary Omega-3 source effect on the fatty acid profile of intramuscular and perimuscular fat—preliminary study on a rat model. Nutrients, 2020, 12(11): 3382. DOI:10.3390/nu12113382
|
| [100] |
Menzel R, Geweiler D, Sass A, et al. Nematodes as important source for Omega-3 long-chain fatty acids in the soil food web and the impact in nutrition for higher trophic levels. Front Ecol Evol, 2018, 6: 96. DOI:10.3389/fevo.2018.00096
|
| [101] |
Cormier RJ, Strang R, Menail H, et al. Systemic and mitochondrial effects of metabolic inflexibility induced by high fat diet in Drosophila melanogaster. Insect Biochem Mol Biol, 2021, 133: 103556. DOI:10.1016/j.ibmb.2021.103556
|
| [102] |
Shi D, Han T, Chu X, et al. An isocaloric moderately high-fat diet extends lifespan in male rats and Drosophila. Cell Metab, 2021, 33(3): 581-597. DOI:10.1016/j.cmet.2020.12.017
|
| [103] |
von Frieling J, Faisal MN, Sporn F, et al. A high-fat diet induces a microbiota-dependent increase in stem cell activity in the Drosophila intestine. PLoS Genet, 2020, 16(5): e1008789. DOI:10.1371/journal.pgen.1008789
|
| [104] |
Arien Y, Dag A, Shafir S. Omega-6: 3 ratio more than absolute lipid level in diet affects associative learning in honey bees. Front Psychol, 2018, 9: 1001. DOI:10.3389/fpsyg.2018.01001
|
| [105] |
Wong AC, Chaston JM, Douglas AE. The inconstant gut microbiota of Drosophila species revealed by 16S rRNA gene analysis. Isme J, 2013, 7(10): 1922-1932. DOI:10.1038/ismej.2013.86
|
| [106] |
Bobay LM, Raymann K. Population genetics of host-associated microbiomes. Curr Mol Biol Rep, 2019, 5(3): 128-139. DOI:10.1007/s40610-019-00122-y
|
| [107] |
Zheng H, Steele MI, Leonard SP, et al. Honey bees as models for gut microbiota research. Lab Anim (NY), 2018, 47(11): 317-325. DOI:10.1038/s41684-018-0173-x
|
| [108] |
Menzel R, Giurfa M. Cognitive architecture of a mini-brain: the honeybee. Trends Cogn Sci, 2001, 5(2): 62-71. DOI:10.1016/S1364-6613(00)01601-6
|
| [109] |
Frasnelli E, Haase A, Rigosi E, et al. The bee as a model to investigate brain and behavioural asymmetries. Insects, 2014, 5(1): 120-138. DOI:10.3390/insects5010120
|
| [110] |
Cox-Foster DL, Conlan S, Holmes EC, et al. A metagenomic survey of microbes in honey bee colony collapse disorder. Science, 2007, 318(5848): 283-287. DOI:10.1126/science.1146498
|
| [111] |
Zheng H, Nishida A, Kwong WK, et al. Metabolism of toxic sugars by strains of the bee gut symbiont Gilliamella apicola. mBio, 2016, 7(6): e013.
|
| [112] |
Zheng H, Powell JE, Steele MI, et al. Honeybee gut microbiota promotes host weight gain via bacterial metabolism and hormonal signaling. Proc Natl Acad Sci USA, 2017, 114(18): 4775-4780. DOI:10.1073/pnas.1701819114
|
| [113] |
Manning R. Fatty acids in pollen: a review of their importance for honey bees. Bee World, 2001, 82(2): 60-75. DOI:10.1080/0005772X.2001.11099504
|
| [114] |
Ma LT, Wang Y, Hang XB, et al. Nutritional effect of alpha-linolenic acid on honey bee colony development (Apis mellifera L.). J Apic Sci, 2015, 59(2): 63-72.
|
| [115] |
Wang XF, Zhong ZP, Chen XY, et al. High-fat diets with differential fatty acids induce obesity and perturb gut microbiota in honey bee. Int J Mol Sci, 2021, 22(2): 834. DOI:10.3390/ijms22020834
|
| [116] |
Arien Y, Dag A, Zarchin S, et al. Omega-3 deficiency impairs honey bee learning. Proc Natl Acad Sci USA, 2015, 112(51): 15761-15766. DOI:10.1073/pnas.1517375112
|
 2021, Vol. 37
2021, Vol. 37




