中国科学院微生物研究所、中国微生物学会主办
文章信息
- 杨昕淳, 吴晓龙, 华进联
- Yang Xinchun, Wu Xiaolong, Hua Jinlian
- 诱导多能干细胞向巨噬细胞分化研究进展
- Induction and differentiation of induced pluripotent stem cells into macrophages: a review
- 生物工程学报, 2021, 37(11): 4001-4014
- Chinese Journal of Biotechnology, 2021, 37(11): 4001-4014
- 10.13345/j.cjb.210134
-
文章历史
- Received: February 9, 2021
- Accepted: June 9, 2021
- Published: July 2, 2021
2006年,Yamanaka等在小鼠成纤维细胞中同时导入Oct3/4、Sox2、c-Myc和Klf4 (简称OSKM) 后获得了与胚胎干细胞(Embryonic stem cells,ESCs) 相似特性的干细胞,并将这些细胞命名为诱导多能干细胞(Induced pluripotent stem cells,iPSCs)[1]。这也为曾经的体细胞核移植时核基因组恢复其分化前的功能状态(重编程) 这一现象提供了分子基础[2]。2007年,Takahashi等将OSKM导入人皮肤成纤维细胞产生了人iPSCs[3],同期James Thomson等使用不同转录因子组合(Oct4,Sox2,Nanog,Lin28) 同样将体细胞重编程为iPSCs[4]。随着研究的深入,越来越多的体细胞都可以被重编程为iPSCs[5],重编程的方法也随之增加。到目前为止,只有转录因子Oct3/4被认为是必不可少的,而Sox2、Klf4、c-Myc则被认为是可以替代的转录因子[6]。与原代分离的多能干细胞相比,iPSCs的细胞来源丰富,操作简便,应用范围广,为许多疾病的研究与治疗提供了全新的思路和技术方法,目前已有使用iPSCs来模拟人体各类组织[7]、器官[8]和其他系统[9]的发生发育发展过程及各类疾病模型(基于iPSCs的疾病建模方法详见图 1)。
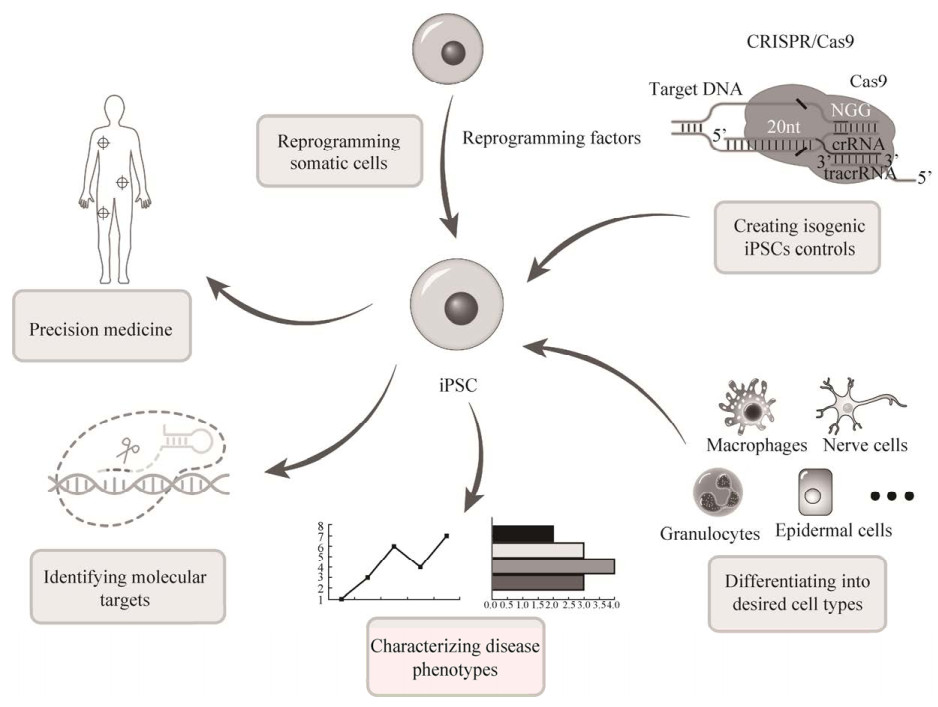
|
| 图 1 基于iPSCs的疾病建模示意图 Fig. 1 Schematic representation for human iPSCs-baseds disease modeling. |
| |
自Yamanaka等将OSKM四种转录因子导入小鼠成纤维细胞中将其重编程为iPSCs后,不同体细胞诱导iPSCs的方法便相继出现[10]。目前,已被证实可诱导为iPSCs的细胞种类不断增多,成纤维细胞[11]、角蛋白细胞[12]、大鼠眼细胞[13]、小鼠[10]和兔的肝脏与胃细胞[14]、胰岛β细胞[15]、神经祖细胞[13]等均可以重编程为iPSCs。但因为在诱导过程中引入了肿瘤基因c-Myc,高致瘤性仍然是一个关键问题[16]。2008年,通过优化重编程方法,获得了不需要c-Myc逆转录病毒的iPSCs[11]。同期,Huangfu等仅使用Oct4和Sox2成功重编程得到iPSCs[17]。人们进一步发现只有Oct4是重编程过程中所必需的转录因子[18],Oct4在ESCs的多能维持和成熟细胞重新编程至iPSCs中起着突出的作用[19]。2009年James A Thomson等使用非整合的表观载体完成了对人体细胞的重编程[20],相比于整合型重编程方法,使用非整合重编程不需要基因组整合或外源重编程因子的持续表达,消除了人类iPSCs临床应用的一个障碍[21]。随后越来越多可用于重编程的方法被研究出来,如使用仙台病毒[22]、附加体[23]、mRNA转染[24]和转座子[25]等。之后,很多学者利用小分子进行体细胞的重编程[26],这种化学重编程避免了基因组的改变,降低了致瘤性,为iPSCs临床应用提供了更多的可能性[27],为人类获取iPSCs提供了新方法、新思路。
1.2 iPSCs应用现状iPSCs广泛应用于构建人类疾病模型、发育模型与再生医学中[28],对分析病因、探究疾病治疗靶点和药物筛选具有重要意义。iPSCs在功能和发育方面更加类似于胚胎干细胞[29],理论上可以诱导成机体的各类细胞[30],且具有无限增殖的能力[31]。因此,可以使用iPSCs及其衍生细胞来模拟身体组织、器官。如今,已经可以将iPSCs诱导为内皮细胞[32]、神经元[9]、自然杀伤细胞[33]、生殖细胞[34]、巨噬细胞[35]等,为疾病模型的建立和分化机制的研究提供了平台。患者与供体ESCs之间的组织不兼容性以及人类ESCs相关的伦理问题是目前限制发展的严重阻碍,而iPSCs技术有望解决这些问题[36]。巨噬细胞作为人体的第一道防线,利用iPSCs诱导分化为巨噬细胞为相关疾病建模及巨噬细胞相关机理研究提供了良好的基础。
2 巨噬细胞 2.1 巨噬细胞起源1893年Élie Metchnikoff等在观察组织炎症和吞噬病原体时,首次发现了嗜中性粒细胞和巨噬细胞吞噬病原体的现象[37]。因为其吞噬病原体的功能,后来的科学家将其归类于组织免疫细胞[38]。1968年,Ralf van Furth和Zanvil Cohn共同定义了单核吞噬细胞系统,认为巨噬细胞是来源于骨髓干细胞起源的单核细胞(Monocytes),具有很强的吞噬能力,且细胞核不分叶。单核吞噬细胞系统是当时巨噬细胞起源的主要模型[39]。但是,最近的研究表明,巨噬细胞存在着不依赖造血干细胞(Hematopoietic stem cells,HSCs) 的起源方式,其中一类是在早期卵黄囊中经原位分化直接产生,并迁移到胚胎各处;另一类则由卵黄囊来源的红系髓系祖细胞(Yolk sac-derived myeloid- biased progenitors,YSMPs) 先形成单核细胞后分化为巨噬细胞,这类巨噬细胞具有自我更新能力,并且不依赖于单核细胞的分化[40],如肝脏(库佛氏细胞)、大脑(小胶质细胞)、表皮(朗格汉斯细胞) 和肺(肺泡巨噬细胞) 中的绝大多数组织驻留巨噬细胞(Tissue-resident macrophage,TRM),是来自与HSCs不同的Tek细胞通路[41] (巨噬细胞起源相关模型见图 2)。然而,形成TRM的YSMPs的确切性质仍然存在争议,使巨噬细胞群体维持的机制也不明确[42]。但这也为巨噬细胞起源阐明了新的途径,也为iPSCs诱导巨噬细胞提供了新的思路与方法。

|
| 图 2 巨噬细胞起源及相关标记模式图(P-Sp:主动脉旁内脏胸膜;AGM:主动脉-性腺-中肾) Fig. 2 Model of macrophage origin and related markers. P-Sp: para-aortic splanchnopleure, AGM: orta-gonad-mesonephros. |
| |
巨噬细胞具有极高的可塑性,可以从一种表型转换到另一种表型[43]。巨噬细胞极化是一种特定的表型,并对每个特定组织中遇到的微环境刺激和信号作出功能性反应的过程[44]。根据巨噬细胞的表型与功能,目前普遍认为巨噬细胞存在一系列连续的功能状态,而经典激活或促炎(M1) 型和交替激活或抗炎(M2) 型巨噬细胞是这一连续状态的两个极端[45]。M1型巨噬细胞通常由Th1细胞因子(如IFN-γ和TNF-α)或细菌脂多糖(Lipopolysaccharide, LPS)诱导,并分泌较高水平的促炎细胞因子TNF-α、IL-1α、IL-1β、IL-6、IL-12、IL-23和环氧合酶-2 (COX-2),并专职提呈抗原,参与正向免疫应答,发挥免疫监视的功能;M2型巨噬细胞通常通过IL-4受体α (IL-4Rα) 激活STAT6,或通过IL-10受体(IL-10R) 激活STAT 3来促进其极化,有较弱抗原提呈能力,并通过分泌抑制性细胞因子IL-10或TGF-β等下调免疫应答,在免疫调节中发挥重要作用[46]。因此,M1型巨噬细胞有较强的抗微生物和抗肿瘤活性,介导活性氧(Reactive oxygen species,ROS) 所致的组织损伤,限制组织再生和伤口愈合。为了防止这种组织损伤,M2型巨噬细胞通过较强的吞噬能力,清除碎片和凋亡的细胞,促进组织修复和伤口愈合,从而抑制慢性炎症反应[47]。然而,M1、M2连续体的界限并不清晰,目前公认的M1型和M2型巨噬细胞表面标记及分泌蛋白见图 2[48]。
