中国科学院微生物研究所、中国微生物学会主办
文章信息
- 高琳惠, 蔡鹏, 周雍进
- Gao Linhui, Cai Peng, Zhou Yongjin J.
- 甲醇酵母代谢工程研究进展
- Advances in metabolic engineering of methylotrophic yeasts
- 生物工程学报, 2021, 37(3): 966-979
- Chinese Journal of Biotechnology, 2021, 37(3): 966-979
- 10.13345/j.cjb.200645
-
文章历史
- Received: October 9, 2020
- Accepted: January 18, 2021
- Published: January 25, 2021
2. 大连市能源生物技术重点实验室,辽宁 大连 116023
2. Dalian Key Laboratory of Energy Biotechnology, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian 116023, Liaoning, China
甲醇是一种十分重要的基础化工原料和清洁能源,可以由煤炭经气化、变换、净化、合成和精馏等步骤大规模制备[1]。我国煤炭储量丰富,因此甲醇原料价格低廉且来源充足。另外,甲醇可由CO2加氢大量制备,因此其被认为是“液态阳光”战略的理想能量储存载体[2]。我国甲醇市场规模位居世界第一,产能已超过8 000万t。甲醇深度加工既有经济价值,也有利于CO2循环利用。因此,发展甲醇化学转化技术[3-4],对煤炭资源的高效清洁利用、发展能源化工新路线以及升级我国能源结构等方面具有重要战略意义。
虽然化学转化技术能将甲醇转化为多种化学品比如短链烯烃,但是化学方法难以合成结构复杂的化合物。而生物转化路线选择性高,有望合成含氧或复杂官能团的精细化学品。另外,甲醇具有很高的还原度,能够为产物生物合成提供更多驱动力,是一种优质生物炼制原料[5]。模式微生物如大肠杆菌、酿酒酵母和谷氨酸棒状杆菌,由于其清晰的遗传背景和成熟的遗传操作工具,受到广泛关注。然而,缺乏高效甲醇代谢途径限制了其作为甲醇生物转化的细胞工厂底盘。近年来,研究者们尝试利用基因工程手段构建甲醇同化途径,在酿酒酵母、大肠杆菌和谷氨酸棒状杆菌中实现了甲醇至中心代谢的转化[6-8],但这些工程菌株仍无法以甲醇为唯一碳源生长。最近,Chen等通过代谢工程与实验室适应性进化技术相结合,实现了大肠杆菌以甲醇为唯一碳源进行生长,但倍增时间长达8.5 h,最大光密度(OD600) 仅为2左右[9],限制了甲醇代谢速率和产物合成效率。此外,自然界中存在一类甲醇酵母(Methylotrophic yeasts),能够利用甲醇作为唯一碳源和能量来源,有望作为甲醇生物转化的另一类理想宿主,甲醇酵母主要分布在有限的3个种属,如毕赤酵母属Komagataella、汉逊酵母属Ogataea、假丝酵母属Candida[10]。
文中首先介绍了甲醇酵母作为宿主菌株的特点和优势,然后以3种最具代表性的甲醇酵母,巴斯德毕赤酵母Komagataella phaffii、多形汉逊酵母Ogataea polymorpha和博伊丁假丝酵母Candida boidinii为例,综述了近年来改造甲醇酵母生物合成蛋白质和化学品的研究进展,并探讨甲醇酵母用于绿色生物制造的一些挑战及潜在对策。
1 甲醇酵母特点与优势与模式微生物酿酒酵母相比,甲醇酵母的遗传分子机制和代谢工程等研究都相对滞后。但由于其独特优势,越来越成为研究热点。其主要特点有:1) 能够在廉价的基础盐培养基中生长;2) 属于Crabtree-negative菌株,发酵过程中不易积累乙醇等副产物,可以实现高密度生长,发酵过程不受噬菌体威胁;3) 具有完整的细胞器结构,尤其是过氧化物酶体,可以作为细胞区室化改造靶点;4) 具有完备的蛋白折叠、修饰以及分泌功能,并且不会产生包涵体。其中,巴斯德毕赤酵母K. phaffii,多形汉逊酵母O. polymorpha和博伊丁假丝酵母C. boidinii是各自种属中最具代表性的甲醇酵母,具有相似的甲醇代谢途径。当细胞响应甲醇诱导时,甲醇同化途径中关键酶醇氧化酶(Alcohol oxidases,Aox1) 与二羟丙酮合酶(Dihydroxyacetone synthase,Das1/Das2) 具有很高的表达水平,其启动子受到甲醇诱导并且具有严格的调控模式,非常适合于表达外源基因[11]。除上述共有优点外,3种甲醇酵母还具有各自的特点,如K. phaffii具有较宽的pH适应范围;O. polymorpha可以耐受50 ℃的高温;O. polymorpha和C. boidinii具有一定的木糖和果胶代谢能力[12],在木质纤维水解液利用方面具有一定潜力。
在进化关系上,O. polymorpha和C. boidinii亲缘关系相对较近(图 1)。目前,两株常用K. phaffii菌株CBS7435和GS115已经完成了全基因组测序及后续拼接和注释[13-15],基因组与转录组相关研究为其代谢工程改造提供了可靠数据[16],因此K. phaffii成为应用最为广泛的甲醇酵母。然而O. polymorpha和C. boidinii基因组尚未完全解析,至今NCBI公布的两组O. polymorpha基因组注释工作均处于拼接阶段,显示其基因组大小约8.97 Mb[17]。NCBI数据库中C. boidinii的测序解析工作共有12组报告,显示其基因组大小约为19 Mb,目前也均处于骨架拼接阶段[18-19]。值得一提的是,O. polymorpha被认为含有7条染色体[17],K. phaffii含有4条染色体[13-15],基因组功能的注释将为甲醇酵母代谢工程提供更多清晰的信息。

|
| 图 1 三种甲醇酵母和酿酒酵母的进化树 Fig. 1 Phylogenetic tree of three methylotrophic yeasts and S. cerevisiae. The 18S rRNA sequences of K. phaffii, O. polymorpha, C. boidinii and S. cerevisiae were downloaded using NCBI (https://www.ncbi.nlm.nih.gov/). Multiple sequence alignments were performed using DNAMAN V6 software (LynnonBiosoft, Quebec, QC, Canada) and the phylogenetic tree was constructed using molecular evolutionary genetics analysis (MEGA 6.0) software based on the results of the homology comparison. |
| |
酿酒酵母作为一种模式酵母,由于在其生理学、遗传学和发酵技术方面积累了全面深入的知识,并且被公认为是安全的菌种(Generally recognized as safe,GRAS),因而在工业生产上使用广泛。近年来,甲醇酵母,尤其是K. phaffii在生产重组蛋白方面的性能显著优于酿酒酵母,成为应用最广的表达宿主,已有超过5 000种重组蛋白使用甲醇酵母进行生产[20]。
2.1.1 启动子改造提高蛋白表达甲醇酵母中甲醇代谢初始阶段位于细胞过氧化物酶体中,过氧化物酶体在甲醇诱导条件下会大量增殖,最多可以占据细胞内部空间的80%[21]。甲醇利用途径中的第一步酶醇氧化酶(Aox) 催化甲醇氧化生成甲醛和过氧化氢(H2O2),H2O2由过氧化氢酶(Catalase,CAT) 催化分解成水和氧气。甲醛则有两个去处:一是进入异化途径,经甲醛脱氢酶(Formaldehyde dehydrogenase,Fld) 和甲酸脱氢酶(Formate dehydrogenase,Fdh) 催化生成CO2并为细胞提供能量;另一部分甲醛则进入同化途径,在二羟丙酮合酶(Das) 的作用下,与木酮糖-5-磷酸反应生成二羟丙酮(DHA) 和三磷酸甘油醛(GAP),之后进入中心代谢[22]。
