中国科学院微生物研究所、中国微生物学会主办
文章信息
- 江政云, 陈敏, 张焜
- Jiang Zhengyun, Chen Min, Zhang Kun
- 运动神经元特异性荧光报告系统的建立
- Development of a motor neurons-specific fluorescence reporter system
- 生物工程学报, 2021, 37(11): 4095-4101
- Chinese Journal of Biotechnology, 2021, 37(11): 4095-4101
- 10.13345/j.cjb.210006
-
文章历史
- Received: January 4, 2021
- Accepted: May 7, 2021
- Published: June 28, 2021
2. 五邑大学 生物科技与大健康学院, 广东 江门 529020;
3. 江门市大健康国际创新研究院, 广东 江门 529080
2. School of Biotechnology and Health Sciences, Wuyi University, Jiangmen 529020, Guangdong, China;
3. International Healthcare Innovation Institute (Jiangmen), Jiangmen 529080, Guangdong, China
人类运动神经元疾病包括一系列退行性疾病,如肌萎缩侧索硬化症(Amyotrophic lateral sclerosis) 和脊髓性肌萎缩症(Spinal muscular atrophy) 等,该类疾病临床表现为严重肌肉萎缩和运动功能失调,最终导致呼吸衰竭和死亡[1-3]。值得注意的是,运动神经元(Motor neurons,MNs)的广泛死亡是最直接、最典型的病理特征[4-7]。解析疾病机制的一个关键障碍是缺乏合适的人类MNs疾病模型,而从人诱导多能干细胞(Human induced pluripotent stem cells,hiPSCs) 获得MNs是得到疾病研究模型的重要途径[8-9]。
获得成熟的MNs是一项具有意义和挑战的工作,对人类神经发育、相关疾病建模和药物开发至关重要[10]。目前,主要通过两种方法将hiPSCs定向分化为成熟的MNs。一个是化学小分子诱导法,模拟人运动神经元发育过程,在相应时间节点加入不同的小分子化合物,可以在体外获得成熟的MNs[9-12]。但是,该方法耗时冗长、操作烦琐且价格昂贵。另外一种是转基因法,通过病毒等载体将MNs定向分化的关键基因转入hiPSCs或人胚胎干细胞(Human embryonic stem cells),可以快速高效地获得运动神经元,该方法比小分子诱导时间缩短一半[9, 13]。无论哪种方法获得的神经元,均需要通过免疫组织化学或者免疫荧光表征MNs相关标记物进行鉴定和分析。为了动态监测MNs的分化过程,可视化的MNs荧光报告系统备受青睐[3]。本文通过转基因技术,在人神经干细胞(Human neural stem cells,hNSCs)中导入MNs特异性启动子HB9控制下的RFP,经嘌呤霉素筛选获得阳性细胞株hNSCs-HB9- RFP-Puro。该细胞株进一步过表达神经原素2 (Neurogenin 2,Ngn2)、Y染色体性别决定域转录因子11 (SYR-box transcription factor 11,Sox11)、胰岛素增强子1 (Insulin gene enhancer 1,Isl1) 和LIM同源框3 (LIM homeobox 3,Lhx3),在定向分化为MNs的过程中,特异性启动子HB9将介导红色荧光蛋白(Red fluorescent protein,RFP)的表达,为运动神经元的定向分化提供了一个可视化的方法,加速了运动神经元疾病体外模型的建立及药物的开发和筛选。
1 材料与方法 1.1 细胞与培养液hiPSCs来自罗格斯大学细胞和DNA资源库(Rutgers University Cell and DNA Repository)。神经干细胞分化培养基:Neuro basal medium 490 mL和Neural induction supplement 10 mL,(Cat. No. A1647801)。神经干细胞扩增培养基:Neuro basal medium 49 mL,advanced DMEM/F12 49 mL和Neural induction supplement 2 mL。运动神经元培养基:Neuro basal medium 24.5 mL,adcanced DMEM/F12 24.5 mL,N-2 (100×) 0.5 mL,B-27 (50×) 1 mL,retinoic acid 1 μmol/L,BDNF 10 ng/mL,GDNF 10 ng/mL,NT-3 10 ng/mL,purmorphamine 500 nmol/L和penicillin/streptomycin (100×) 0.5 mL。
