| [1] |
Newman DJ, Cragg GM. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J Nat Prod, 2020, 83(3): 770-803. DOI:10.1021/acs.jnatprod.9b01285
|
|
| [2] |
Catteau L, Zhu L, Van Bambeke F, et al. Natural and hemi-synthetic pentacyclic triterpenes as antimicrobials and resistance modifying agents against Staphylococcus aureus: a review. Phytochem Rev, 2018, 17(5): 1129-1163. DOI:10.1007/s11101-018-9564-2
|
|
| [3] | |
|
| [4] |
Niu G, Tan H. Nucleoside antibiotics: biosynthesis, regulation, and biotechnology. Trends Microbiol, 2015, 23(2): 110-119. DOI:10.1016/j.tim.2014.10.007
|
|
| [5] | |
|
| [6] |
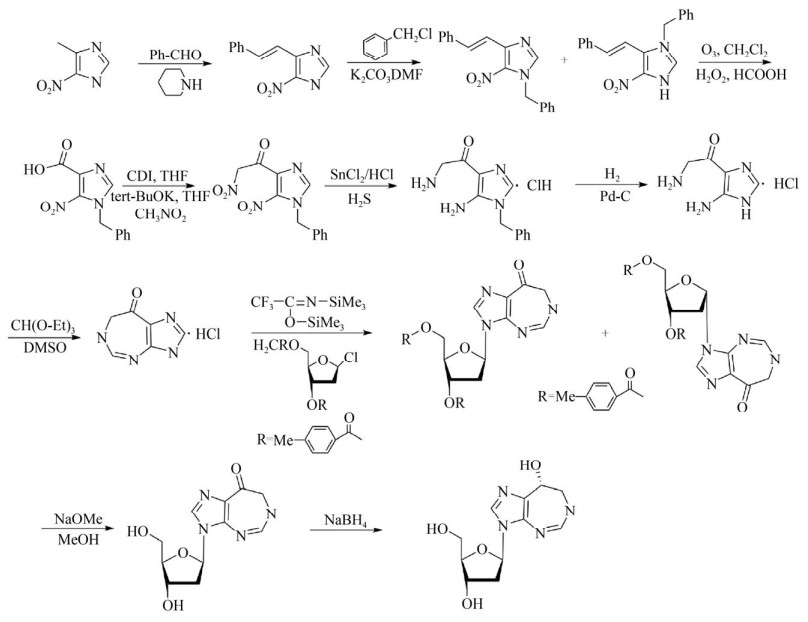
Baker DC, Putt SR. A total synthesis of pentostatin, the potent inhibitor of adenosine deaminase. J Am Chem Soc, 1979, 101(20): 6127-6128. DOI:10.1021/ja00514a048
|
|
| [7] |
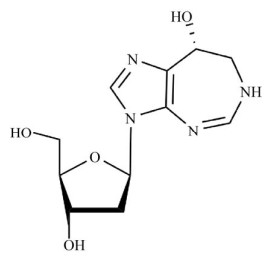
Woo PWK, Dion HW, Lange SM, et al. A novel adenosine and Ara—a deaminase inhibitor, (R)-3-(2-deoxy-β-D-erythro-pentofuranosyl)-3, 6, 7, 8-tetrahydroimidazo[4, 5-d][1, 3]diazepin-8-ol. J Heterocycl Chem, 1974, 11(4): 641-643. DOI:10.1002/jhet.5570110438
|
|
| [8] |
Agarwal RP, Spector T, Parks RE Jr. Tight-binding inhibitors—Ⅳ. Inhibition of adenosine deaminases by various inhibitors. Biochem Pharmacol, 1977, 26(5): 359-367. DOI:10.1016/0006-2952(77)90192-7
|
|
| [9] |
徐积恩. 抗肿瘤药——喷司他丁. 国外医药合成药生化药制剂分册, 1994, 15(3): 175-176.
Xu JE. Pentostatin, an antitumor drugs. World pharm, 1994, 15(3): 175-176 (in Chinese).
|
|
| [10] |
French JC, Edmunds CR, Mcdonnell P, et al. Process for purifying pentostatin. Biotechnol Adv, 1996, 14(4): 609.
|
|
| [11] |
王鹏. 抗肿瘤药——喷司他丁. 中国药学杂志, 1999, 34(10): 3-5.