甲醇代谢相关基因受到甲醇强烈诱导,其启动子为蛋白质表达提供了高效元件(表 1)。例如,K. phaffii含有两个醇氧化酶Aox1和Aox2,两者氨基酸序列相似性达97%,但Aox2的表达量远低于Aox1。PAOX1是公认的强甲醇诱导型启动子,其表达强度受严格调控,在葡萄糖、乙醇或甘油存在条件下表达强度受到严格阻遏作用[23-24]。而在O. polymorpha和C. boidinii中均只有一个醇氧化酶,分别为OpMox和CbAod1,其启动子调控模式与K. phaffii十分相似,都受到葡萄糖或乙醇的强烈阻遏,但在甘油存在条件下去阻遏作用情况不尽相同。在O. polymorpha中,在甘油存在条件下,PMOX表达强度可以达到单一甲醇诱导条件下的80%[21, 25],而C. boidinii中,醇氧化酶(Alcohol oxidases,Aod) 基因启动子PAOD1在甘油存在条件下表达强度仅为单一甲醇诱导时的20%[21, 26]。与醇氧化酶一样,甲醛脱氢酶的启动子PFLD1也是强甲醇诱导型启动子,并且可以受甲胺诱导[27-29]。PFLD1调控也发生在转录水平,其表达强度同样受到葡萄糖阻遏,在去阻遏阶段会有少量表达[22]。有趣的是,在K. phaffii和C. boidinii中如果以甲胺为唯一氮源、葡萄糖为碳源进行培养时,PFLD1可以正常高表达[27, 29]。因此,PFLD1可以作为PAOX1一种替代选择用于蛋白质高效表达。甲酸脱氢酶启动子PFDH可以被甲醇或是甲酸盐高效诱导,其阻遏规律与PFLD1相近。在K. phaffii和C. boidinii中,二羟基丙酮合成酶基因启动子PDAS甲醇诱导表达强度均高于PAOX1[22, 30]。甲醇同化途径产生的H2O2对细胞具有毒性,需要过氧化氢酶不断分解来减小其对细胞损伤,因此PCAT的表达与甲醇代谢也有直接响应关系,此外PCAT还可被油酸诱导表达[22]。
| Proteins | Titer | Host strain | Promoter | Methanol concentration (%) | References |
| Keratinocyte growth factor-2 | 1 g/L | K. phaffii | PAOX1 | 0.5 | [38] |
| Plectasin | 0.75 g/L | K. phaffii | PAOX1 | 0.5 | [39] |
| Human serum albumin | 8.9 g/L | K. phaffii | PAOX1 | 0.5 | [31] |
| Hispidalin | 98.6 mg/L | K. phaffii | PAOX1 | 1.5 | [40] |
| Trypsin | 185.7 mg/L | K. phaffii | PAOX1 | 1.8 | [41] |
| Staphylokinase | 0.7 g/L | K. phaffii | PAOX1 | 3.0 | [42] |
| (HPV-16) L1-L2 proteins | 132.1 mg/L | O. polymorpha | PMOX | 1.0 | [43] |
| Streptavidin | 0.75 g/L | O. polymorpha | PFMD | 0.5 | [33] |
| Ferritin | 1.9 g/L | O. polymorpha | PFMD | 1.0 | [44] |
| Staphylokinase | 1.2 g/L | O. polymorpha | PFMD | 1.0 | [45] |
| Transglutaminase | 90 mg/L | C. boidinii | PAOD1 | 0.7 | [46] |
| Glucoamylase | 3.4 g/L | C. boidinii | PAOD1 | 1.2 | [32] |
| Cathepsin C | 12 mg/L | C. boidinii | PFDH1 | 1.5 | [34] |
| PAOX1, PMOX and PAOD1 are the promoters of alcohol oxidase genes from K. phaffii, O. polymorpha and C. boidinii, respectively. PFDH1 and PFMD are the promoters of formate dehydrogenase genes from O. polymorpha and C. boidinii, respectively. | |||||
这些启动子为构建高效甲醇酵母蛋白表达细胞工厂提供了元件(表 1)。比如:以质粒形式在PAOX1启动子下表达人血清白蛋白(Human serum albumin,HSA) 蛋白,通过优化诱导条件和甲醇添加速率,摇瓶中HSA最高产率达到1.6 g/L。发酵罐高密度发酵时,甲醇诱导96 h后最高产量为8.9 g/L[31],相比之前报道的工业应用生产速率提升了1倍。Sakai等在C. boidinii中利用PAOD1启动子表达葡糖淀粉酶,在1.2%的甲醇浓度诱导条件下,发酵12 h蛋白产量可达3.4 g/L[32]。除了PAOX外,甲酸脱氢酶(Formate dehydrogenase,Fmd) 基因启动子PFMD是最多的用于重组蛋白生产的启动子,在O. polymorpha中使用该启动子进行链霉亲和素的生产,通过甘油的添加增加了细胞生物量,去阻遏阶段,通过0.5%的甲醇诱导分批补料发酵,最终产量可以达到0.75 g/L[33]。而在C. boidinii中,采用PFDH1启动子成功用于组织蛋白酶C的生产,但产量较低,发酵90 h产物滴度仅有12 mg/L[34]。虽然目前有一系列甲醇响应启动子的报道,但实际生产过程中,PAOX和PFDH1仍然是使用最多的甲醇诱导型启动子。
对甲醇酵母的调控机制进行改造,缓解其他碳源的阻遏作用是进一步提高蛋白质表达的有效策略。在K. phaffii中敲除PAOX1的3个反式作用因子MIG1、MIG2和NRG1基因,同时过表达转录激活因子MIT1基因,显著解除了甘油对PAOX1的阻遏效应[35]。通过对K. phaffii启动子PAOX1核心区域序列进行设计改造,创建了一系列人工PAOX1突变体,表达强度范围为野生型的0.3%-176%,这些启动子可以用来对基因表达进行精细调控,从而平衡蛋白表达水平或代谢流分布[36-37]。
2.1.2 过程优化强化甲醇酵母蛋白生产虽然对菌株进行改造,如增强宿主细胞的蛋白分泌能力[47]、减少过度糖基化修饰可以显著提高宿主蛋白质表达效率,生物过程参数优化同样十分重要,其依赖于对细胞内代谢和生理变化的深入了解。生物工艺参数变化可以改变细胞生长状态,从而影响产量、质量和生产成本[48]。甲醇酵母生产重组蛋白过程复杂,尤其在生物反应器培养时,甲醇浓度、细胞培养时间、温度和通气量等都是重要参数。甲醇酵母过程优化主要从以下三方面考虑:一是控制甲醇浓度以平衡基因表达与细胞生长。甲醇是主要碳源及蛋白表达诱导物。在发酵过程中监测甲醇含量并进行调节,可以加快细胞生长,提升重组蛋白生产效率,同时避免甲醇对细胞的毒性。为了实现重组蛋白诱导表达,甲醇浓度至少要达到0.5% (W/V)。若要获得高效表达,甲醇浓度需达到2.0%-2.5% (W/V)[49]。甲醇酵母可耐受5%浓度甲醇,更高浓度甲醇会使细胞大量积累甲醛和H2O2,对细胞产生较大毒性并使生产过程停滞[50]。根据细胞生长情况控制甲醇补加速率使得甲醇维持在一定的浓度,既能达到足够好的蛋白质诱导表达水平,又不会对细胞生长产生毒害作用[51-52]。二是要维持高密度培养的高耗氧代谢并降低副产物积累。细胞高密度发酵需要消耗大量氧气,若氧气供应不足既会影响细胞生长,又会造成副产物积累。通过增加搅拌速率、通气速率、通气压力或者使用纯氧能增加氧气供应[53]。三是要解决工业规模发酵热控制,如前所述,甲醇酵母高密度发酵会产生大量热量,影响细胞生长和产物表达。有研究报道,控制温度发酵时,能显著提高K. phaffii中纤维素酶和酯酶的表达,其主要原因是低温时PAOX1的表达水平更高且蛋白酶的表达量更低,提高了蛋白稳定性[54]。工业生产中一般通过使用冷却水来控制发酵温度。另外,通过控制甲醇或其他碳源补料来控制温度是一个经济可行方案。当然,生产过程温度需要根据特定目标重组蛋白加以考察,以确定最佳培养条件。
恒化器培养是生物过程精确和可重复表征的可靠策略。通过恒化培养对O. polymorpha甲醇利用途径相关酶进行研究时发现:甲醇代谢基因启动子表达强度与恒化培养稀释率密切相关,其中PFLD和PFDH表达强度随着稀释率增加而增加,CAT的活性在培养过程中保持恒定,而Aox的活性却显著降低[55]。采用恒化培养器研究K. phaffii的甘油与甲醇代谢时发现:在低稀释率条件下,两种碳源都能被完全消耗,而在高稀释率条件下,甲醇并未进入中心碳代谢[56]。这些研究表明,细胞生长速度会影响甲醇诱导型启动子的活性,从而影响重组蛋白的表达效率。