巨噬细胞是先天免疫系统的关键组成部分,在发育过程中分布在不同的组织和器官中,并通过局部增殖和稳态募集而终生维持[49]。这些组织驻留巨噬细胞群体在各种组织特异性的生理和病理过程中起着核心作用[50]。巨噬细胞还提供了抵御入侵病原体的一道防线,协调先天免疫反应,并激活适应性免疫应答[51]。而且在大多数癌症中,巨噬细胞与肿瘤浸润和预后不良相关,并会导致化疗耐药性,这使得肿瘤相关巨噬细胞无论是通过肿瘤消融还是从亲肿瘤状态向抗肿瘤状态的再分化,都是人类抗癌治疗的良好靶点[52]。因此,在生物学以及人类疾病的背景下,巨噬细胞的功能及表征是许多研究者的主要重点。而实验性人类巨噬细胞主要有两个来源:肿瘤衍生细胞系,如U937、THP-1细胞等,或原代细胞,如外周血单核细胞(Peripheral blood mononuclear cell,PBMC)、单核细胞源性巨噬细胞(Human- monocyte derived macrophages,HMDM)。前者虽具有无限增殖的能力,但核型异常、表型不成熟,不能进行特定基因型的研究[53]。后者是一种应用广泛的巨噬细胞功能实验模型[54],虽然相对容易获得,但HMDM是终末分化的,不能进行自我更新,缺乏增殖能力[55],且较难对其进行基因编辑[42]。利用iPSCs诱导产生的巨噬细胞(Induced pluripotent stem cell-derived macrophages,IPSDM) 可以解决巨噬细胞的获取问题,并可利用先前构建的基因型特异性的iPSCs来衍生出核型正常和基因型稳定的巨噬细胞[56]。这种方法为研究巨噬细胞特定功能、免疫应答机制、活化和极化分子及巨噬细胞相关疾病或肿瘤治疗提供了良好的工具。
3 诱导多能干细胞向巨噬细胞分化的方法目前巨噬细胞研究主要通过使用血液单核细胞衍生的巨噬细胞[57],这些不仅需要来自献血者大量的血液,并且相对难以进行基因编辑。因此,IPSDM为人类巨噬细胞研究提供了一个有力的解决方法。通过对小鼠和人iPSCs产生的畸胎瘤进行分析,证实了iPSCs具有分化为三胚层不同细胞的潜力[58]。此外,iPSCs来源的细胞能够嵌合到多种组织、器官甚至生殖系[59]。小鼠和人类iPSCs已被广泛用于研究造血和免疫系统的发展[60]。虽然并非所有细胞都可以以相同的效率获得,但免疫细胞,如巨噬细胞和自然杀伤细胞等,可以较便捷地利用iPSCs获得,并利用其研发新的细胞疗法[61]。iPSCs衍生的巨噬细胞也可以作为癌症免疫治疗的新方法以及修复或再生患病/受损组织和器官的新策略。图 3展示了iPSCs诱导分化巨噬细胞的主要体外模型。

|
| 图 3 iPSCs诱导分化巨噬细胞体外模型(GM-CSF:粒细胞-巨噬细胞集落刺激因子;M-CSF:巨噬细胞集落刺激因子;IL:白介素;BMP4:骨形态发生蛋白4;Activin A:激活素A;VEGF:血管内皮生长因子;bFGF:成纤维细胞生长因子;SCF:干细胞因子;TPO:血小板生成素;IFN:干扰素;TGF:转化生长因子;TNF:肿瘤坏死因子) Fig. 3 Model of macrophage differentiation induced by iPSCs in vitro. GM-CSF: granulocyte-macrophage colony stimulating factor; M-CSF: macrophage colony stimulating factor; IL: interleukin; BMP4: bone morphogenetic protein 4;; VEGF: vascular endothelial growth factor; bFGF: basic fibroblast growth factor; SCF: stem cell factor; TPO: thermoplastic polyolefin; IFN: interferon; TGF: transforming growth factor; TNF: tumor necrosis factor. |
| |
拟胚体生成法(Embryoid bodies,EBs) 是目前最常用的使iPSCs诱导分化为巨噬细胞的方法。EBs生成法是使未分化的克隆状iPSCs在悬液中生长,形成一种被称为EBs的结构[62]。EBs可以分化为内胚层、中胚层、外胚层的三胚层结构[63]。一旦形成,EBs就可以使用特定的酶分离,获得一个细胞群体,该细胞群体利用特定诱导条件可以分化为三胚层中所有类型的细胞[64]。常见的EBs生产方法主要有静态悬浮培养、旋转悬浮培养[65]、悬挂滴片法[66]、微孔和微模型芯片法[67]等。将由iPSCs产生的EBs在无血清状态下添加特定的细胞因子,如GM-CSF、M-CSF、IL-3、IL-4等将EBs诱导分化为巨噬细胞[68]。Choi等将EBs诱导为髓样前体细胞,再加入M-CSF和IL-1诱导分化为巨噬细胞[69]。Mukherjee等则利用由iPSCs衍生出的EBs,并加入M-CSF、IL-3产生髓样前体细胞,最后用高浓度的M-CSF将髓样前体细胞发育成熟为功能性巨噬细胞,并可使用IFN-γ和IL-4刺激后分别极化为M1或M2型巨噬细胞,且在功能上,这些巨噬细胞能对微生物或病原体刺激作出吞噬反应[70]。Gao等使用M-CSF、IL-3和IL-6先使EBs向中胚层分化产生单核细胞(通过诱导得到的CD14+单核细胞起初只占细胞总量的10%–25%,在诱导25–40 d后可达90%–95%),经过流式细胞荧光分选技术(Fluorescence activated cell sorting,FACS) 分选,再使用M-CSF诱导至M0型巨噬细胞,在有M-CSF的条件下可使用LPS、GM-CSF或IFN-γ诱导M0型巨噬细胞向M1型巨噬细胞转变,使用IL-4向M2型巨噬细胞转变[71]。仅从一个6孔板培养的iPSCs细胞中便可以生成4×106个IPSDM[72],证明了现阶段诱导方案的可行性与高效性。
3.2 单层细胞诱导法单层细胞诱导法是以2D培养的方法将iPSCs在多种细胞因子的作用下直接诱导至髓系祖细胞而产生巨噬细胞,并可将M0型巨噬细胞分化为M1或M2型巨噬细胞[73]。此种方法将iPSCs通过中胚层分化至HSCs形成与PBMC具有相同基因表型的单核细胞[74];也能通过将iPSCs诱导至YSMPs进而产生巨噬细胞[75],相比于HSCs来源的巨噬细胞,YSMPs来源的巨噬细胞缺乏HOXA基因,故其更类似于TRM[76],并能够在低温下长期保存。Hansen等使用该体系无需饲养层,以一种新的单细胞来源的iPSCs集落分化为基础模拟体内造血步骤,通过多种细胞因子在8–10 d内产生多潜能的HSCs,经过谱系特异性生长因子的补充,这些细胞可以诱导为红系细胞、巨噬细胞和髓系细胞[77]。Vordenbäumen等发现人αS1酪蛋白可以促进iPSCs诱导的PBMC向巨噬细胞分化,具体表现在能刺激PBMC促炎细胞因子GM-CSF的表达[78]。Amanda等使用iPSCs模拟YSMPs产生组织驻留巨噬细胞,并使用IL-34、GM-CSF向小胶质细胞诱导,并能对Ca2+响应,展现出与HMDM的区别[79]。单层细胞诱导法很好地避免了EBs的形成,无需特定的EBs进行诱导,并可以经历YSMPs或HSCs分别诱导至TRM或HMDM。且分化步骤清晰,杂细胞比例小,对认清巨噬细胞起源与分化机理具有重大意义,也为巨噬细胞相关疾病提供了一个良好的研究平台,但每一步需要的细胞因子多、成本高且诱导周期长,不利于用于大规模的诱导策略,无法利用巨噬细胞作为“工厂细胞”生产各类细胞制品。