1.2 慢病毒包装慢病毒(Lentivirus,LV) 包装采用TaKaRa Bio Company的Lenti-X Packaging Single Shots Kit (货号:631275)。重组慢病毒质粒来自Addgene的pCSC-NGN2-IRES-GFP-T2A-Sox11和pCSC-ISL- T2A-LHX3,质粒编号:90214和90215[14]。参照Lenti-X Packaging Single Shots Kit使用手册制备慢病毒LV-Ngn2-Sox11-GFP和LV-Isl1-Lhx3-Hygro。
质粒pLVDP-HB9-RFP-Puro的慢病毒载体来自Addgene,质粒编号为:89762,由Stelios Andreadis和Pedro Lei教授实验室开发和共享[15-16]。HB9-RFP来自Addgene,质粒编号:37081[17]。pLVDP-HB9-RFP-Puro质粒包含慢病毒包装识别序列,HB9启动子介导的RFP基因和PGK启动子介导的嘌呤霉素(Puromycin,Puro) 抗性基因等,通过基因工程技术合成得到。参照Lenti-X Packaging Single Shots Kit使用手册制备慢病毒LV-HB9-RFP-Puro。
1.3 慢病毒感染和hNSCs-HB9-RFP-Puro细胞筛选将hiPSCs分化得到的hNSC接种于Matrigel包被的24孔板,每孔4×104个。第2天,在iPSCs生长至约30%融合度时,向神经干细胞扩增培养基中加入200 μL LV-HB9-RFP-Puro病毒溶液和polybrene (6 μg/mL)。感染过夜,更换新鲜的神经干细胞扩增培养基,继续培养至细胞融合度为50%。此时,加入Puro (1 μg/mL) 筛选阳性细胞,直至没有细胞死亡为止,所得到的细胞全部为感染上LV-HB9-RFP-Puro的hNSCs,即阳性细胞株hNSCs-HB9-RFP-Puro。
1.4 hNSCs的免疫荧光染色将hNSCs接种在放入24孔细胞培养板中的细胞爬片上,培养至融合度达到约60%后,弃去培养液,磷酸缓冲盐溶液(Phosphate buffered saline,PBS) 洗2次;加4%多聚甲醛溶液,室温固定15 min;而后PBS洗3次,每次10 min。加通透封闭液(0.2% Triton X-100、3%牛血清白蛋白(Bovine serum albumin,BSA) 的PBS),室温通透和封闭1 h,去除通透封闭液,按照1︰400稀释一抗Rabbit anti-Sox2 antibody (Cell Signaling Technology,货号:2748S) 和Mouse anti-Pax6 antibody (ThermoFisher Scientific,货号:MA1109),4 ℃孵育过夜。弃去一抗溶液,PBS洗3次,每次10 min。根据一抗来源,按照1︰2 000稀释荧光二抗Anti-rabbit IgG (H+L) 488,Cell Signaling Technology,货号:4412和Anti-mouse IgG (H+L) 555 (Cell Signaling Technology,货号:4409),室温避光孵育1 h。弃去二抗溶液,用PBS洗3次,每次10 min。滴加2 µg/mL 4′, 6-二脒基-2-苯基吲哚(4′, 6-diamidino-2-phenylindole,DAPI) 溶液,室温孵育3 min。用PBS洗2次,每次5 min。载玻片上滴90%的甘油,将细胞爬片倒扣于甘油上,封片完成后在荧光显微镜下进行观察和拍照记录。
1.5 成熟的MNs的免疫组织化学显色将hNSCs-HB9-RFP-Puro接种在放入24孔细胞培养板中的细胞爬片上,生长至50%融合状态后,感染慢病毒LV-Ngn2-Sox11-GFP和LV-Isl1- Lhx3-Hygro;次日,更换运动神经元成熟培养基培养分化至所需时间后,PBS洗2次,之后加4%多聚甲醛,室温固定15 min;而后PBS洗3次,每次10 min。加通透封闭液(0.2% Triton X-100,3% BSA的PBS),室温通透和封闭1 h,去除通透封闭液,分别孵育一抗羊抗胆碱乙酰转移酶抗体Goat anti- choline acetyltransferase antibody (Merck Millipore,货号:AB144P,稀释比例为1︰200) 或小鼠抗β-微管蛋白抗体Mouse anti-β-tubulin antibody (Merck Millipore,货号:T8328,稀释比例为1︰1 000),4 ℃孵育过夜。弃去一抗溶液,PBS洗3次,每次10 min。孵育对应的二抗Rabbit anti-goat IgG antibody (HRP conjugate,Merck Millipore,货号:AP106-P,稀释比例为1︰1 000) 或Goat anti- mouse IgG (HRP conjugate,Merck Millipore,货号:DC02L,稀释比例为1︰1 000),室温孵育1 h。弃去二抗溶液,用PBS洗3次,每次10 min。滴加适量的3-二氨基联苯胺(3-diaminobenzidine,DAB) 显色液,铺满细胞爬片,置于平式摇荡仪上,室温避光孵育2–5 min,直至棕褐色沉淀出现后,用去离子水清洗3次,在空气中晾干。在显微镜下进行观察和拍照记录。
