
中国科学院微生物研究所、中国微生物学会主办
文章信息
- 张香燕, 申晓林, 孙新晓, 王佳, 袁其朋
- Zhang Xiangyan, Shen Xiaolin, Sun Xinxiao, Wang Jia, Yuan Qipeng
- 甲基转移酶在微生物合成天然产物中的应用
- Application of methyltransferases in microbial synthesis of natural products
- 生物工程学报, 2021, 37(6): 1869-1886
- Chinese Journal of Biotechnology, 2021, 37(6): 1869-1886
- 10.13345/j.cjb.200742
-
文章历史
- Received: November 23, 2020
- Accepted: April 19, 2021
- Published: April 29, 2021
天然产物在人类健康中扮演着重要的角色,是吗啡、青蒿素、麻黄碱和紫杉醇等著名药物的重要来源[1],在食品、保健品、化妆品和药品等领域有着广泛的应用[2]。常见的后修饰反应包括羟基化、糖基化、甲基化和戊烯基化,在扩大天然产物分子多样性方面起着重要作用[3]。其中,S-腺苷甲硫氨酸(S-adenosy-L-methionine,SAM)依赖的甲基转移酶催化的甲基化修饰可显著改善天然产物的生物学特性,包括稳定性、溶解性和生物活性[4],是生成阿魏酸、杜鹃花素、木质素等多种重要天然产物的关键步骤。
甲基转移酶(Methyltransferases,MTs) 是一种广泛存在于植物、动物和微生物中的重要酶类[5],通常以SAM作为甲基供体,催化生物体中的甲基化反应,形成核酸、蛋白质、多糖和脂质的甲基化产物[6]。甲基化是生命体中最基本、最主要的修饰反应之一,从原核生物到真核生物,MTs通过对底物的甲基化修饰来调节细胞的生长发育、增殖分化、基因损伤的修复、基因突变、基因组印迹、基因表达调控及代谢物的合成与降解,由于其具有重要的生物学意义而被广泛研究[7]。根据甲基化的靶原子的不同,可将MTs分为氧-甲基转移酶(O-MTs)、碳-甲基转移酶(C-MTs)、氮-甲基转移酶(N-MTs)、硫-甲基转移酶(S-MTs)、无机砷甲基转移酶(Cyt19)[8]。目前报道的MTs的研究中,大多为O-MTs和N-MTs,与其他3种MTs相关的研究较少。O-MTs催化甲基供体的甲基转移到底物的氧原子上形成氧甲基化合物,动物中的O-MTs主要参与儿茶酚胺类神经递质和一些激素的生物合成[9],还将黄酮类物质甲基化降低其在体内的致癌作用[10],植物中的O-MTs通常以苯丙烷类等含羟基结构的化合物为底物,催化次生代谢产物的生物合成,进而调控植物中的多种生理过程[11]。N-MTs催化的甲基化反应在动物的脂肪组织、肝脏、癌组织等的代谢过程中起着非常重要的作用,与许多疾病的发生、发展密切相关[12],在植物中主要参与黄嘌呤核苷到咖啡碱的生物合成[13]。目前,国内外关于甲基转移酶催化应用的综述主要集中在表观遗传调控[14-15]、肿瘤及代谢性疾病的发生、诊断和治疗[16-17]、医药研发[18]等领域。合成生物学技术的不断发展为MTs的应用提供了一个全新的平台,通过将MTs应用到人工构建的微生物合成途径中,可实现多种高附加值产品的生物合成[19]。本文将重点对植物天然产物的O-甲基化修饰在微生物细胞工厂中的最新研究进展进行总结和展望。
近年来,通过在微生物中异源表达MTs,实现了苯丙烷类化合物、香料类化合物、激素和抗生素等多种重要天然产物的生物合成[20],但大多数甲基转移酶催化活性低,特异性不高,使其成为天然产物高效合成的限制性因素。针对这一问题,研究者们通过合理的蛋白质工程对甲基转移酶进行改造,增强其与底物的结合能力[21],或者采用代谢工程策略对人工构建的天然产物生物合成途径进行优化[22-23],进一步促进天然产物的生物合成。这就要求研究者们更深入地解析甲基转移酶的催化机制及其结构和功能之间的联系,而甲基转移酶的底物灵活性和庞大的种类数量为这项工作带来了一定的困难。因此,本文根据天然产物的种类对MTs在微生物合成中的应用进行综述和展望。