3.3 类器官或组织细胞共培养类器官或组织细胞共培养是使用iPSCs与骨髓基质细胞(Bone marrow stromal cells, BMSCs) 共培养使其向造血方向诱导分化得到巨噬细胞或将M0型巨噬细胞与其他原代组织、iPSCs来源的类器官共培养得到类似TRM的巨噬细胞[80]。目前,已被证实其可以产生小胶质细胞[81]、肺巨噬细胞,并有发展为其他TRM的潜力[35]。此方法的主要优点是可以在短时间内(8–9 d)实现iPSCs的高效定向分化,诱导效率可达85%以上,而且不需要添加细胞因子[82],但诱导步骤及促进其分化的细胞因子种类不明确也对后续科学研究造成了一定的困扰。Choi等于2011年使用小鼠骨髓基质细胞与iPSCs共培养得到巨噬细胞[70],BMSCs共培养可用于获取造血干细胞和成熟细胞,包括T、B淋巴细胞和巨核细胞,虽然BMSCs共培养最初是为ESCs的分化而开发的,但这种方法也可以有效地从iPSCs生成造血细胞,进而分化为巨噬细胞、粒细胞等,并稳定表达其特异性表面标记,通过FACS可以特异性地分离出目的细胞[83],此外该方法通过抑制lnk[84]、强制激活STAT5A[85]、SOX17[86]等均可促进iPSCs向造血分化,提高ESCs的造血潜力及IPSDM的诱导效率。IPSDM共培养系统可能成为研究组织驻留性巨噬细胞的一种非常有价值的工具。
3.4 大规模诱导策略目前,iPSCs向巨噬细胞的大规模分化策略主要仍为EBs生成法,基于生物反应器可以一次性收获大量IPSDM,且收获期较长,细胞因子需求种类较少,通过BMP4、VEGF、SCF得到EB后再添加M-CSF和IL-3便可得到巨噬细胞,较适合商业化生产[35]。Ackermann等开发了一种基于悬浮(3D)、连续(4D) 的造血分化方案,将EBs诱导为髓系细胞形成复合体,其可以连续生产IPSDM并进行肺内移植,可以有效抑制细菌感染[87]。Gutbier等基于生物反应器利用iPSCs建立了一种可大规模生产类似TRM的方法,并克服了巨噬细胞前体及巨噬细胞冷冻保存导致的低存活率,且单次收获产量高达6×108个细胞[88]。几种诱导分化的特点和优缺点见表 1[89]。
| Induction strategy | Induced way | Induced content | The advantages and disadvantages |
| Small scale strategy | Embryoid body formation | iPSCs was generated into EBs, and then induced to differentiate into macrophages by corresponding cytokines | Advantages: it is beneficial to the control of cell differentiation or the study and optimization of the differentiation conditions of a single variable Disadvantages: low differentiation efficiency and high cost |
| Monolayer culture | iPSCs was induced into myeloid progenitor cells to produce macrophages step by step under the action of many factors, and the induction efficiency could be increased by using CSN1S1, and the steps were clear | ||
| Co-culture induction method | iPSCs was co-cultured with bone marrow stromal cells to induce hematopoiesis, and the induction efficiency could be improved by inhibiting Ink or enhancing STAT5 | ||
| Large scale strategy | 2D monolayer culture method | No concentration gradient of cytokines is directly allowed to enter the cells, reducing the differentiation operation | Advantages: it can be used for large-scale production of target cells with low induction cost and high efficiency Disadvantages: need to rely on expensive production equipment, the investment is large, and is not conducive to research |
| 3D suspension culture method | IPSCs can be differentiated into macrophages on a large scale, but the environment is not conducive to the formation of dynamic balance | ||
| Bioreactor culture method | It can improve the use efficiency of raw materials, the product is easy to collect, and the differentiation efficiency is high |
巨噬细胞分布广泛,因其功能状态不同而变化,一般为圆形或椭圆形,并有短小突起,功能活跃者常伸出较长伪足而呈不规则形[90]。M1型巨噬细胞形态常为具有多个突起的星形,而M2型巨噬细胞形态较圆,体外培养时可附着在玻璃和塑料表面[44]。利用吞噬及释放活性氧和一氧化氮的特性同样可以确定其为巨噬细胞,巨噬细胞利用吞噬作用吞噬带荧光标记的细菌或蛋白可以定位巨噬细胞,如Dil-乙酰化低密度脂蛋白或大肠杆菌等,并可实时示踪其活动轨迹[91]。巨噬细胞表达CD11b、CD14、CD18、CD45和CD64标记,但相较于外周血来源的巨噬细胞,CD18和CD45的表达量偏低,M1型巨噬细胞表达CD80和CD86,而M2型巨噬细胞表达CD163和CD206,成熟/激活的巨噬细胞表达CD195和HLA-DR[92]。巨噬细胞的另一种免疫调节功能是刺激后释放亲炎或抗炎细胞因子,这使得其释放的各种细胞因子也能成为验证靶点,亲炎细胞因子(称为M1相关) 包括IL-6、IL-8、TNF-α、CXCL8、CXCL10、CCL2和CCL4等,抗炎因子(称为M2相关) 包括IL-1RA、IL-10、VEGF和CCL22等,均可作为验证模型[93]。单核-巨噬细胞形态学特征及主要表面标记见表 2和图 2[49]。
| Types | Morphological features | Main surface marks |