2.2 代谢工程改造甲醇酵母生产化学品甲醇酵母作为底盘细胞生产蛋白优势明显,已用于生产多种蛋白质。由于甲醇酵母的一些优良特性,如Crabtree-negative和强鲁棒性等,被认为是合成重要化学品的潜在宿主[57]。另外,甲醇酵母底物谱广,除甲醇外,还可利用葡萄糖[58]、甘油[59],甚至是半纤维素水解产物[60]。由于甲醇代谢比葡萄糖代谢慢,甲醇酵母细胞工厂以葡萄糖为底物的研究比较广泛(表 2)。
| Products | Species | Carbon source | Medium | Cultivation | Yield | References |
| Ethanol | K. phaffii | CMC | Complex | Shake flask | 5.1 g/L | [64] |
| O. polymorpha | Glycerol | Complex | Shake flask | 5 g/L | [65] | |
| C. boidinii | MPHH | Complex | Shake flask | 12 g/L | [66] | |
| Lactic acid | K. phaffii | Glycerol | Minimal | Bioreactor, fed-batch | 24 g/L | [59] |
| C. boidinii | Glucose | Complex | Bioreactor, fed-batch | 85.9 g/L | [61] | |
| Xylitol | K. phaffii | Glucose | Complex | Bioreactor, fed-batch | 17.3 g/L | [58] |
| C. boidinii | CPHHH | Complex | Shake flask | 11.3 g/L | [60] | |
| FABCEs | K. phaffii | Glucose | Complex | Shake flask | 169 mg/L | [82] |
| Glucaric acid | K. phaffii | Glucose, myo-inositol | Complex | Bioreactor, fed-batch | 6.6 g/L | [83] |
| Riboflavin | K. phaffii | Glycerol | Complex | Bioreactor, fed-batch | 175 mg/L | [84] |
| Isobutanol | K. phaffii | Glucose | Minimal | Shake flask | 2.2 g/L | [72] |
| 2, 3-butanediol | K. phaffii | Glucose | Minimal | Bioreactor, fed-batch | 74.5 g/L | [73] |
| 1, 3-propandiol | O. polymorpha | Glucose or glycerol | Complex | Shake flask | 2.4 g/L or 0.8 g/L | [85] |
| Glutathione | O. polymorpha | Glucose or methanol | Minimal | Bioreactor, fed-batch | 2.3 g/L or 0.25 g/L | [74] |
| 6-methylsalicylic acid | K. phaffii | Glycerol, methanol | Minimal | Bioreactor, fed-batch | 2.2 g/L | [75] |
| Ricinoleic acid | K. phaffii | Glucose, galactose | Minimal | Shake flask | 125.4 mg/L | [76] |
| (+)-Nootkatone | K. phaffii | Glucose, methanol | Minimal | Bioreactor, fed-batch | 208 mg/L | [62] |
| Dammarenediol-Ⅱ | K. phaffii | Methanol | Complex | Shake flask | 1 mg/g | [63] |
| Lycopene | K. phaffii | Glucose | Minimal | Bioreactor, fed-batch | 73.9 mg/L | [78] |
| β-carotene | K. phaffii | Glucose | Complex | Shake flask | 339 μg/g | [79] |
| Lovastatin | K. phaffii | Glycerol, methanol | Minimal | Bioreactor, fed-batch | 419 mg/L | [81] |
| Monacolin J | K. phaffii | Glycerol, methanol | Minimal | Bioreactor, fed-batch | 594 mg/L | [80] |
| CMC: carboxymethyl cellulose; MPHH: macaú ba presscake hemicellulosic hydrolysate; CPHHH: cocoa pod husk hemicellulose hydrolysate; FABCEs: fatty acid branched-chain esters. | ||||||
与其他微生物宿主相似,在甲醇酵母中,初级代谢产物如乳酸等产量可达较高水平[61],而次级代谢产物如萜类化合物等产量较低[62-63]。由于遗传背景清晰,基因操作工具相对完备,K. phaffii是应用最广泛的宿主,而O. polymorpha及C. boidinii应用较少。
2.2.1 改造甲醇酵母合成初级代谢产物乙醇、乳酸和木糖醇是最常见的初级代谢产物(表 2)。虽然甲醇酵母为Crabtree-negative菌株,不易积累乙醇,但通过构建乙醇生物合成途径,可实现乙醇合成[64-66],不过其产量(< 20 g/L)远远低于酿酒酵母的乙醇产量[67] (表 3)。在K. phaffii中,通过表面展示系统构建了羧甲基纤维素利用途径,将羧甲基纤维素转化为乙醇,拓展了底物谱[64],且其产量与酿酒酵母以羧甲基纤维素为底物合成的乙醇产量相当[68]。在甲醇酵母中构建乳酸生物合成途径,实现了以葡萄糖或者甘油为底物高效合成乳酸[59, 61],特别是C. boidinii中乳酸产量(85.9 g/L) 和生产强度(1.79 g/(L·h)) 远远高于酿酒酵母[69]。最近,在K. phaffii中整合表达乳酸脱氢酶基因,首次实现了以甲醇为碳源合成D-乳酸[70],但是其产量和生产强度分别仅有3.5 g/L和0.036 g/(L·h),主要原因可能是甲醇同化效率不够高。相似地,通过构建木糖醇代谢途径,在K. phaffii与C. boidinii中分别以葡萄糖和半纤维素水解物为碳源合成了木糖醇,产量分别达到17.3 g/L[58]和11.3 g/L[60],但低于酿酒酵母中47 g/L的产量[71]。
| Products | Host | Titer | References | Host | Titer | References |
| Ethanol | K. phaffii | 5.1 g/L | [64] | S. cerevisiae | 93.1 g/L | [67] |
| O. polymorpha | 5 g/L | [65] | ||||
| C. boidinii | 12 g/L | [66] | ||||
| Lactic acid | K. phaffii | 24 g/L | [59] | S. cerevisiae | 60.3 g/L | [69] |
| C. boidinii | 85.9 g/L | [61] | ||||
| Xylitol | K. phaffii | 17.3 g/L | [58] | S. cerevisiae | 47 g/L | [71] |
| C. boidinii | 11.3 g/L | [60] | ||||