Wang P. Antitumor drugs—pentostatin. Chin Pharmacol J, 1999, 34(10): 3-5 (in Chinese).
|
|
| [12] | |
|
| [13] |
Gao YJ, Xu GD, Wu P, et al. Biosynthesis of 2'-chloropentostatin and 2'-amino-2'-deoxyadenosine highlights a single gene cluster responsible for two independent pathways in Actinomadura sp. strain ATCC 39365. Appl Environ Microbiol, 2017, 83(10): e00078-17.
|
|
| [14] |
Cha S, Agarwal RP, Parks RE. Tight-binding inhibitors-Ⅱ. non-steady state nature of inhibition of milk xanthine oxidase by allopurinol and alloxanthine and of human erythrocytic adenosine deaminase by coformycin. Biochem Pharmacol, 1975, 24(23): 2187-2197. DOI:10.1016/0006-2952(75)90051-9
|
|
| [15] |
Schramm VL, Baker DC. Spontaneous epimerization of (S)-deoxycoformycin and interaction of (R)-deoxycoformycin, (S)-deoxycoformycin, and 8-ketodeoxycoformycin with adenosine deaminase. Biochemistry, 1985, 24(3): 641-646. DOI:10.1021/bi00324a016
|
|
| [16] |
Caron N, Lee SH, Kimball AP. Effects of 2'-deoxycoformycin, 9-beta-D-arabinofuranosyladenine 5'-phosphate, and 1-beta-D-arabinofuranosylcytosine triple combination therapy on intracerebral leukemia 1210. Cancer Res, 1977, 37(9): 3274-3279.
|
|
| [17] |
Alfayez M, Thakral B, Jain P, et al. First report of clinical response to venetoclax combination with pentostatin in T-cell-prolymphocytic leukemia (T-PLL). Leuk Lymphoma, 2020, 61(2): 445-449. DOI:10.1080/10428194.2019.1660967
|
|
| [18] |
Lamanna N, Kalaycio M, Maslak P, et al. Pentostatin and cyclophosphamide with or without rituximab has significant activity in patients with previously treated chronic lymphocytic leukemia and other low grade lymphoid neoplasms. Blood, 2011, 104(11): 3484.
|
|
| [19] |
Chan E, Putt SR, Showalter HDH, et al. Total synthesis of (8R)-3-(2-deoxy-β-D-erythro-pentofuranosyl)-3, 6, 7, 8-tetrahydroimidazo[4, 5-d][1, 3] diazepin-8-ol(pentostatin), the potent inhibitor of adenosine deaminase. J Org Chem, 1982, 47(18): 3457-3464. DOI:10.1021/jo00139a015
|
|
| [20] |
Thien VT, Henry R. Chirospecific synthesis of the tetrahydroimidazodiazepinol aglycon of pentostatin and its analogs. J Org Chem, 1993, 58(22): 6090-6096. DOI:10.1021/jo00074a041
|
|
| [21] |
Chen BC, Chao ST, Sundeen JE, et al. Vicarious nucleophilic substitution of 1-benzyl-5-nitroimidazole, application to the synthesis of 6, 7-dihydroimidazo[4, 5-d][1, 3] diazepin-8(3H)-one. Tetrahedron Lett, 2002, 43(9): 1595-1596. DOI:10.1016/S0040-4039(02)00053-9
|
|
| [22] |
Nadji S, James S, Umashanker S. Process for the production of pentostatin aglycone and pentostatin: US, 7393954. 2002-12-12.
|
|
| [23] |
Phiasivongsa P, Redkar S. Azacytosine analogs and derivatives: US, 20060205687. 2006-9-14.
|
|
| [24] |
高丽萍. 喷司他汀的合成与质量控制研究[D]. 杭州: 浙江大学, 2010.
Gao LP. Studies of pentostatin synthesis and quality control[D]. Hangzhou: Zhejiang University, 2010 (in Chinese).