| Monocytes | It is large in size, 15-20 μm. The nuclei are often eccentric, chromatin is loose and reticular, more chromatin than shallow cytoplasm, and basophilic | CD14, CD45 |
| Macrophages | It is generally round or elliptical, and has short protuberance, and the functional active person often stretches out longer pseudopod and shows irregular shape. The nucleus is small, round or elliptical, and darker in color | CD11b, CD14, CD18, CD45, CD64, CD195, HLA-DR |
| M1 macrophages | It is usually a star with multiple prominences | CD80, CD86 |
| M2 macrophages | Cell morphology is round, basically no protuberance | CD163, CD206 |
巨噬细胞是代谢、免疫、心血管疾病及肿瘤等领域中的关键细胞类型,而IPSDM可以无限增殖并表现特定基因型,且易于通过CRISPR/Cas9进行基因操作。与HMDM相比,IPSDM不仅有与其相媲美的基因表达特征[75],且在免疫反应、抗原处理和呈递及对刺激的反应中,IPSDM均表现出更强的能力[57],并能分化为TRM,为研究疾病建模、药物筛选和细胞治疗提供了一个良好的平台,为研究巨噬细胞生物学提供了重要工具[35]。
利用功能基因组学分析和转录组学分析,IPSDM在疾病模型建立方面拥有着无限的潜力[94]。通过体外刺激IPSDM以确定个体遗传差异如何改变免疫细胞对环境刺激的影响,建立模型预测人类巨噬细胞相关疾病的易感性与恢复力[57],在孟德尔病的建模与研究中起到了关键的作用,如遗传性肺泡蛋白沉积症[95]、丹吉尔病[54]等。对于各类传染病,巨噬细胞吞噬作用是抵抗病原体侵袭的重要防御机制,利用IPSDM建立免疫疾病模型也展现了其极大的优势,Bernard等利用IPSDM研究其对结核杆菌的感染反应及ESX-1 Ⅶ型分泌系统的作用,揭示了细菌逃逸时的膜动力学[96]。
IPSDM在细胞治疗,特别是肿瘤相关治疗方面也展现出其独有的优势。CAR-IPSDM利用肿瘤特异性靶向作用为巨噬细胞清除癌细胞提供了新方法[97]。并利用其表达的干扰素β显著抑制了植入免疫功能低下小鼠腹腔的人胃癌和胰腺癌的生长,展现出了抗肿瘤能力[98]。Ackermann等将IPSDM作为针对细菌性呼吸道感染的细胞治疗方案,有效治疗下呼吸道的急性感染[88]。IPSDM相比HMDM表现出HLA-DRdim,是低活性低极化细胞[94],略微偏向于抗炎活性,具有多种功能活性,是一种研究病原体和宿主之间的相互作用及研究其抗菌能力的宝贵模型,用于评估多种遗传背景下的宿主反应[99]。在移植或输血过程中使用IPSDM能够有效避免免疫排斥和疾病传播风险,并能增强其功能或赋予其新的治疗特性[100]。
利用IPSDM可以分化为类似组织驻留巨噬细胞的特性,通过单细胞测序或CRISPR/Cas9,更有利于验证TRM所涉及的关键转录因子在机体中的作用及其如何调节组织免疫功能[69],例如小胶质细胞的SALL1[101]、肺泡巨噬细胞的PPARγ[102]、朗格汉斯细胞的RUNX3[103]、库弗氏细胞的ld3[104],利用IPSDM进行此类研究很可能为巨噬细胞相关疾病(如肺泡蛋白沉积症[105]、阿尔茨海默病[106]等) 带来新的治疗靶点。且当移植进体内时,IPSDM能够适应并获得与相应组织驻留巨噬细胞相同的表型和功能特征,这为研究组织驻留巨噬细胞提供了极佳的模型[35]。IPSDM的发展为YSMPs来源的巨噬细胞提供了一个良好的替代品,为区分哪些属性是所有巨噬细胞固有的、哪些是组织驻留巨噬细胞特有的提供了一个强大的工具。
因此,IPSDM为巨噬细胞相关疾病建模、新疗法的开发以及作为治疗特定疾病患者的细胞来源提供了新的机遇。再加上易于通过基因组编辑方法(如CRISPR/Cas9和TALEN)进行操作,IPSDM代表了一种潜在的、优质的巨噬细胞来源,可用于大规模生成特定研究材料[107]。
5 总结与展望iPSCs的相关问题已经经过了10余年的研究,取得了一定的研究进展,与胚胎干细胞相比,iPSCs解决了免疫排斥问题和伦理道德问题,为相关领域的研究开辟了新方向、新思路,但仍有很多问题亟需解决,重编程效率、致瘤风险、分化多样性与多能性不足的问题仍困扰着当代研究者。笔者研究组建立了猪人工诱导多能干细胞(piPSCs),解析了决定多能性干细胞发育分化的一系列关键因子的调控机理[108-113]。然而,猪诱导多能干细胞虽然具有三胚层分化能力并在体内能够产生畸胎瘤,但不能得到生殖系嵌合体后代,说明piPSCs的多能性可能存在缺陷[114-115]。虽然人和小鼠的多能干细胞能够被定性诱导至巨噬细胞,但猪诱导多能干细胞能否定向分化成巨噬细胞,仍是一个未知的问题。而且,在多能信号通路中,猪也表现出与人和小鼠的不同[116-117],人和小鼠的诱导体系是否也适用于猪,也是值得探索的。
巨噬细胞作为人体内重要的免疫细胞,iPSCs向巨噬细胞分化为研究者获取巨噬细胞并在体外培养提供了一个便捷、可靠的来源,为研究巨噬细胞发育规律建立了良好的模型,且对IPSDM进行基因修饰可以针对一些免疫相关疾病提供基因分析工具,但目前对其相关分化机制的研究还十分有限,探究其分化机制为人类免疫系统的研究提供了很好的借鉴意义,且过程可视化。笔者利用猪诱导性多能干细胞作为种子细胞,利用小分子结合CRISPR/Cas9基因编辑技术尝试建立高效的iPSCs诱导分化为肺泡巨噬细胞,挖掘iPSCs向免疫细胞的高效体系和决定因子,进一步筛选用于抗病育种的靶标基因和抗病药物筛选或用作大批量制作疫苗的载体细胞。目前采用多种方法优化提高其诱导分化效率,其中改进其诱导分化途径、基因敲除技术的联合使用、分化机制的探究都将是今后研究的热点问题。
| [1] |
Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell, 2006, 126(4): 663-676. DOI:10.1016/j.cell.2006.07.024
|
| [2] |
Brumbaugh J, Di Stefano B, Hochedlinger K. Reprogramming: identifying the mechanisms that safeguard cell identity. Development, 2019, 146(23): dev182170.. DOI:10.1242/dev.182170
|
| [3] |
Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell, 2007, 131(5): 861-872. DOI:10.1016/j.cell.2007.11.019
|
| [4] |
Yu J, Vodyanik MA, Smuga-Otto K, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science, 2007, 318(5858): 1917-1920. DOI:10.1126/science.1151526
|
| [5] |
Wang HF, Yang YC, Liu JD, et al. Direct cell reprogramming: approaches, mechanisms and progress. Nat Rev Mol Cell Biol, 2021, 1-15.