2 结果与分析 2.1 神经干细胞株HNSCS-HB9-RFP-PURO的建立按照图 1的实验流程,将hiPSCs在神经干细胞分化培养基中培养7 d,得到hNSCs。hNSCs感染慢病毒LV-HB9-RFP-Puro过夜,而后使用Puro进行筛选,得到具有Puro抗性的阳性神经干细胞,命名为hNSCs-HB9-RFP-Puro。对hNSCs进行免疫荧光染色,所得的hNSCs大量表达神经干细胞相关标志物SOX2 (SRY-box transcription factor 2) 和PAX6 (Paired box gene 6),二者重合性好,证明所得的hNSCs纯度较高[18]。
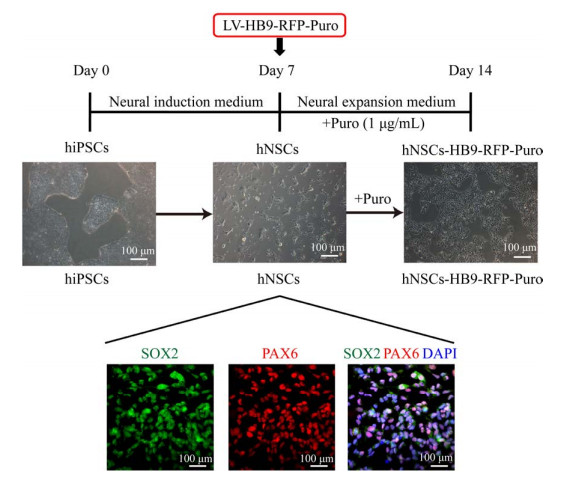
|
| 图 1 神经干细胞株hNSCs-HB9-RFP-Puro建立的实验流程图 Fig. 1 Flow chart of establishing the neural stem cell line hNSCs-HB9-RFP-Puro. |
| |
Ngn2、Sox11、Isl1和Lhx3是MNs定向分化的关键调控因子,大量研究通过转入这4个基因,可以在体外诱导分化获得高纯度的MNs[19]。因此,将阳性的神经干细胞hNSCs-HB9- RFP-Puro感染慢病毒LV-Ngn2-Sox11-GFP和LV-Isl1-Lhx3- Hygro进行定向分化,并在MNs培养基中进一步分化5 d后,得到MNs[14],继续培养至第15天,获得成熟的MNs (图 2A)。在荧光显微镜下观察,分化得到的细胞展现出神经元样结构,并在MNs特异性启动子HB9的作用下,成功介导了RFP表达。同时,感染上LV-Ngn2-Sox11-GFP的细胞表达绿色荧光蛋白(Green fluorescent protein,GFP),二者具有很好的共定位(图 2B),说明过表达Ngn2、Sox11、Isl1和Lhx3可促进其分化。对诱导分化得到的MNs进行免疫组化染色(图 2C),发现它们表达了神经元通用标志物β微管蛋白(β-tubulin) 和成熟神经元标志物乙酰胆碱转移酶(Choline acetyltransferase,ChAT),证明分化得到的细胞为成熟的MNs。在对照组中,未经慢病毒LV-Ngn2-Sox11-GFP和LV-Isl1-Lhx3-Hygro感染的hNSCs在第5天和第15天均无法分化得到MNs。

|
| 图 2 运动神经元的定向分化及特异性荧光RFP的表达 Fig. 2 Directed differentiation of MNs and specific expression of RFP. (A) Positive cell line hNSCs-HB9-RFP-Puro differentiated to MNs after infected with lentiviruses LV-Ngn2-Sox11-GFP and LV-Isl1-Lhx3-Hygro. MNs were cultured in motor neuron medium, mature MNs were obtained after further differentiation and maturation. (B) MNs expressed RFP from HB9 promoter control and GFP from lentivirus LV-Ngn2-Sox11-GFP on day 5 after lentivirus infection. RFP and GFP had good co-localization. Control group without lentivirus infection could not differentiate to MNs. (C) Immunohistochemistry staining of mature MNs. β-tubulin, the neuronal universal marker, and ChAT, the MNs maturation marker, were expressed in mature MNs on day 15 after lentivirus infection. Control group showed weak β-tubulin activity and no ChAT activity. Three replicates were performed for all experiments. Scale bars, B: 200 μm, C: 100 μm. |