| (+)-Nootkatone | K. phaffii | 208 mg/L | [62] | S. cerevisiae | 59.8 mg/L | [77] |
| Dammarenediol-Ⅱ | K. phaffii | 1 mg/g | [63] | S. cerevisiae | 10.9 mg/g | [86] |
| Lycopene | K. phaffii | 73.9 mg/L | [78] | S. cerevisiae | 5.9 g/L | [87] |
| β-carotene | K. phaffii | 339 μg/g | [79] | S. cerevisiae | 14.3 mg/L | [88] |
近年来,甲醇酵母进一步改造用于生产异丁醇、2, 3-丁二醇等化合物(图 2),其前体均为丙酮酸。其中,在K. phaffii构建异丁醇生物合成途径,并进一步强化内源缬氨酸生物合成途径实现了以葡萄糖为底物从头合成异丁醇,其产量为2.2 g/L,得率仅为0.02 g/g葡萄糖[72],表明其初级代谢需要进一步强化。进一步表达甲基转移酶实现了乙酸异丁酯的从头合成,产量为0.05 g/L。然而,在K. phaffii构建2, 3-丁二醇途径,批式补料发酵产量可达74.5 g/L,该发酵过程利用了复杂培养基酵母粉及蛋白胨,增加了发酵成本[73]。以上发酵碳源为葡萄糖、甘油或半纤维素水解产物,而在O. polymorpha中实现了以甲醇为底物合成谷胱甘肽(glutathione)[74],但产量(0.25 g/L) 远远低于以葡萄糖为碳源时产量(2.3 g/L),推测甲醇同化效率低是限制产物合成效率的关键因素之一。从中心代谢前体丙酮酸、乙酰辅酶A到目标产物生物合成途径构建可借鉴成熟模式生物酿酒酵母中的代谢工程策略。但是甲醇同化利用进入中心代谢过程复杂,且涉及到过氧化物酶体调控,限制了其代谢通量。后续研究应该着重于甲醇同化途径调控改造,并实现与中心代谢的适配。
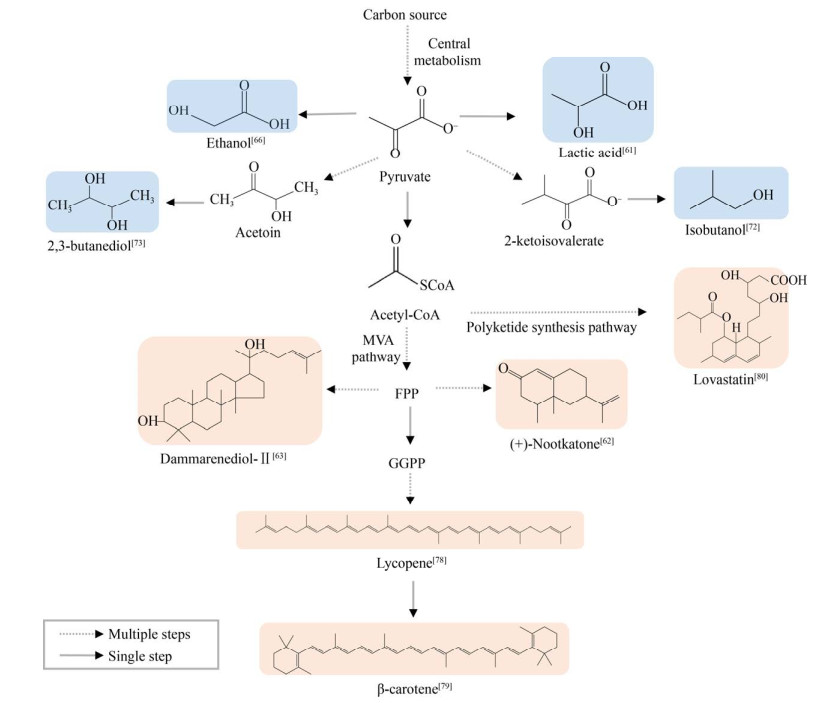
|
| 图 2 甲醇酵母生产代谢物以及合成途径 Fig. 2 Methylotrophic yeasts produces metabolites and biosynthetic pathways. Blue modules are the primary metabolites, pink modules are the secondary metabolite. |
| |
基于甲醇酵母的次级代谢产物多见于K. phaffii中,O. polymorpha及C. boidinii的研究并不多(表 2)。主要次级代谢产物包括聚酮类[75]、萜类[62]、脂肪酸衍生物[76]等(图 2)。
次级代谢产物合成途径一般较长,重构其生物合成途径通常需要对多个基因进行改造。诺卡酮是一种高价值倍半萜化合物,其合成需要细胞色素P450酶参与。在K. phaffii中构建诺卡酮生物合成途径,利用PAOX1启动子过表达植物天仙子的P450酶基因hpo、拟南芥细胞色素P450还原酶基因以及过表达毕赤酵母醇脱氢酶(Alcohol dehydrogenase,ADH)、酿酒酵母甲羟戊酸-CoA还原酶截短蛋白突变体(Truncated hydroxy- methylglutaryl-CoA reductase 1,tHMG1),诺卡酮产量达到208 mg/L[62]。其生物合成相关基因启动子均为PAOX1,先以葡萄糖为碳源进行细胞生长,然后再以甲醇为碳源和诱导剂合成诺卡酮。虽然该工程菌并不能以甲醇为单一碳源,但其产量超过目前酿酒酵母中的最高产量59.8 mg/L[77],证明了甲醇酵母作为宿主合成萜类化合物的潜力。相似地,在K. phaffii中实现了三萜化合物人参皂苷前体达玛烯二醇-Ⅱ[63]、四萜化合物番茄红素[78]和β-胡萝卜素[79]的生物合成,不过其产量都远远低于酿酒酵母中的产量(表 3)。其主要原因是次级代谢化合物生物合成途径复杂,需要对多种途径多个基因进行调节,目前对甲醇酵母的代谢认识还不够深入,难以对细胞代谢进行全局系统优化。近期,将洛伐他汀生物合成途径分步构建在两株K. phaffii中,通过混菌发酵实现了洛伐他汀高产量生物合成,批式补料发酵产量达到251 mg/L[80],前体monacolin J的产量则达到了594 mg/L。在加强洛伐他汀合成途径基础上,过表达洛伐他汀外排膜蛋白TapA,洛伐他汀产量达到419 mg/L[81]。
次级代谢途径复杂,涉及到多个基因。因此,优化生物合成途径除了要考虑前体和辅因子以外,还要考虑到次级代谢途径与初级代谢途径的平衡。比如,目前研究多用甲醇诱导型启动子,往往给细胞代谢带来负担,后续需要挖掘更多的启动子来调控次级代谢生物合成途径。
3 总结与展望 3.1 缺乏高效遗传操作工具在以往的甲醇酵母重组蛋白研究中,外源基因数目较少,一般通过质粒游离表达的形式[89]或是经整合质粒整合至酵母染色体的特定位点,如AOX1和GAP位点[90]。质粒表达时,基因拷贝数较高有助于提高蛋白表达水平,而染色体整合模式,有助于蛋白稳定表达。若要将甲醇酵母改造成为性能优良的底盘细胞进行重组蛋白或是化学品的生产,往往需要对宿主细胞的多个基因进行调控,且构建的外源途径基因可能多达几十个[91],因此高效可靠的遗传操作工具至关重要。
目前,改造甲醇酵母用于绿色生物制造刚刚兴起,其生物合成效率远远低于酿酒酵母,其主要原因是缺乏高效遗传操作工具,限制了对细胞代谢的全局优化。高效遗传操作工具主要有3个方面:1) 高效基因编辑工具;2) 充足的基因元件如启动子和终止子;3) 充足的染色体整合位点(中性位点)。近年来,CRISPR-Cas9技术的兴起给基因编辑带来了巨大便捷。CRISPR-Cas9系统通过小向导RNA (Small guide RNA,sgRNA) 的靶向序列定位至含有前间隔序列邻近基序(Protospacer adjacent motif,PAM) 序列的特定位点,引导Cas9蛋白切割DNA形成双链断裂缺口,再依靠同源重组(HR) 或者非同源末端连接(NHEJ) 修复的方式进行连接,从而引发基因编辑[92]。甲醇酵母属于非传统酵母,NHEJ是其主要的修复方式。Thomas等首先基于NHEJ修复的模式,通过96种不同组合筛选,最终确定使用Ⅱ型双向启动子PHTX1表达人源密码子优化的Cas9蛋白以及gRNA,建立了K. phaffii中的CRISPR-Cas9编辑系统,该系统下DNA切割效率达到90%以上[93]。然而,甲醇酵母中同源重组效率很低,限制了代谢途径理性精确改造[92]。敲除NHEJ关键基因KU70显著提升了同源重组效率,但是降低了整合效率[94]。相似地,在O. polymorpha中用内源PSNR6启动子表达tRNACUG-sgRNA融合表达盒,实现了基因编辑,但效率有待于进一步提高[95]。目前尚未有C. boidinii中关于CRISPR-Cas9编辑系统的报道。虽然CRISPR-Cas9是实现DNA高效剪切的关键,未来在不影响细胞鲁棒性的情况下提高同源重组效率才能实现甲醇酵母的理性改造。虽然CRISPR-Cas9编辑技术在K. phaffii等甲醇酵母中应用不断增多,但与酿酒酵母相比,其低同源重组效率严重限制了系统代谢工程改造。除高效基因编辑系统外,足够的启动子等基因表达原件有利于对甲醇酵母细胞代谢进行精确调控,从而提高生物合成效率。虽然已有报道利用微阵列芯片对K. phaffii中系列启动子进行了表征[22],但是其他甲醇酵母仍然缺乏足够的启动子。另外,基因组整合位点不足限制了基因整合,不利于构建稳定的细胞工厂。未来借鉴酿酒酵母代谢工程策略,鉴定系列整合位点,才能对甲醇酵母进行多基因整合,从而对其代谢全局进行优化[96]。
3.2 甲醇利用效率低利用甲醇为碳源或诱导剂是甲醇酵母的一大优势,用其生产的蛋白应用也非常广泛,但由于蛋白生产时,甲醇仅仅是作为诱导剂[97],往往会添加辅助碳源或者营养成分丰富的培养基组分。然而,与蛋白产物相比,生物化学品附加值不够高,甲醇利用效率和转化率决定了生物过程经济性[98]。甲醇对细胞有一定毒性,且甲醇同化效率比葡萄糖代谢效率低,目前甲醇生物转化合成生物化学品的效率远远低于基于葡萄糖的生物合成效率(表 3)。随着对甲醇酵母基因组、蛋白质组、代谢组等组学研究的完善,甲醇代谢的关键基因及调控机制越来越清晰[99-102],这些信息将为甲醇转化效率强化提供代谢工程改造靶点。另外,优化培养基与发酵过程也是提高甲醇利用效率的可行策略[103]。