|
| [6] |
Ma T, Xie M, Laurent T, et al. Progress in the reprogramming of somatic cells. Circ Res, 2013, 112(3): 562-574. DOI:10.1161/CIRCRESAHA.111.249235
|
| [7] |
Marin Navarro A, Pronk RJ, Van der Geest AT, et al. p53 controls genomic stability and temporal differentiation of human neural stem cells and affects neural organization in human brain organoids. Cell Death Dis, 2020, 11(1): 52. DOI:10.1038/s41419-019-2208-7
|
| [8] |
Olgasi C, Cucci A, Follenzi A. iPSC-derived liver organoids: a journey from drug screening, to disease modeling, arriving to regenerative medicine. Int J Mol Sci, 2020, 21(17): 6215. DOI:10.3390/ijms21176215
|
| [9] |
Engle SJ, Blaha L, Kleiman RJ. Best practices for translational disease modeling using human iPSC-derived neurons. Neuron, 2018, 100(4): 783-797. DOI:10.1016/j.neuron.2018.10.033
|
| [10] |
Aoi T, Yae K, Nakagawa M, et al. Generation of pluripotent stem cells from adult mouse liver and stomach cells. Science, 2008, 321(5889): 699-702. DOI:10.1126/science.1154884
|
| [11] |
Okita K, Nakagawa M, Hyenjong H, et al. Generation of mouse induced pluripotent stem cells without viral vectors. Science, 2008, 322(5903): 949-953. DOI:10.1126/science.1164270
|
| [12] |
Aasen T, Raya A, Barrero MJ, et al. Efficient and rapid generation of induced pluripotent stem cells from human keratinocytes. Nat Biotechnol, 2008, 26(11): 1276-1284. DOI:10.1038/nbt.1503
|
| [13] |
Balasubramanian S, Babai N, Chaudhuri A, et al. Non cell-autonomous reprogramming of adult ocular progenitors: generation of pluripotent stem cells without exogenous transcription factors. Stem Cells, 2009, 27(12): 3053-3062. DOI:10.1002/stem.242
|
| [14] |
Honda A, Hirose M, Hatori M, et al. Generation of induced pluripotent stem cells in rabbits: potential experimental models for human regenerative medicine. J Biol Chem, 2010, 285(41): 31362-31369. DOI:10.1074/jbc.M110.150540
|
| [15] |
Stadtfeld M, Brennand K, Hochedlinger K. Reprogramming of pancreatic beta cells into induced pluripotent stem cells. Curr Biol, 2008, 18(12): 890-894. DOI:10.1016/j.cub.2008.05.010
|
| [16] |
Abad M, Mosteiro L, Pantoja C, et al. Reprogramming in vivo produces teratomas and iPS cells with totipotency features. Nature, 2013, 502(7471): 340-345. DOI:10.1038/nature12586
|
| [17] |
Huangfu D, Osafune K, Maehr R, et al. Induction of pluripotent stem cells from primary human fibroblasts with only Oct4 and Sox2. Nat Biotechnol, 2008, 26(11): 1269-1275. DOI:10.1038/nbt.1502
|
| [18] |
Pessôa LVF, Bressan FF, Freude KK. Induced pluripotent stem cells throughout the animal kingdom: availability and applications. World J Stem Cells, 2019, 11(8): 491-505. DOI:10.4252/wjsc.v11.i8.491
|
| [19] |
Chew JL, Loh YH, Zhang W, et al. Reciprocal transcriptional regulation of Pou5f1 and Sox2 via the Oct4/Sox2 complex in embryonic stem cells. Mol Cell Biol, 2005, 25(14): 6031-6046. DOI:10.1128/MCB.25.14.6031-6046.2005
|
| [20] |
Yu J, Hu K, Smuga-Otto K, et al. Human induced pluripotent stem cells free of vector and transgene sequences. Science, 2009, 324(5928): 797-801. DOI:10.1126/science.1172482
|
| [21] |
Schlaeger TM, Daheron L, Brickler TR, et al. A comparison of non-integrating reprogramming methods. Nat Biotechnol, 2015, 33(1): 58-63. DOI:10.1038/nbt.3070
|
| [22] |
Miyamoto K, Akiyama M, Tamura F, et al. Direct in vivo reprogramming with Sendai virus vectors improves cardiac function after myocardial infarction. Cell Stem Cell, 2018, 22(1): 91-103. DOI:10.1016/j.stem.2017.11.010
|
| [23] |
Okita K, Matsumura Y, Sato Y, et al. A more efficient method to generate integration-free human iPS cells. Nat Methods, 2011, 8(5): 409-412. DOI:10.1038/nmeth.1591
|
| [24] |
Miyoshi N, Ishii H, Nagano H, et al. Reprogramming of mouse and human cells to pluripotency using mature microRNAs. Cell Stem Cell, 2011, 8(6): 633-638. DOI:10.1016/j.stem.2011.05.001
|
| [25] |
Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature, 2007, 448(7151): 313-317. DOI:10.1038/nature05934
|
| [26] |
Hou P, Li Y, Zhang X, et al. Pluripotent stem cells induced from mouse somatic cells by small-molecule compounds. Science, 2013, 341(6146): 651-654. DOI:10.1126/science.1239278
|
| [27] |
Chou BK, Cheng LZ. And then there were none: no need for pluripotency factors to induce reprogramming. Cell Stem Cell, 2013, 13(3): 261-262. DOI:10.1016/j.stem.2013.08.004
|
| [28] |
Karagiannis P, Takahashi K, Saito M, et al. Induced pluripotent stem cells and their use in human models of disease and development. Physiol Rev, 2019, 99(1): 79-114. DOI:10.1152/physrev.00039.2017
|
| [29] |
Yamanaka S. Induced pluripotent stem cells: past, present, and future. Cell Stem Cell, 2012, 10(6): 678-684. DOI:10.1016/j.stem.2012.05.005
|
| [30] |
Warren L, Manos PD, Ahfeldt T, et al. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell, 2010, 7(5): 618-630. DOI:10.1016/j.stem.2010.08.012
|
| [31] |
Mertens J, Marchetto MC, Bardy C, et al. Evaluating cell reprogramming, differentiation and conversion technologies in neuroscience. Nat Rev Neurosci, 2016, 17(7): 424-437. DOI:10.1038/nrn.2016.46
|
| [32] |
Lin Y, Gil CH, Yoder MC. Differentiation, evaluation, and application of human induced pluripotent stem cell-derived endothelial cells. Arterioscler Thromb Vasc Biol, 2017, 37(11): 2014-2025. DOI:10.1161/ATVBAHA.117.309962
|
| [33] |