1 MTs在苯丙烷衍生物合成过程的应用苯丙烷代谢途径是莽草酸途径衍生的植物次生产物代谢的一条重要途径,含苯丙烷骨架的化合物基本都是由该途径直接或间接产生的,这些苯丙烷类物质对植物的细胞分化、生长发育和植物抗病性等都有密切的关联[24]。MTs催化的甲基化反应是苯丙烷类化合物及其衍生物生物合成的一部分重要反应,大多数苯丙烷类化合物含有多个羟基结构,通过MTs对羟基的甲基化修饰可以显著改善其代谢稳定性和细胞膜穿透性,将其转化成具有生理和药理活性的化合物[25]。苯丙烷类化合物的生物合成通常以葡萄糖或甘油等为简单碳源,经莽草酸途径转化为分支酸,然后顺序转化为苯丙氨酸和酪氨酸,随后在苯丙氨酸解氨酶(Phenylalanine ammonia lyase,PAL)、酪氨酸氨裂解酶(Tyrosine ammonia lyase,TAL)、肉桂酸-4-羟化酶(Cinnamic acid hydroxylase,C4H)、4-香豆酸-CoA连接酶(4-coumarate-CoA ligase,4CL) 和O-MTs等的作用下,将苯丙氨酸和酪氨酸进一步转化为咖啡酸、香豆酸、阿魏酸、芥子酸、类黄酮、花青苷等多种生物活性物质[26]。
咖啡醇、对香豆醇和松柏醇等单体醇类是生物合成木质素的非常重要的原料[27],具有抗癌[28]、抗氧化、抗炎[29]等多种生理和药理活性,研究其高效合成方法具有重要意义。2017年,Chen等通过在大肠杆菌Escherichia coli中过表达TAL、4CL、肉桂酰-CoA还原酶(Cinnamoyl-CoA reductase,CCR) 和乙醇脱氢酶6 (Alcohol dehydrogenase 6,ADH6),实现了对香豆醇的生物合成(图 1),并采用代谢工程策略将竞争途径的碳通量重定向到香豆醇合成途径,使其产量提高至501.8 mg/L。在进一步过表达大肠杆菌内源酶4-羟基丙-3-羟化酶(4-hydroxypropanoic acid 3-hydroxylase,HpaBC) 和来源于拟南芥Arabidopsis thaliana的咖啡酸氧甲基转移酶(Caffeic acid O-methyltransferase,AtCOMT)、咖啡酰-CoA氧甲基转移酶(Caffeoyl- CoA 3-O-methyltransferase,AtCCoAOMT) 后,将该途径进一步扩展,首次在微生物中实现了咖啡醇和松柏醇的生物合成。为了减少途径中副产物的产生,采用微生物共培养的策略限制了杂蛋白的活性,经代谢工程改造的最佳菌株在摇瓶中生产了854.1 mg/L咖啡醇和124.9 mg/L松柏醇[30] (图 1)。但是在该途径中,有大量咖啡酸的积累,松柏醇的合成效率受到MTs活性的限制,为了解决这个问题,未来还需通过蛋白质工程对MTs进行合理的设计改造,以期提高MTs的活性。研究表明,AtCOMT除了催化以上反应外,还可将5-羟基松柏醛转化为芥子醛,5-羟基松柏醇转化为芥子醇,5-羟基阿魏酸转化为芥子酸,进而参与S-木质素的合成[31]。

|
| 图 1 利用MTs合成木质素单体 Fig. 1 Biosynthesis of monolignols through MTs. TAL: tyrosine ammonia lyase; 4CL: p-coumarate-CoA ligase; CCR: cinnamoyl-CoA reductase; ADH6: alcohol dehydrogenase; HpaBC: 4-hydroxypropanoic acid 3-hydroxylase; AtCOMT: Arabidopsis thaliana caffeate 3-O-methyltransferase; AtCCoAOMT: Arabidopsis thaliana caffeoyl-CoA O-methyltransferase. |
| |