虽然面临基因操作工具相对缺乏、碳源利用效率低、代谢背景不够清晰等难题,但是其独特的优势使得甲醇酵母在生物制造中仍具有广阔的前景。相信随着进一步优化基因操作平台及系统解析甲醇代谢途径,甲醇酵母将在蛋白质及化学品等的绿色生物制造发挥重要作用。
| [1] |
崔普选. 煤制甲醇技术发展评述. 现代化工, 2020, 40(5): 4-9. Cui PX. Review on development of coal-to-methanol technologies. Mod Chem Ind, 2020, 40(5): 4-9 (in Chinese). |
| [2] |
Shih CF, Zhang T, Li J, et al. Powering the future with liquid sunshine. Joule, 2018, 2(10): 1925-1949. DOI:10.1016/j.joule.2018.08.016
|
| [3] |
高兴, 田文莉, 刘军战. 甲醇制烯烃技术进展. 工业催化, 2020, 28(8): 21-23. Gao X, Tian WL, Liu JZ. Progress on methanol-to-olefins technology. Ind Catal, 2020, 28(8): 21-23 (in Chinese). |
| [4] |
高教琪, 周雍进. 甲醇生物转化的机遇与挑战. 合成生物学, 2020, 1(2): 158-173. Gao JQ, Zhou YJ. Advances in methanol bio-transformation. Synth Biol J, 2020, 1(2): 158-173 (in Chinese). |
| [5] |
Zhou YJ, Kerkhoven EJ, Nielsen J. Barriers and opportunities in bio-based production of hydrocarbons. Nat Energy, 2018, 3(11): 925-935. DOI:10.1038/s41560-018-0197-x
|
| [6] |
Dai Z, Gu H, Zhang S, et al. Metabolic construction strategies for direct methanol utilization in Saccharomyces cerevisiae. Bioresour Technol, 2017, 245(Pt B): 1407-1412.
|
| [7] |
Muller JEN, Meyer F, Litsanov B, et al. Engineering Escherichia coli for methanol conversion. Metab Eng, 2015, 28: 190-201. DOI:10.1016/j.ymben.2014.12.008
|
| [8] |
Tuyishime P, Wang Y, Fan LW, et al. Engineering Corynebacterium glutamicum for methanol- dependent growth and glutamate production. Metab Eng, 2018, 49: 220-231. DOI:10.1016/j.ymben.2018.07.011
|
| [9] |
Chen FY, Jung HW, Tsuei CY, et al. Converting Escherichia coli to a synthetic methylotroph growing solely on methanol. Cell, 2020, 182(4): 933-946. DOI:10.1016/j.cell.2020.07.010
|
| [10] |
Yurimoto H, Oku M, Sakai Y. Yeast methylotrophy: metabolism, gene regulation and peroxisome homeostasis. Int J Microbiol, 2011, 2011: 101298.
|
| [11] |
Hollenberg CP, Gellissen G. Production of recombinant proteins by methylotrophic yeast. Curr Opin Biotech, 1997, 8(5): 554-560. DOI:10.1016/S0958-1669(97)80028-6
|
| [12] |
Vasylyshyn R, Kurylenko O, Ruchala J, et al. Engineering of sugar transporters for improvement of xylose utilization during high-temperature alcoholic fermentation in Ogataea polymorpha yeast. Microb Cell Fact, 2020, 19(1): 96-107. DOI:10.1186/s12934-020-01354-9
|
| [13] |
Sturmberger L, Chappell T, Geier M, et al. Refined Pichia pastoris reference genome sequence. J Biotechnol, 2016, 235: 121-131. DOI:10.1016/j.jbiotec.2016.04.023
|
| [14] |
Valli M, Tatto NE, Peymann A, et al. Curation of the genome annotation of Pichia pastoris (Komagataella phaffii) CBS7435 from gene level to protein function. FEMS Yeast Res, 2016, 16(6): fow051. DOI:10.1093/femsyr/fow051
|
| [15] |
De Schutter K, Lin YC, Tiels P, et al. Genome sequence of the recombinant protein production host Pichia pastoris. Nat Biotechnol, 2009, 27(6): 561-566. DOI:10.1038/nbt.1544
|
| [16] |
Love KR, Shah KA, Whittaker CA, et al. Comparative genomics and transcriptomics of Pichia pastoris. BMC Genomics, 2016, 17: 550-566. DOI:10.1186/s12864-016-2876-y
|
| [17] |
Ravin NV, Eldarov MA, Kadnikov VV, et al. Genome sequence and analysis of methylotrophic yeast Hansenula polymorpha DL1. BMC Genomics, 2013, 14: 837-856. DOI:10.1186/1471-2164-14-837
|
| [18] |
Borelli G, Jose J, Teixeira PJ, et al. De novo assembly of Candida sojae and Candida boidinii genomes, unexplored xylose-consuming yeasts with potential for renewable biochemical production. Genome Announc, 2016, 4(1): e01551.
|
| [19] |
Somani A, Smith D, Hegarty M, et al. Draft genome assemblies of xylose-utilizing Candida tropicalis and Candida boidinii with potential application in biochemical and biofuel production. Genome Announc, 2018, 6(7): e01594.