Zeng J, Tang SY, Toh LL, et al. Generation of "off-the-shelf" natural killer cells from peripheral blood cell-derived induced pluripotent stem cells. Stem Cell Reports, 2017, 9(6): 1796-1812. DOI:10.1016/j.stemcr.2017.10.020
|
| [34] |
Amini Mahabadi J, Sabzalipoor H, Kehtari M, et al. Derivation of male germ cells from induced pluripotent stem cells by inducers: a review. Cytotherapy, 2018, 20(3): 279-290. DOI:10.1016/j.jcyt.2018.01.002
|
| [35] |
Takata K, Kozaki T, Lee CZW, et al. Induced-pluripotent-stem-cell-derived primitive macrophages provide a platform for modeling tissue-resident macrophage differentiation and function. Immunity, 2017, 47(1): 183-198. DOI:10.1016/j.immuni.2017.06.017
|
| [36] |
Crow D. Could iPSCs enable "off-the-shelf" cell therapy?. Cell, 2019, 177(7): 1667-1669. DOI:10.1016/j.cell.2019.05.043
|
| [37] |
Wynn TA, Vannella KM. Macrophages in tissue repair, regeneration, and fibrosis. Immunity, 2016, 44(3): 450-462. DOI:10.1016/j.immuni.2016.02.015
|
| [38] |
Van den Bossche J, O'Neill LA, Menon D. Macrophage immunometabolism: where are we (going)?. Trends Immunol, 2017, 38(6): 395-406. DOI:10.1016/j.it.2017.03.001
|
| [39] |
Franken L, Schiwon M, Kurts C. Macrophages: sentinels and regulators of the immune system. Cell Microbiol, 2016, 18(4): 475-487. DOI:10.1111/cmi.12580
|
| [40] |
Bian Z, Gong Y, Huang T, et al. Deciphering human macrophage development at single-cell resolution. Nature, 2020, 582(7813): 571-576. DOI:10.1038/s41586-020-2316-7
|
| [41] |
Gomez Perdiguero E, Klapproth K, Schulz C, et al. Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature, 2015, 518(7540): 547-551. DOI:10.1038/nature13989
|
| [42] |
Gentek R, Molawi K, Sieweke MH. Tissue macrophage identity and self-renewal. Immunol Rev, 2014, 262(1): 56-73. DOI:10.1111/imr.12224
|
| [43] |
Yang D, Wan Y. Molecular determinants for the polarization of macrophage and osteoclast. Semin Immunopathol, 2019, 41(5): 551-563. DOI:10.1007/s00281-019-00754-3
|
| [44] |
Murray PJ. Macrophage polarization. Annu Rev Physiol, 2017, 79(1): 541-566. DOI:10.1146/annurev-physiol-022516-034339
|
| [45] |
Donadon M, Torzilli G, Cortese N, et al. Macrophage morphology correlates with single-cell diversity and prognosis in colorectal liver metastasis. J Exp Med, 2020, 217(11): e20191847. DOI:10.1084/jem.20191847
|
| [46] |
Shapouri-Moghaddam A, Mohammadian S, Vazini H, et al. Macrophage plasticity, polarization, and function in health and disease. J Cell Physiol, 2018, 233(9): 6425-6440. DOI:10.1002/jcp.26429
|
| [47] |
Atri C, Guerfali FZ, Laouini D. Role of human macrophage polarization in inflammation during infectious diseases. Int J Mol Sci, 2018, 19(6): E1801. DOI:10.3390/ijms19061801
|
| [48] |
Varol C, Mildner A, Jung S. Macrophages: development and tissue specialization. Annu Rev Immunol, 2015, 33: 643-675. DOI:10.1146/annurev-immunol-032414-112220
|
| [49] |
Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature, 2013, 496(7446): 445-455. DOI:10.1038/nature12034
|
| [50] |
Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol, 2011, 11(11): 723-737. DOI:10.1038/nri3073
|
| [51] |
Okabe Y, Medzhitov R. Tissue biology perspective on macrophages. Nat Immunol, 2016, 17(1): 9-17. DOI:10.1038/ni.3320
|
| [52] |
Cassetta L, Pollard JW. Targeting macrophages: therapeutic approaches in cancer. Nat Rev Drug Discov, 2018, 17(12): 887-904. DOI:10.1038/nrd.2018.169
|
| [53] |
Chanput W, Peters V, Wichers H. THP-1 and U937 cells. The impact of food bioactives on health. Cham: Springer International Publishing, 2015: 147-159.
|
| [54] |
Zhang H, Xue C, Shah R, et al. Functional analysis and transcriptomic profiling of iPSC-derived macrophages and their application in modeling Mendelian disease. Circ Res, 2015, 117(1): 17-28. DOI:10.1161/CIRCRESAHA.117.305860
|
| [55] |
Shi J, Xue C, Liu W, et al. Differentiation of human-induced pluripotent stem cells to macrophages for disease modeling and functional genomics. Curr Protoc Stem Cell Biol, 2019, 48(1): e74.
|
| [56] |
Zhang HR, Reilly MP. Human induced pluripotent stem cell-derived macrophages for unraveling human macrophage biology. Arterioscler Thromb Vasc Biol, 2017, 37(11): 2000-2006. DOI:10.1161/ATVBAHA.117.309195
|
| [57] |
Hoeffel G, Chen J, Lavin Y, et al. C-Myb(+) erythro-myeloid progenitor-derived fetal monocytes give rise to adult tissue-resident macrophages. Immunity, 2015, 42(4): 665-678. DOI:10.1016/j.immuni.2015.03.011
|
| [58] |
Zakrzewski W, Dobrzyński M, Szymonowicz M, et al. Stem cells: past, present, and future. Stem Cell Res Ther, 2019, 10(1): 68. DOI:10.1186/s13287-019-1165-5
|
| [59] |
Stadtfeld M, Nagaya M, Utikal J, et al. Induced pluripotent stem cells generated without viral integration. Science, 2008, 322(5903): 945-949. DOI:10.1126/science.1162494
|
| [60] |
Omole AE, Fakoya AOJ. Ten years of progress and promise of induced pluripotent stem cells: historical origins, characteristics, mechanisms, limitations, and potential applications. PeerJ, 2018, 6: e4370. DOI:10.7717/peerj.4370
|
| [61] |
Bernareggi D, Pouyanfard S, Kaufman DS. Development of innate immune cells from human pluripotent stem cells. Exp Hematol, 2019, 71: 13-23. DOI:10.1016/j.exphem.2018.12.005
|
| [62] |
Yoshida Y, Yamanaka S. iPS cells: a source of cardiac regeneration. J Mol Cell Cardiol, 2011, 50(2): 327-332. DOI:10.1016/j.yjmcc.2010.10.026
|
| [63] |
Brickman JM, Serup P. Properties of embryoid bodies. WIREs Dev Biol, 2017, 6(2): e259.