| |
MNs的病变是运动神经元疾病发病的根本原因,有关MNs的保护和替代治疗的研究日益受到关注[20]。虽然相关的动物疾病模型已经得到有效的建立,但这些动物模型尚未能完全模拟相关的人类疾病特征[21]。值得注意的是,通过转基因技术将特异性转录因子转入病人来源的hiPSCs和成纤维细胞,可以分化为几乎所有类型的细胞,被认为是一种潜力强大的体外疾病模型[22]。其中,Goto等通过仙台病毒转入Ngn2-Isl1-Lhx3的3个转录因子,成功将健康志愿者和ALS病人来源的hiPSCs定向分化为MNs[23]。此外,Liu等发现通过慢病毒导入外源性的Ngn2、Sox11、Isl1和Lhx3至健康志愿者和ALS病人来源的原代成纤维细胞后,在条件性神经元诱导培养基中,也可以分化获得MNs[14]。HB9是运动神经元特异性表达基因,被广泛用于干细胞来源的运动神经元的鉴定和表征。无论是小分子或是转基因诱导得到的神经元,均需要考察HB9的表达水平。为此,Haidet-Philips和Wichterle等通过转基因技术,构建了HB9:GFP小鼠,而后从该小鼠中分离出携带HB9:GFP胚胎干细胞(Murine embryonic stem cells,mESCs),该细胞系mESCs-HB9-GFP在体外诱导运动神经元定向分化过程中,特异性启动子HB9介导了GFP表达,从而可以实时观察小鼠MNs的分化[24-25]。
为考察人MNs的分化过程,本研究通过慢病毒载体LV-HB9-RFP-Puro,将特异性启动子HB9控制下的RFP稳定导入hNSCs基因组中,经Puro筛选,得到阳性神经干细胞株hNSCs-HB9- RFP-Puro。在感染定向分化慢病毒LV-Ngn2- Sox11-GFP和LV-Isl1-Lhx3-Hygro后,继续培养为成熟的MNs。所得成熟的MNs同时表达由HB9介导的RFP和LV-Ngn2-Sox11-GFP携带的GFP,且二者具有很好的共定位。同时,对成熟的MNs进行免疫组化染色,其表达了神经元通用标志物β-tubulin和运动神经元成熟标志物ChAT,证明该细胞为成熟的MNs。
综上所述,本研究成功建立了运动神经元特异性荧光报告系统,可通过直接观察RFP和GFP的表达水平,判断阳性神经干细胞hNSCs-HB9- RFP-Puro已经定向分化为MNs。该系统可实时观察MNs的定向分化、减少鉴定时间、缩短实验周期,为运动神经元疾病的治疗和药物筛选提供更快速的细胞模型方案选择。
| [1] |
Schorling DC, Pechmann A, Kirschner J. Advances in treatment of spinal muscular atrophy — new phenotypes, new challenges, new implications for care. J Neuromuscul Dis, 2020, 7(1): 1-13. DOI:10.3233/JND-190424
|
| [2] |
Tsokolas G, Tsaousis KT, Diakonis VF, et al. Optical coherence tomography angiography in neurodegenerative diseases: a review. Eye Brain, 2020, 12: 73-87. DOI:10.2147/EB.S193026
|
| [3] |
Kanning KC, Kaplan A, Henderson CE. Motor neuron diversity in development and disease. Annu Rev Neurosci, 2010, 33: 409-440. DOI:10.1146/annurev.neuro.051508.135722
|
| [4] |
Hudish LI, Bubak A, Triolo TM, et al. Modeling hypoxia-induced neuropathies using a fast and scalable human motor neuron differentiation system. Stem Cell Rep, 2020, 14(6): 1033-1043. DOI:10.1016/j.stemcr.2020.04.003
|
| [5] |