|
| [20] |
Schwarzhans JP, Luttermann T, Geier M, et al. Towards systems metabolic engineering in Pichia pastoris. Biotechnol Adv, 2017, 35(6): 681-710. DOI:10.1016/j.biotechadv.2017.07.009
|
| [21] |
Hartner FS, Glieder A. Regulation of methanol utilisation pathway genes in yeasts. Microb Cell Fact, 2006, 5(1): 39-59. DOI:10.1186/1475-2859-5-39
|
| [22] |
Vogl T, Sturmberger L, Kickenweiz T, et al. A toolbox of diverse promoters related to methanol utilization: functionally verified parts for heterologous pathway expression in Pichia pastoris. ACS synth biol, 2016, 5(2): 172-186. DOI:10.1021/acssynbio.5b00199
|
| [23] |
Vogl T, Glieder A. Regulation of Pichia pastoris promoters and its consequences for protein production. N Biotechnol, 2013, 30(4): 385-404. DOI:10.1016/j.nbt.2012.11.010
|
| [24] |
Vogl T, Sturmberger L, Fauland PC, et al. Methanol independent induction in Pichia pastoris by simple derepressed overexpression of single transcription factors. Biotechnol Bioeng, 2018, 115(4): 1037-1050. DOI:10.1002/bit.26529
|
| [25] |
Manfrao-Netto JHC, Gomes AMV, Parachin NS. Advances in using Hansenula polymorpha as chassis for recombinant protein production. Front Bioeng Biotechnol, 2019, 7: 94-106. DOI:10.3389/fbioe.2019.00094
|
| [26] |
Yurimoto H, Sakai Y. Methanol-inducible gene expression and heterologous protein production in the methylotrophic yeast Candida boidinii. Biotechnol Appl Biochem, 2009, 53(Pt 2): 85-92.
|
| [27] |
Shen S, Sulter G, Jeffries TW, et al. A strong nitrogen source-regulated promoter for controlled expression of foreign genes in the yeast Pichia pastoris. Gene, 1998, 216(1): 93-102. DOI:10.1016/S0378-1119(98)00315-1
|
| [28] |
Baerends RJ, Sulter GJ, Jeffries TW, et al. Molecular characterization of the Hansenula polymorpha FLD1 gene encoding formaldehyde dehydrogenase. Yeast, 2002, 19(1): 37-42. DOI:10.1002/yea.805
|
| [29] |
Lee B, Yurimoto H, Sakai Y, et al. Physiological role of the glutathione-dependent formaldehyde dehydrogenase in the methylotrophic yeast Candida boidinii. Microbiology, 2002, 149(Pt 9): 2697-2704.
|
| [30] |
Yurimoto H, Komeda T, Lim CR, et al. Regulation and evaluation of five methanol-inducible promoters in the methylotrophic yeast Candida boidinii. Biochim Biophys Acta, 2000, 1493(1/2): 56-63.
|
| [31] |
Zhu W, Gong GH, Pan J, et al. High level expression and purification of recombinant human serum albumin in Pichia pastoris. Protein Expres Purif, 2018, 147: 61-68. DOI:10.1016/j.pep.2018.02.003
|
| [32] |
Sakai Y, Akiyama M, Kondoh H, et al. High-level secretion of fungal glucoamylase using the Candida boidinii gene expression system. Biochim Biophys Acta, 1996, 1308(1): 81-87. DOI:10.1016/0167-4781(96)00075-9
|
| [33] |
Wetzel D, Muller JM, Flaschel E, et al. Fed-batch production and secretion of streptavidin by Hansenula polymorpha: Evaluation of genetic factors and bioprocess development. J Biotechnol, 2016, 225: 3-9. DOI:10.1016/j.jbiotec.2016.03.017
|
| [34] |
Komeda T, Tazumi K, Shimada H, et al. Production of active bovine cathepsin C (dipeptidyl aminopeptidase Ⅰ) in the methylotrophic yeast Candida boidinii. Appl Microbiol Biotechnol, 2002, 59(2/3): 252-258. DOI:10.1007/s00253-002-1010-z
|
| [35] |
Wang J, Wang X, Shi L, et al. Methanol-independent protein expression by AOX1 promoter with trans-acting elements engineering and glucose- glycerol-shift induction in Pichia pastoris. Sci Rep, 2017, 7: 41850. DOI:10.1038/srep41850
|
| [36] |
Vogl T, Ruth C, Pitzer J, et al. Synthetic core promoters for Pichia pastoris. ACS Synth Biol, 2014, 3(3): 188-191. DOI:10.1021/sb400091p
|
| [37] |
Portela RM, Vogl T, Kniely C, et al. Synthetic core promoters as universal parts for fine-tuning expression in different yeast species. ACS Synth Biol, 2017, 6(3): 471-484. DOI:10.1021/acssynbio.6b00178
|
| [38] |
Wang Y, Yuan S, Wang P, et al. Expression, purification, and characterization of recombinant human keratinocyte growth factor-2 in Pichia pastoris. J Biotechnol, 2007, 132(1): 44-48. DOI:10.1016/j.jbiotec.2007.08.024
|
| [39] |
Zhang J, Yang YL, Teng D, et al. Expression of plectasin in Pichia pastoris and its characterization as a new antimicrobial peptide against Staphyloccocus and Streptococcus. Protein Expres Purif, 2011, 78(2): 189-196. DOI:10.1016/j.pep.2011.04.014
|
| [40] |
Meng DM, Li WJ, Shi LY, et al. Expression, purification and characterization of a recombinant antimicrobial peptide Hispidalin in Pichia pastoris. Protein Expres Purif, 2019, 160: 19-27. DOI:10.1016/j.pep.2019.03.007
|
| [41] |
Zhang YF, Huang H, Yao XH, et al. High-yield secretory production of stable, active trypsin through engineering of the N-terminal peptide and self-degradation sites in Pichia pastoris. Bioresour Technol, 2018, 247: 81-87. DOI:10.1016/j.biortech.2017.08.006
|
| [42] |
Faraji H, Ramezani M, Sadeghnia HR, et al. High-level expression of a biologically active staphylokinase in Pichia pastoris. Prep Biochem Biotechnol, 2017, 47(4): 379-387. DOI:10.1080/10826068.2016.1252924
|
| [43] |
Bredell H, Smith JJ, Gorgens JF, et al. Expression of unique chimeric human papilloma virus type 16 (HPV-16) L1-L2 proteins in Pichia pastoris and Hansenula polymorpha. Yeast, 2018, 35(9): 519-529. DOI:10.1002/yea.3318
|
| [44] |
Eilert E, Hollenberg CP, Piontek M, et al. The use of highly expressed FTH1 as carrier protein for cytosolic targeting in Hansenula polymorpha. J Biotechnol, 2012, 159(3): 172-176. DOI:10.1016/j.jbiotec.2011.12.014
|
| [45] |
Moussa M, Ibrahim M, El Ghazaly M, et al. Expression of recombinant staphylokinase in the methylotrophic yeast Hansenula polymorpha. BMC Biotechnol, 2012, 12: 96-108. DOI:10.1186/1472-6750-12-96
|
| [46] |
Yurimoto H, Yamane M, Kikuchi Y, et al. The pro-peptide of Streptomyces mobaraensis transglutaminase functions in cis and in trans to mediate efficient secretion of active enzyme from methylotrophic yeasts. Biosci Biotechnol Biochem, 2004, 68(10): 2058-2069. DOI:10.1271/bbb.68.2058
|
| [47] |
Eilert E, Rolf T, Heumaier A, et al. Improved processing of secretory proteins in Hansenula polymorpha by sequence variation near the processing site of the alpha mating factor prepro sequence. J Biotechnol, 2013, 167(2): 94-100. DOI:10.1016/j.jbiotec.2012.08.024
|
| [48] |
Nieto-Taype MA, Garcia-Ortega X, Albiol J, et al. Continuous cultivation as a tool toward the rational bioprocess development with Pichia pastoris cell factory. Front Bioeng Biotechnol, 2020, 8: 632-652. DOI:10.3389/fbioe.2020.00632
|
| [49] |
Wang Z, Wang Y, Zhang D, et al. Enhancement of cell viability and alkaline polygalacturonate lyase production by sorbitol co-feeding with methanol in Pichia pastoris fermentation. Bioresour Technol, 2010, 101(4): 1318-1323. DOI:10.1016/j.biortech.2009.09.025
|
| [50] |
Karbalaei M, Rezaee SA, Farsiani H. Pichia pastoris: A highly successful expression system for optimal synthesis of heterologous proteins. J Cell Physiol, 2020, 235(9): 5867-5881. DOI:10.1002/jcp.29583
|
| [51] |
Barrigón JM, Montesinos JL, Valero F. Searching the best operational strategies for Rhizopus oryzae lipase production in Pichia pastoris Mut+ phenotype: Methanol limited or methanol non-limited fed-batch cultures?. Biochem Eng J, 2013, 75: 47-54. DOI:10.1016/j.bej.2013.03.018
|
| [52] |
Gurramkonda C, Polez S, Skoko N, et al. Application of simple fed-batch technique to high-level secretory production of insulin precursor using Pichia pastoris with subsequent purification and conversion to human insulin. Microb Cell Fact, 2010, 9(1): 31-41. DOI:10.1186/1475-2859-9-31
|
| [53] |
Liu WC, Gong T, Wang QH, et al. Scaling-up fermentation of Pichia pastoris to demonstration- scale using new methanol-feeding strategy and increased air pressure instead of pure oxygen supplement. Sci Rep, 2016, 6: 18439. DOI:10.1038/srep18439
|
| [54] |
Jahic M, Wallberg F, Bollok M, et al. Temperature limited fed-batch technique for control of proteolysis in Pichia pastoris bioreactor cultures. Microb Cell Fact, 2003(1), 2: 6-16.