|
|
| [25] |
Shiraishi T, Kuzuyama T. Recent advances in the biosynthesis of nucleoside antibiotics. J Antibiot (Tokyo), 2019, 72(12): 913-923. DOI:10.1038/s41429-019-0236-2
|
|
| [26] |
Lacalle RA, Tercero JA, Jiménez A. Cloning of the complete biosynthetic gene cluster for an aminonucleoside antibiotic, puromycin, and its regulated expression in heterologous hosts. EMBO J, 1992, 11(2): 785-792. DOI:10.1002/j.1460-2075.1992.tb05112.x
|
|
| [27] |
Wu P, Wan D, Xu G, et al. An unusual protector-protégé strategy for the biosynthesis of purine nucleoside antibiotics. Cell Chem Biol, 2017, 24(2): 171-181. DOI:10.1016/j.chembiol.2016.12.012
|
|
| [28] |
Xia Y, Luo F, Shang Y, et al. Fungal cordycepin biosynthesis is coupled with the production of the safeguard molecule pentostatin. Cell Chem Biol, 2017, 24(12): 1479-1489.e4. DOI:10.1016/j.chembiol.2017.09.001
|
|
| [29] |
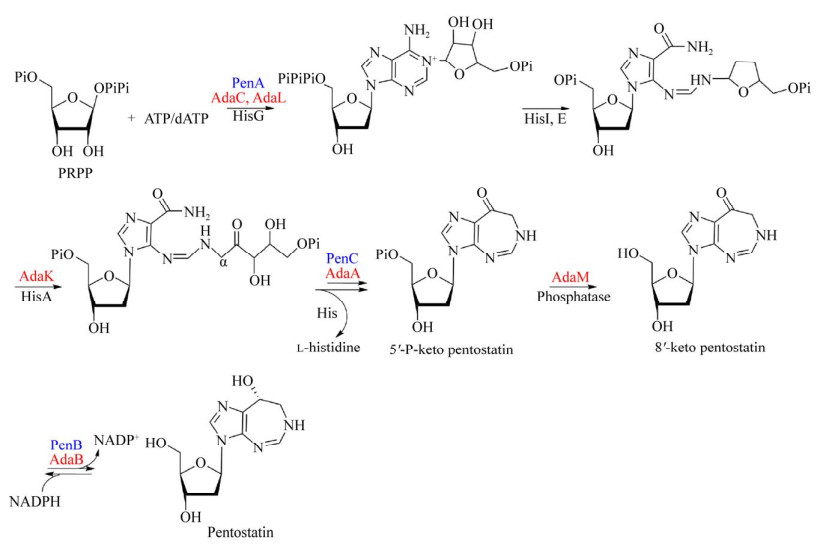
Hanvey JC, Hawkins ES, Tunac JB, et al. Biosynthesis of 2'-deoxycoformycin: evidence for ring expansion of the adenine moiety of adenosine to a tetrahydroimidazo[4, 5-d][1, 3]diazepine system. Biochemistry, 1987, 26(18): 5636-5641. DOI:10.1021/bi00392a008
|
|
| [30] | |
|
| [31] |
Hanvey JC, Hawkins ES, Baker DC, et al. 8-ketodeoxycoformycin and 8-ketocoformycin as intermediates in the biosynthesis of 2'-deoxycoformycin and coformycin. Biochemistry, 1988, 27(15): 5790-5795. DOI:10.1021/bi00415a059
|
|
| [32] |
Ren DA, Ruszczycky MW, Ko Y, et al. Characterization of the coformycin biosynthetic gene cluster in Streptomyces kaniharaensis. Proc Natl Acad Sci USA, 2020, 117(19): 10265-10270. DOI:10.1073/pnas.2000111117
|
|
| [33] | |
|
| [34] | |
|
| [35] |
Zhao X, Zhang G, Li C, et al. Cordycepin and pentostatin biosynthesis gene identified through transcriptome and proteomics analysis of Cordyceps kyushuensis Kob. Microbiol Res, 2019, 218: 12-21. DOI:10.1016/j.micres.2018.09.005
|
|
| [36] |
李晓辉. 喷司他丁生物合成的发酵优化[D]. 天津: 天津科技大学, 2014.
Li XH. Optimization of fermentation for pentostatin biosynthesis[D]. Tianjin: Tianjin University of Science & Technology, 2014 (in Chinese).
|
|
| [37] |
Woo PW, Dion HW, Ryder A, et al. (R)-3-(2-deoxy-β-D-erythro-pentofuranosyl)-3, 6, 7, 8-tetrahydroimidazo[4, ][1, 3]diazepin-8-ol: US, 3923785. 1974-04-22.
|
|
| [38] |
Showalter HD, Bunge RH, French JC, et al. Improved production of pentostatin and identification of fermentation cometabolites. J Antibiot (Tokyo), 1992, 45(12): 1914-1918. DOI:10.7164/antibiotics.45.1914
|
|
| [39] |
朱健, 陈晓霞, 谢祥茂, 等. 一种链霉菌及其应用: 中国, 101792722A. 2010-08-04.
Zhu J, Chen XX, Xie XM, et al. Streptomycin and application thereof: CN, 101792722A. 2010-08-04 (in Chinese).
|
|
| [40] |
陈少欣, 王岩, 杨鹏. 抗生链霉菌发酵生产喷司他丁的发酵培养基以及发酵方法: 中国, 102234673A. 2011-11-09.