董思其, 徐和, 景乃禾, 等. 人类多能干细胞向脊髓前角运动神经元定向分化方法研究进展: 从发育生物学到临床转化. 中国细胞生物学学报, 2020, 42(8): 1395-1405. Dong SQ, Xu H, Jing NH, et al. Human pluripotent stem cell-derived spinal cord motor neuron: from developmental biology to therapeutic applications. Chin J Cell Biol, 2020, 42(8): 1395-1405 (in Chinese). DOI:10.11844/cjcb.2020.08.0012(inChinese) |
| [6] |
Payne NL, Sylvain A, O'Brien C, et al. Application of human induced pluripotent stem cells for modeling and treating neurodegenerative diseases. New Biotechnol, 2015, 32(1): 212-228. DOI:10.1016/j.nbt.2014.05.001
|
| [7] |
Taylor JP, Brown RH Jr, Cleveland DW. Decoding ALS: from genes to mechanism. Nature, 2016, 539(7628): 197-206. DOI:10.1038/nature20413
|
| [8] |
Shimojo D, Onodera K, Doi-Torii Y, et al. Rapid, efficient, and simple motor neuron differentiation from human pluripotent stem cells. Mol Brain, 2015, 8: 79. DOI:10.1186/s13041-015-0172-4
|
| [9] |
Karumbayaram S, Novitch BG, Patterson M, et al. Directed differentiation of human-induced pluripotent stem cells generates active motor neurons. Stem Cells, 2009, 27(4): 806-811. DOI:10.1002/stem.31
|
| [10] |
Patani R. Generating diverse spinal motor neuron subtypes from human pluripotent stem cells. Stem Cells Int, 2016, 2016: 1036974. DOI:10.1155/2016/1036974
|
| [11] |
Li XJ, Hu BY, Jones SA, et al. Directed differentiation of ventral spinal progenitors and motor neurons from human embryonic stem cells by small molecules. Stem Cells, 2008, 26(4): 886-893. DOI:10.1634/stemcells.2007-0620
|
| [12] |
Amoroso MW, Croft GF, Williams DJ, et al. Accelerated high-yield generation of limb-innervating motor neurons from human stem cells. J Neurosci, 2013, 33(2): 574-586. DOI:10.1523/JNEUROSCI.0906-12.2013
|
| [13] |
Faravelli I, Bucchia M, Rinchetti P, et al. Motor neuron derivation from human embryonic and induced pluripotent stem cells: experimental approaches and clinical perspectives. Stem Cell Res Ther, 2014, 5(4): 87. DOI:10.1186/scrt476
|
| [14] |
Liu ML, Zang T, Zhang CL. Direct lineage reprogramming reveals disease-specific phenotypes of motor neurons from human ALS patients. Cell Rep, 2016, 14(1): 115-128. DOI:10.1016/j.celrep.2015.12.018
|
| [15] |