|
| [64] |
Barzegari A, Gueguen V, Omidi Y, et al. The role of Hippo signaling pathway and mechanotransduction in tuning embryoid body formation and differentiation. J Cell Physiol, 2020, 235(6): 5072-5083. DOI:10.1002/jcp.29455
|
| [65] |
Carpenedo RL, Sargent CY, McDevitt TC. Rotary suspension culture enhances the efficiency, yield, and homogeneity of embryoid body differentiation. Stem Cells, 2007, 25(9): 2224-2234. DOI:10.1634/stemcells.2006-0523
|
| [66] |
Wu HW, Hsiao YH, Chen CC, et al. A PDMS-based microfluidic hanging drop chip for embryoid body formation. Molecules, 2016, 21(7): E882. DOI:10.3390/molecules21070882
|
| [67] |
Spelke DP, Ortmann D, Khademhosseini A, et al. Methods for embryoid body formation: the microwell approach. Methods Mol Biol, 2011, 690: 151-162.
|
| [68] |
Lee CZW, Kozaki T, Ginhoux F. Studying tissue macrophages in vitro: are iPSC-derived cells the answer?. Nat Rev Immunol, 2018, 18(11): 716-725. DOI:10.1038/s41577-018-0054-y
|
| [69] |
Choi KD, Vodyanik M, Slukvin Ⅱ. Hematopoietic differentiation and production of mature myeloid cells from human pluripotent stem cells. Nat Protoc, 2011, 6(3): 296-313. DOI:10.1038/nprot.2010.184
|
| [70] |
Mukherjee C, Hale C, Mukhopadhyay S. A simple multistep protocol for differentiating human induced pluripotent stem cells into functional macrophages. Methods Mol Biol, 2018, 1784: 13-28.
|
| [71] |
Gao WX, Sun YQ, Shi JB, et al. Effects of mesenchymal stem cells from human induced pluripotent stem cells on differentiation, maturation, and function of dendritic cells. Stem Cell Res Ther, 2017, 8(1): 48. DOI:10.1186/s13287-017-0499-0
|
| [72] |
Quarta A, Le Blon D, D'aes T, et al. Murine iPSC-derived microglia and macrophage cell culture models recapitulate distinct phenotypical and functional properties of classical and alternative neuro-immune polarisation. Brain Behav Immun, 2019, 82: 406-421. DOI:10.1016/j.bbi.2019.09.009
|
| [73] |
Douvaras P, Sun B, Wang M, et al. Directed differentiation of human pluripotent stem cells to microglia. Stem Cell Reports, 2017, 8(6): 1516-1524. DOI:10.1016/j.stemcr.2017.04.023
|
| [74] |
Cao X, Yakala GK, Van den Hil FE, et al. Differentiation and functional comparison of monocytes and macrophages from hiPSCs with peripheral blood derivatives. Stem Cell Reports, 2019, 12(6): 1282-1297. DOI:10.1016/j.stemcr.2019.05.003
|
| [75] |
Abud EM, Ramirez RN, Martinez ES, et al. iPSC-derived human microglia-like cells to study neurological diseases. Neuron, 2017, 94(2): 278-293. DOI:10.1016/j.neuron.2017.03.042
|
| [76] |
Dou DR, Calvanese V, Sierra MI, et al. Medial HOXA genes demarcate haematopoietic stem cell fate during human development. Nat Cell Biol, 2016, 18(6): 595-606. DOI:10.1038/ncb3354
|
| [77] |
Hansen M, Varga E, Aarts C, et al. Efficient production of erythroid, megakaryocytic and myeloid cells, using single cell-derived iPSC colony differentiation. Stem Cell Res, 2018, 29: 232-244. DOI:10.1016/j.scr.2018.04.016
|
| [78] |
Vordenbäumen S, Braukmann A, Altendorfer I, et al. Human casein alpha s1 (CSN1S1) skews in vitro differentiation of monocytes towards macrophages. BMC Immunol, 2013, 14: 46. DOI:10.1186/1471-2172-14-46
|
| [79] |
McQuade A, Coburn M, Tu CH, et al. Development and validation of a simplified method to generate human microglia from pluripotent stem cells. Mol Neurodegener, 2018, 13(1): 67. DOI:10.1186/s13024-018-0297-x
|
| [80] |
Kaufman DS, Hanson ET, Lewis RL, et al. Hematopoietic colony-forming cells derived from human embryonic stem cells. Proc Natl Acad Sci USA, 2001, 98(19): 10716-10721. DOI:10.1073/pnas.191362598
|
| [81] |
Guttikonda SR, Sikkema L, Tchieu J, et al. Fully defined human pluripotent stem cell-derived microglia and tri-culture system model C3 production in Alzheimer's disease. Nat Neurosci, 2021, 24(3): 343-354. DOI:10.1038/s41593-020-00796-z
|
| [82] |
Wang HT, Wang MG, Wang Y, et al. MSX2 suppression through inhibition of TGFβ signaling enhances hematopoietic differentiation of human embryonic stem cells. Stem Cell Res Ther, 2020, 11(1): 147. DOI:10.1186/s13287-020-01653-3
|
| [83] |
Choi KD, Yu J, Smuga-Otto K, et al. Hematopoietic and endothelial differentiation of human induced pluripotent stem cells. Stem Cells, 2009, 27(3): 559-567. DOI:10.1634/stemcells.2008-0922
|
| [84] |
Tashiro K, Omori M, Kawabata K, et al. Inhibition of lnk in mouse induced pluripotent stem cells promotes hematopoietic cell generation. Stem Cells Dev, 2012, 21(18): 3381-3390. DOI:10.1089/scd.2012.0100
|
| [85] |
Schuringa JJ, Wu K, Morrone G, et al. Enforced activation of STAT5A facilitates the generation of embryonic stem-derived hematopoietic stem cells that contribute to hematopoiesis in vivo. Stem Cells, 2004, 22(7): 1191-1204. DOI:10.1634/stemcells.2004-0033
|
| [86] |
Nakajima-Takagi Y, Osawa M, Oshima M, et al. Role of SOX17 in hematopoietic development from human embryonic stem cells. Blood, 2013, 121(3): 447-458. DOI:10.1182/blood-2012-05-431403
|
| [87] |
Ackermann M, Kempf H, Hetzel M, et al. Bioreactor-based mass production of human iPSC-derived macrophages enables immunotherapies against bacterial airway infections. Nat Commun, 2018, 9(1): 5088. DOI:10.1038/s41467-018-07570-7
|
| [88] |
Gutbier S, Wanke F, Dahm N, et al. Large-scale production of human iPSC-derived macrophages for drug screening. Int J Mol Sci, 2020, 21(13): 4808. DOI:10.3390/ijms21134808
|
| [89] |
Guilliams M, Thierry GR, Bonnardel J, et al. Establishment and maintenance of the macrophage niche. Immunity, 2020, 52(3): 434-451. DOI:10.1016/j.immuni.2020.02.015
|
| [90] |
Chen YN, Hu MR, Wang L, et al. Macrophage M1/M2 polarization. Eur J Pharmacol, 2020, 877: 173090. DOI:10.1016/j.ejphar.2020.173090
|
| [91] |
Neupane AS, Willson M, Chojnacki AK, et al. Patrolling alveolar macrophages conceal bacteria from the immune system to maintain homeostasis. Cell, 2020, 183(1): 110-125. DOI:10.1016/j.cell.2020.08.020
|
| [92] |
Roussel M, Ferrell PB, Greenplate AR, et al. Mass cytometry deep phenotyping of human mononuclear phagocytes and myeloid-derived suppressor cells from human blood and bone marrow. J Leukoc Biol, 2017, 102(2): 437-447. DOI:10.1189/jlb.5MA1116-457R
|
| [93] |
Nenasheva T, Gerasimova T, Serdyuk Y, et al. Macrophages derived from human induced pluripotent stem cells are low-activated "naïve-like" cells capable of restricting Mycobacteria growth. Front Immunol, 2020, 11: 1016. DOI:10.3389/fimmu.2020.01016
|
| [94] |
Buchrieser J, James W, Moore MD. Human induced pluripotent stem cell-derived macrophages share ontogeny with MYB-independent tissue-resident macrophages. Stem Cell Reports, 2017, 8(2): 334-345. DOI:10.1016/j.stemcr.2016.12.020
|
| [95] |
Mucci A, Kunkiel J, Suzuki T, et al. Murine iPSC-derived macrophages as a tool for disease modeling of hereditary pulmonary alveolar proteinosis due to Csf2rb deficiency. Stem Cell Reports, 2016, 7(2): 292-305. DOI:10.1016/j.stemcr.2016.06.011
|
| [96] |
Bernard EM, Fearns A, Bussi C, et al. Mycobacteriam tuberculosis infection of human iPSC-derived macrophages reveals complex membrane dynamics during xenophagy evasion. J Cell Sci, 2021, 134(5): jcs252973.