Chen SX, Wang Y, Yang P. Fermentation culture medium and fermentation method for producing pentostatin by fermentation of Streptomyces antibioticus: CN, 102234673A. 2011-11-09 (in Chinese).
|
|
| [41] |
邹昕. 喷司他丁发酵条件的优化. 生物技术进展, 2013, 3(5): 371-376. Zou X. Optimization of pentostatin fermentation conditions. Curr Biotechnol, 2013, 3(5): 371-376 (in Chinese). DOI:10.3969/j.issn.2095-2341.2013.05.12
|
|
| [42] |
郑孝贤. 抗生素链霉菌合成喷司他汀发酵工艺的关键原料研究. 中国抗生素杂志, 2019, 44(12): 1371-1376. Zhen XX. Studies on the critical materials in the fermentation process of pentastatin produced by Streptomyce antibioticus. Chin J Antibio, 2019, 44(12): 1371-1376 (in Chinese). DOI:10.3969/j.issn.1001-8689.2019.12.011
|
|
| [43] |
Tunac JB, Underhill M. 2'-chloropentostatin: discovery, fermentation and biological activity. J Antibiot, 1985, 38(10): 1344-1349. DOI:10.7164/antibiotics.38.1344
|
|
| [44] |
Schaumberg JP, Hokanson GC, French JC, et al. 2'-chloropentostatin, a new inhibitor of adenosine deaminase. J Org Chem, 1985, 50(10): 1651-1656. DOI:10.1021/jo00210a018
|
|
| [45] |
李晓辉, 姜文侠, 张笑然, 等. 变温及控制pH提高喷司他丁发酵产量. 药物生物技术, 2014, 21(5): 433-436. Li XH, Jiang WX, Zhang XR, et al. Effects of temperature and pH on fermentation of pentostatin production. Pharm Biotechnol, 2014, 21(5): 433-436 (in Chinese).
|
|
| [46] |
周秋玲. 复合育种策略选育喷司他丁高产菌株[D]. 天津: 天津商业大学, 2019.
Zhou QL. Breeding the strain with high-yield of pentostatin by combined mutation strategy[D]. Tianjin: Tianjin University of Commerce, 2019 (in Chinese).
|
|
| [47] | |
|
| [48] |
Ko YS, Kim JW, Lee JA, et al. Tools and strategies of systems metabolic engineering for the development of microbial cell factories for chemical production. Chem Soc Rev, 2020, 49(14): 4615-4636. DOI:10.1039/D0CS00155D
|
|
| [49] |
Gong R, Yu L, Qin Y, et al. Harnessing synthetic biology-based strategies for engineered biosynthesis of nucleoside natural products in actinobacteria. Biotechnol Adv, 2021, 46: 107673. DOI:10.1016/j.biotechadv.2020.107673
|
|
| [50] |
肖丽萍, 邓子新, 刘天罡. 链霉菌底盘细胞的开发现状及其应用. 微生物学报, 2016, 56(3): 441-453, 330. Xiao LP, Deng ZX, Liu TG. Progress in developing and applying Streptomyces chassis. Acta Microbiol Sin, 2016, 56(3): 441-453, 330 (in Chinese).
|
|
| [51] |
Ahmed Y, Rebets Y, Estévez MR, et al. Engineering of Streptomyces lividans for heterologous expression of secondary metabolite gene clusters. Microb Cell Fact, 2020, 19(1): 5. DOI:10.1186/s12934-020-1277-8
|
|
| [52] |
Lee SY, Kim HU, Chae TU, et al. A comprehensive metabolic map for production of bio-based chemicals. Nat Catal, 2019, 2(1): 18-33. DOI:10.1038/s41929-018-0212-4
|
|
| [53] |
Bu X, Lin JY, Cheng J, et al. Engineering endogenous ABC transporter with improving ATP supply and membrane flexibility enhances the secretion of β-carotene in Saccharomyces cerevisiae. Biotechnol Biofuels, 2020, 13: 168. DOI:10.1186/s13068-020-01809-6
|
|
| [54] |
Yuan SF, Yi X, Johnston TG, et al. De novo resveratrol production through modular engineering of an Escherichia coli-Saccharomyces cerevisiae co-culture. Microb Cell Fact, 2020, 19(1): 143. DOI:10.1186/s12934-020-01401-5
|
|
| [55] |
于政, 申晓林, 孙新晓, 等. 动态调控策略在代谢工程中的应用研究进展. 合成生物学, 2020, 1(4): 440-453. Yu Z, Shen XL, Sun XX, et al. Application of dynamic regulation strategies in metabolic engineering. Synth Biol J, 2020, 1(4): 440-453 (in Chinese).
|
|
 2021, Vol. 37
2021, Vol. 37