Moharil J, Lei P, Tian J, et al. Lentivirus live cell array for quantitative assessment of gene and pathway activation during myogenic differentiation of mesenchymal stem cells. PLoS ONE, 2015, 10(10): e0141365. DOI:10.1371/journal.pone.0141365
|
| [16] |
Mistriotis P, Bajpai VK, Wang XY, et al. NANOG reverses the myogenic differentiation potential of senescent stem cells by restoring ACTIN filamentous organization and SRF-dependent gene expression. Stem Cells, 2017, 35(1): 207-221. DOI:10.1002/stem.2452
|
| [17] |
Marchetto MC, Muotri AR, Mu YL, et al. Non-cell-autonomous effect of human SOD1G37R astrocytes on motor neurons derived from human embryonic stem cells. Cell Stem Cell, 2008, 3(6): 649-657. DOI:10.1016/j.stem.2008.10.001
|
| [18] |
Ladran I, Tran N, Topol A, et al. Neural stem and progenitor cells in health and disease. WIREs Syst Biol Med, 2013, 5(6): 701-715. DOI:10.1002/wsbm.1239
|
| [19] |
Wichterle H, Lieberam I, Porter JA, et al. Directed differentiation of embryonic stem cells into motor neurons. Cell, 2002, 110(3): 385-397. DOI:10.1016/s0092-8674(02)00835-8
|
| [20] |
Tiryaki E, Horak HA. ALS and other motor neuron diseases. Continuum (Minneap Minn), 2014, 20: 1185-1207. DOI:10.1212/01.CON.0000455886.14298.a4
|
| [21] |
Arbab M, Baars S, Geijsen N. Modeling motor neuron disease: the matter of time. Trends Neurosci, 2014, 37(11): 642-652. DOI:10.1016/j.tins.2014.07.008
|
| [22] |
Sharma A, Sane H, Gokulchandran Net al. Stem cell therapy in motor neuron disease//Sibat H F. Novel aspects on motor neuron disease. London: Intech Open, 2019: 1-24. DOI: 10.5772/intechopen.87116.
|
| [23] |
Goto K, Imamura K, Komatsu K, et al. Simple derivation of spinal motor neurons from ESCs/iPSCs using sendai virus vectors. Mol Ther Methods Clin Dev, 2017, 4: 115-125. DOI:10.1016/j.omtm.2016.12.007
|
| [24] |
Haidet-Phillips AM, Hester ME, Miranda CJ, et al. Astrocytes from familial and sporadic ALS patients are toxic to motor neurons. Nat Biotechnol, 2011, 29(9): 824-828. DOI:10.1038/nbt.1957
|
| [25] |
Wichterle H, Lieberam I, Porte JA, et al. Directed differentiation of embryonic stem cells into motor neurons. Cell, 2002, 110(3): 385-397.
|
 2021, Vol. 37
2021, Vol. 37