|
| [55] |
van Dijken JP, Otto R, Harder W. Growth of Hansenula polymorpha in a methanol-limited chemostat. Physiological responses due to the involvement of methanol oxidase as a key enzyme in methanol metabolism. Arch Microbiol, 1976, 111(1/2): 137-144. DOI:10.1007/BF00446560
|
| [56] |
Sola A, Jouhten P, Maaheimo H, et al. Metabolic flux profiling of Pichia pastoris grown on glycerol/methanol mixtures in chemostat cultures at low and high dilution rates. Microbiology, 2007, 153(Pt 1): 281-290.
|
| [57] |
Pena DA, Gasser B, Zanghellini J, et al. Metabolic engineering of Pichia pastoris. Metab Eng, 2018, 50: 2-15. DOI:10.1016/j.ymben.2018.04.017
|
| [58] |
Cheng H, Lv J, Wang H, et al. Genetically engineered Pichia pastoris yeast for conversion of glucose to xylitol by a single-fermentation process. Appl Microbiol Biotechnol, 2014, 98(8): 3539-3552. DOI:10.1007/s00253-013-5501-x
|
| [59] |
Melo NTM, Pontes GC, Procopio DP, et al. Evaluation of product distribution in chemostat and batch fermentation in lactic acid-producing Komagataella phaffii strains utilizing glycerol as substrate. Microorganisms, 2020, 8(5): 781-792. DOI:10.3390/microorganisms8050781
|
| [60] |
Santana NB, Dias JCT, Rezende RP, et al. Production of xylitol and bio-detoxification of cocoa pod husk hemicellulose hydrolysate by Candida boidinii XM02G. PLoS ONE, 2018, 13(4): e0195206. DOI:10.1371/journal.pone.0195206
|
| [61] |
Osawa F, Fujii T, Nishida T, et al. Efficient production of L-lactic acid by Crabtree-negative yeast Candida boidinii. Yeast, 2009, 26(9): 485-496. DOI:10.1002/yea.1702
|
| [62] |
Wriessnegger T, Augustin P, Engleder M, et al. Production of the sesquiterpenoid (+)-nootkatone by metabolic engineering of Pichia pastoris. Metab Eng, 2014, 24: 18-29. DOI:10.1016/j.ymben.2014.04.001
|
| [63] |
Liu XB, Liu M, Tao XY, et al. Metabolic engineering of Pichia pastoris for the production of dammarenediol-Ⅱ. J Biotechnol, 2015, 216: 47-55. DOI:10.1016/j.jbiotec.2015.10.005
|
| [64] |
Dong C, Qiao J, Wang X, et al. Engineering Pichia pastoris with surface-display minicellulosomes for carboxymethyl cellulose hydrolysis and ethanol production. Biotechnol Biofuels, 2020, 13: 108-116. DOI:10.1186/s13068-020-01749-1
|
| [65] |
Kata I, Semkiv MV, Ruchala J, et al. Overexpression of the genes PDC1 and ADH1 activates glycerol conversion to ethanol in the thermotolerant yeast Ogataea (Hansenula) polymorpha. Yeast, 2016, 33(8): 471-478. DOI:10.1002/yea.3175
|
| [66] |
Goncalves DB, Batista AF, Rodrigues M, et al. Ethanol production from macauba (Acrocomia aculeata) presscake hemicellulosic hydrolysate by Candida boidinii UFMG14. Bioresour Technol, 2013, 146: 261-266. DOI:10.1016/j.biortech.2013.07.075
|
| [67] |
Caspeta L, Coronel J, Montes de Oca A, et al. Engineering high-gravity fermentations for ethanol production at elevated temperature with Saccharomyces cerevisiae. Biotechnol Bioeng, 2019, 116(10): 2587-2597. DOI:10.1002/bit.27103
|
| [68] |
Song X, Li Y, Wu Y, et al. Metabolic engineering strategies for improvement of ethanol production in cellulolytic Saccharomyces cerevisiae. FEMS Yeast Res, 2018, 18(8): 1-10.
|
| [69] |
Yamada R, Wakita K, Mitsui R, et al. Enhanced D-lactic acid production by recombinant Saccharomyces cerevisiae following optimization of the global metabolic pathway. Biotechnol Bioeng, 2017, 114(9): 2075-2084. DOI:10.1002/bit.26330
|
| [70] |
Yamada R, Ogura K, Kimoto Y, et al. Toward the construction of a technology platform for chemicals production from methanol: D-lactic acid production from methanol by an engineered yeast Pichia pastoris. World J Microbiol Biotechnol, 2019, 35(2): 37-45. DOI:10.1007/s11274-019-2610-4
|
| [71] |
Kogje AB, Ghosalkar A. Xylitol production by genetically modified industrial strain of Saccharomyces cerevisiae using glycerol as co-substrate. J Ind Microbiol Biotechnol, 2017, 44(6): 961-971. DOI:10.1007/s10295-017-1914-3
|
| [72] |
Siripong W, Wolf P, Kusumoputri TP, et al. Metabolic engineering of Pichia pastoris for production of isobutanol and isobutyl acetate. Biotechnol Biofuels, 2018, 11: 1-16. DOI:10.1186/s13068-017-1003-x
|
| [73] |
Yang Z, Zhang Z. Production of (2R, 3R)-2, 3- butanediol using engineered Pichia pastoris: strain construction, characterization and fermentation. Biotechnol Biofuels, 2018, 11: 35-50. DOI:10.1186/s13068-018-1031-1
|
| [74] |
Ubiyvovk VM, Ananin VM, Malyshev AY, et al. Optimization of glutathione production in batch and fed-batch cultures by the wild-type and recombinant strains of the methylotrophic yeast Hansenula polymorpha DL-1. BMC Biotechnol, 2011, 11: 8-19. DOI:10.1186/1472-6750-11-8
|
| [75] |
Gao LM, Cai MH, Shen W, et al. Engineered fungal polyketide biosynthesis in Pichia pastoris: a potential excellent host for polyketide production. Microb Cell Fact, 2013, 12(1): 77-90. DOI:10.1186/1475-2859-12-77
|
| [76] |
Meesapyodsuk D, Chen Y, Ng SH, et al. Metabolic engineering of Pichia pastoris to produce ricinoleic acid, a hydroxy fatty acid of industrial importance. J Lipid Res, 2015, 56(11): 2102-2019. DOI:10.1194/jlr.M060954
|
| [77] |
Meng X, Liu H, Xu W, et al. Metabolic engineering Saccharomyces cerevisiae for de novo production of the sesquiterpenoid (+)-nootkatone. Microb Cell Fact, 2020, 19(1): 21-34. DOI:10.1186/s12934-020-1295-6
|
| [78] |
Bhataya A, Schmidt-Dannert C, Lee PC. Metabolic engineering of Pichia pastoris X-33 for lycopene production. Process Biochem, 2009, 44(10): 1095-1102. DOI:10.1016/j.procbio.2009.05.012
|