|
| [97] |
Zhang L, Tian L, Dai X, et al. Pluripotent stem cell-derived CAR-macrophage cells with antigen-dependent anti-cancer cell functions. J Hematol Oncol, 2020, 13(1): 153. DOI:10.1186/s13045-020-00983-2
|
| [98] |
Senju S, Koba C, Haruta M, et al. Application of iPS cell-derived macrophages to cancer therapy. Oncoimmunology, 2014, 3(1): e27927.
|
| [99] |
Hale C, Yeung A, Goulding D, et al. Induced pluripotent stem cell derived macrophages as a cellular system to study Salmonella and other pathogens. PLoS ONE, 2015, 10(5): e0124307. DOI:10.1371/journal.pone.0124307
|
| [100] |
Hansen M, von Lindern M, van den Akker E, et al. Human-induced pluripotent stem cell-derived blood products: state of the art and future directions. FEBS Lett, 2019, 593(23): 3288-3303. DOI:10.1002/1873-3468.13599
|
| [101] |
Buttgereit A, Lelios I, Yu X, et al. Sall1 is a transcriptional regulator defining microglia identity and function. Nat Immunol, 2016, 17(12): 1397-1406. DOI:10.1038/ni.3585
|
| [102] |
Schneider C, Nobs SP, Kurrer M, et al. Induction of the nuclear receptor PPAR-γ by the cytokine GM-CSF is critical for the differentiation of fetal monocytes into alveolar macrophages. Nat Immunol, 2014, 15(11): 1026-1037. DOI:10.1038/ni.3005
|
| [103] |
Fainaru O, Woolf E, Lotem J, et al. Runx3 regulates mouse TGF-beta-mediated dendritic cell function and its absence results in airway inflammation. EMBO J, 2004, 23(4): 969-979. DOI:10.1038/sj.emboj.7600085
|
| [104] |
Mass E, Ballesteros I, Farlik M, et al. Specification of tissue-resident macrophages during organogenesis. Science, 2016, 353(6304): aaf4238. DOI:10.1126/science.aaf4238
|
| [105] |
Happle C, Lachmann N, Ackermann M, et al. Pulmonary transplantation of human induced pluripotent stem cell-derived macrophages ameliorates pulmonary alveolar proteinosis. Am J Respir Crit Care Med, 2018, 198(3): 350-360. DOI:10.1164/rccm.201708-1562OC
|
| [106] |
Hanger B, Couch A, Rajendran L, et al. Emerging developments in human induced pluripotent stem cell-derived microglia: implications for modelling psychiatric disorders with a neurodevelopmental origin. Front Psychiatry, 2020, 11: 789.
|
| [107] |
Zhang H, Shi J, Hachet MA, et al. CRISPR/Cas9-mediated gene editing in human iPSC-derived macrophage reveals lysosomal acid lipase function in human macrophages-brief report. Arterioscler Thromb Vasc Biol, 2017, 37(11): 2156-2160. DOI:10.1161/ATVBAHA.117.310023
|
| [108] |
Shen QY, Yu S, Zhang Y, et al. Characterization of porcine extraembryonic endoderm cells. Cell Prolif, 2019, 52(3): e12591. DOI:10.1111/cpr.12591
|
| [109] |
Zhu Z, Pan Q, Zhao W, et al. BCL2 enhances survival of porcine pluripotent stem cells through promoting FGFR2. Cell Prolif, 2021, 54(1): e12932.
|
| [110] |
Zhu Z, Wu X, Li Q, et al. Histone demethylase complexes KDM3A and KDM3B cooperate with OCT4/SOX2 to define a pluripotency gene regulatory network. FASEB journal, 2021, 35(6): e21664.
|
| [111] |
Yu S, Zhang R, Shen Q, et al. ESRRB Facilitates the Conversion of Trophoblast-Like Stem Cells From Induced Pluripotent Stem Cells by Directly Regulating CDX2. Front Cell Dev Biol, 2021, 9: 712224. DOI:10.3389/fcell.2021.712224
|
| [112] |
Wu XL, Zhu ZS, Xiao X, et al. LIN28A inhibits DUSP family phosphatases and activates MAPK signaling pathway to maintain pluripotency in porcine induced pluripotent stem cells. Zool Res, 2021, 42(3): 377-388. DOI:10.24272/j.issn.2095-8137.2020.375
|
| [113] |
Yue W, Sun J, Zhang J, et al. Mir-34c affects the proliferation and pluripotency of porcine induced pluripotent stem cell (piPSC)-like cells by targeting c-Myc. Cells Dev, 2021, 166: 203665. DOI:10.1016/j.cdev.2021.203665
|
| [114] |
Esteban MA, Xu JY, Yang JY, et al. Generation of induced pluripotent stem cell lines from Tibetan miniature pig. J Biol Chem, 2009, 284(26): 17634-17640. DOI:10.1074/jbc.M109.008938
|
| [115] |
Ezashi T, Telugu BP, Alexenko AP, et al. Derivation of induced pluripotent stem cells from pig somatic cells. Proc Natl Acad Sci USA, 2009, 106(27): 10993-10998. DOI:10.1073/pnas.0905284106
|
| [116] |
Ramos-Ibeas P, Sang F, Zhu Q, et al. Pluripotency and X chromosome dynamics revealed in pig pre-gastrulating embryos by single cell analysis. Nat Commun, 2019, 10(1): 500. DOI:10.1038/s41467-019-08387-8
|
| [117] |
Gao X, Nowak-Imialek M, Chen X, et al. Establishment of porcine and human expanded potential stem cells. Nat Cell Biol, 2019, 21(6): 687-699. DOI:10.1038/s41556-019-0333-2
|
 2021, Vol. 37
2021, Vol. 37