| [79] |
Araya-Garay JM, Feijoo-Siota L, Rosa-dos-Santos F, et al. Construction of new Pichia pastoris X-33 strains for production of lycopene and β-carotene. Appl Microbiol Biotechnol, 2012, 93(6): 2483-2492. DOI:10.1007/s00253-011-3764-7
|
| [80] |
Liu Y, Tu X, Xu Q, et al. Engineered monoculture and co-culture of methylotrophic yeast for de novo production of monacolin J and lovastatin from methanol. Metab Eng, 2018, 45: 189-199. DOI:10.1016/j.ymben.2017.12.009
|
| [81] |
Liu Y, Bai C, Xu Q, et al. Improved methanol- derived lovastatin production through enhancement of the biosynthetic pathway and intracellular lovastatin efflux in methylotrophic yeast. Bioresour and Bioprocess, 2018, 5(1): 22-32. DOI:10.1186/s40643-018-0202-z
|
| [82] |
Tao H, Guo D, Zhang Y, et al. Metabolic engineering of microbes for branched-chain biodiesel production with low-temperature property. Biotechnol Biofuels, 2015, 8: 92-102. DOI:10.1186/s13068-015-0270-7
|
| [83] |
Liu Y, Gong X, Wang C, et al. Production of glucaric acid from myo-inositol in engineered Pichia pastoris. Enzyme Microb Technol, 2016, 91: 8-16. DOI:10.1016/j.enzmictec.2016.05.009
|
| [84] |
Marx H, Mattanovich D, Sauer M. Overexpression of the riboflavin biosynthetic pathway in Pichia pastoris. Microb Cell Fact, 2008, 7(1): 23-33. DOI:10.1186/1475-2859-7-23
|
| [85] |
Hong WK, Kim CH, Heo SY, et al. 1, 3-propandiol production by engineered Hansenula polymorpha expressing dha genes from Klebsiella pneumoniae. Bioprocess Biosyst Eng, 2011, 34(2): 231-236. DOI:10.1007/s00449-010-0465-z
|
| [86] |
Dai Z, Liu Y, Zhang X, et al. Metabolic engineering of Saccharomyces cerevisiae for production of ginsenosides. Metab Eng, 2013, 20: 146-156. DOI:10.1016/j.ymben.2013.10.004
|
| [87] |
Wang Z, Li X, Yu C, et al. Continuous self-cycling fermentation leads to economical lycopene production by Saccharomyces cerevisiae. Front Bioeng Biotechnol, 2020, 8: 420-431. DOI:10.3389/fbioe.2020.00420
|
| [88] |
Lange N, Steinbuchel A. β-carotene production by Saccharomyces cerevisiae with regard to plasmid stability and culture media. Appl Microbiol Biotechnol, 2011, 91(6): 1611-1622. DOI:10.1007/s00253-011-3315-2
|
| [89] |
宋小平, 王雅洁, 蔡晶晶, 等. 基因拷贝数对重组毕赤酵母产谷氨酰胺转胺酶的影响. 生物工程学报, 2020, 36(8): 1679-1688. Song XP, Wang YJ, Cai JJ, et al. Impact of gene dosage on recombinant transglutaminase production of Pichia pastoris. Chin J Biotech, 2020, 36(8): 1679-1688 (in Chinese). |
| [90] |
Verçosa JV, Carmo EJ, Filho SA. Genetic transformation to integrate two expression cassettes into the genome of yeast Pichia pastoris. BMC Proceed, 2014, 8: 254. DOI:10.1186/1753-6561-8-S4-P254
|
| [91] |
Srinivasan P, Smolke CD. Biosynthesis of medicinal tropane alkaloids in yeast. Nature, 2020, 585(7826): 614-619.
|
| [92] |
Cai P, Gao J, Zhou Y. CRISPR-mediated genome editing in non-conventional yeasts for biotechnological applications. Microb Cell Fact, 2019, 18(1): 63-74. DOI:10.1186/s12934-019-1112-2
|
| [93] |
Weninger A, Hatzl AM, Schmid C, et al. Combinatorial optimization of CRISPR/Cas9 expression enables precision genome engineering in the methylotrophic yeast Pichia pastoris. J Biotechnol, 2016, 235: 139-149. DOI:10.1016/j.jbiotec.2016.03.027
|
| [94] |
Weninger A, Fischer JE, Raschmanova H, et al. Expanding the CRISPR/Cas9 toolkit for Pichia pastoris with efficient donor integration and alternative resistance markers. J Cell Biochem, 2018, 119(4): 3183-3198. DOI:10.1002/jcb.26474
|
| [95] |
Numamoto M, Maekawa H, Kaneko Y. Efficient genome editing by CRISPR/Cas9 with a tRNA-sgRNA fusion in the methylotrophic yeast Ogataea polymorpha. J Biosci Bioeng, 2017, 124(5): 487-492. DOI:10.1016/j.jbiosc.2017.06.001
|
| [96] |
Mikkelsen MD, Buron LD, Salomonsen B, et al. Microbial production of indolylglucosinolate through engineering of a multi-gene pathway in a versatile yeast expression platform. Metab Eng, 2012, 14(2): 104-111. DOI:10.1016/j.ymben.2012.01.006
|
| [97] |
槐强强, 贾禄强, 丁健, 等. 通过在低细胞浓度下启动甲醇诱导、优化碳/能量代谢模式促进毕赤酵母表达Monellin. 生物工程学报, 2018, 34(2): 282-293. Huai QQ, Jia LQ, Ding J, et al. Optimizing carbon/energy metabolism to enhance monellin production by Pichia pastoris. Chin J Biotech, 2018, 34(2): 282-293 (in Chinese). |
| [98] |
高教琪, 周雍进. 甲醇生物转化的机遇与挑战. 合成生物学, 2020, 1(2): 158-173. Gao JQ, Zhou YJ. Advances in methanol bio-transformation. Syn Biol J, 2020, 1(2): 158-173 (in Chinese). |
| [99] |
Rußmayer H, Buchetics M, Gruber C, et al. Systems-level organization of yeast methylotrophic lifestyle. BMC Biol, 2015, 13: 80-104. DOI:10.1186/s12915-015-0186-5
|
| [100] |
Liang S, Wang B, Pan L, et al. Comprehensive structural annotation of Pichia pastoris transcriptome and the response to various carbon sources using deep paired-end RNA sequencing. BMC Genomics, 2012, 13: 738-751. DOI:10.1186/1471-2164-13-738
|
| [101] |
Valli M, Grillitsch K, Grunwald-Gruber C, et al. A subcellular proteome atlas of the yeast Komagataella phaffii. FEMS Yeast Res, 2020, 20(1): foaa001. DOI:10.1093/femsyr/foaa001
|
| [102] |
Chung BK, Selvarasu S, Camattari A, et al. Genome-scale metabolic reconstruction and in silico analysis of methylotrophic yeast Pichia pastoris for strain improvement. Microb Cell Fact, 2010(1), 9: 50-64.
|
| [103] |
刘爽, 高教琪, 薛闯, 等. 多形汉逊酵母提高生长性能的培养基优化. 生物加工过程, 2020, 18(1): 117-126. Liu S, Gao JQ, Xue C, et al. Medium optimization for growth of Ogataea polymorpha. Chin J Bioprocess Eng, 2020, 18(1): 116-125 (in Chinese). DOI:10.3969/j.issn.1672-3678.2020.01.014 |
 2021, Vol. 37
2021, Vol. 37