黄酮类化合物是来源于苯丙烷途径的一类重要的植物次生代谢产物,广泛存在于维管植物中[32],包括查尔酮、黄酮、黄酮醇、黄烷、黄烷酮、黄烷醇、异黄酮和花青素等[33-34]。大量研究表明,黄酮类化合物具有抗氧化、抗肿瘤、抗炎、抗结核、抗菌和抗病毒等多种药理活性[35],被广泛用于化妆品、保健品和药品的生产[36-37]。由于天然提取法和化学合成法生产黄酮化合物存在收率较低、操作复杂等难题,微生物合成法成为当前研究的热点[38]。黄酮类氧甲基转移酶(Flavonoid O-methyltransferases,FOMTs) 可以催化多种底物且具有高区域选择性,可以选择性地将黄酮类化合物特定位置的羟基甲基化,专一性地生成人们需要的化合物。利用FOMTs的这一特点,Willits等在大肠杆菌中过表达了6个来自薄荷Mentha piperita的O-MTs (MpOMTs),以槲皮素为底物在大肠杆菌体内进行转化实验,MpOMT1A可将底物转化成槲皮素7-甲基醚(鼠李素),利用MpOMT3将底物转化成槲皮素3′-甲基醚(异鼠李素),而MpOMT4将底物转化为槲皮素4′-甲基醚[39],该研究成功地增加了鼠李素类化合物的多样性。白杨氧甲基转移酶(Populus deltoids O-methyltransferases,PdPOMTs) 中的PdPOMT-7也有相同的特点,Kim等通过将PdPOMT-7在大肠杆菌中过表达,发现PdPOMT-7能够特异性地将类黄酮化合物的C-7羟基转化为甲氧基,与黄酮和异黄酮相比,PdPOMT-7对芹菜素、山奈酚、木犀草素和槲皮素的亲和力更高,可将其转化为相应的7-O-甲基化化合物。其中,以木犀草素和山奈酚为底物时,转化率可达80%,对芹菜素和槲皮素的转化率达到90%以上[40],该研究为生产更多种类的芹菜素、山奈酚、木犀草素和槲皮素衍生物奠定了理论基础。为了进一步提高PdPOMT-7的催化活性,Lee等利用同源建模的方法构建了PdPOMT-7的3D分子模型,检测其与辅因子SAM和槲皮素结合位点,构建PdPOMT-7 V119L突变体,该突变位点增加了该酶的疏水性,增强了该酶与底物的结合力,最终以槲皮素为底物,生产了111 mg/L鼠李素,与携带野生型PdPOMT-7的菌株相比,产量增加了6倍[41]。除了PdPOMT-7外,链霉菌7-氧-甲基转移酶(Streptomyces avermitilis 7-O-methyltransferase,SaOMT) 也可催化7位羟基甲基化。7-O-甲基香橙素(7-O-methyl- aromadendrin,7-OMA) 是类黄酮糖苷中的配基部分,具有多种医学应用。为了大量生产这种有价值的天然类黄酮,Malla等采用微生物合成法,通过在大肠杆菌中过表达4CL、CHS和查尔酮异构酶(Chalcone isomerase,CHI),将关键前体对香豆酸转化为柚皮素(Naringenin,NRN),NRN在黄烷酮-3-羟化酶的作用下其3位被羟基化,随后在SaOMT作用下,进一步被甲基化生成7-OMA (图 2)。在该途径中通过引入酰基-CoA羧化酶的α和β亚基、生物素连接酶和来自诺卡菌Nocardia farcinica的乙酰-CoA合成酶增强7-OMA的关键前体丙二酰-CoA的合成,可以进一步提高7-OMA的产量,最终添加500 μmol/L的对香豆酸时产生了30 mg/L的7-OMA[42]。在另一项研究中,Kim等通过在大肠杆菌过表达大豆氧甲基转移酶(Glycine max soybean O-methyltransferase,GmSOMT2) 和水稻柚皮素氧甲基转移酶(Oryza sativa naringenin O-methyltransferase,OsNOMT) 以及其他的合成途径关键酶,实现了樱花素(Sakurarin)和4′-甲氧基柚皮素(Ponciretin) 的生物合成,并通过干扰竞争途径,将碳通量重定向到莽草酸途径,在摇瓶中生产了42.5 mg/L的樱花素和40.1 mg/L的4′-甲氧基柚皮素(图 2)[43]。近来,Cress等发现葡萄花青素氧甲基转移酶(Vitis vinifera anthocyanin O-methyltransferase,VvAOMT1) 和仙客来花青素氧甲基转移酶(Fragrant cyclamen, ‘Kaori-no-mai’ anthocyanin O-methyltransferase,CkmOMT2) 是生产芍药素3-O-葡萄糖(Peonidin 3-O-glucoside,P3G) 最有效的O-MTs,分别将VvAOMT1和CkmOMT2与矮牵牛花青素合成酶(PhANS)、拟南芥花青素3-O-葡萄糖基转移酶(At3GT) 在大肠杆菌中共表达,P3G产量分别为2.4 mg/L和2.7 mg/L,为了进一步提高产量,利用CRISPRi下调转录抑制因子metJ,解除其对甲硫氨酸的生物合成途径的控制来提高SAM的供应,最终P3G产量达到56 mg/L[44]。
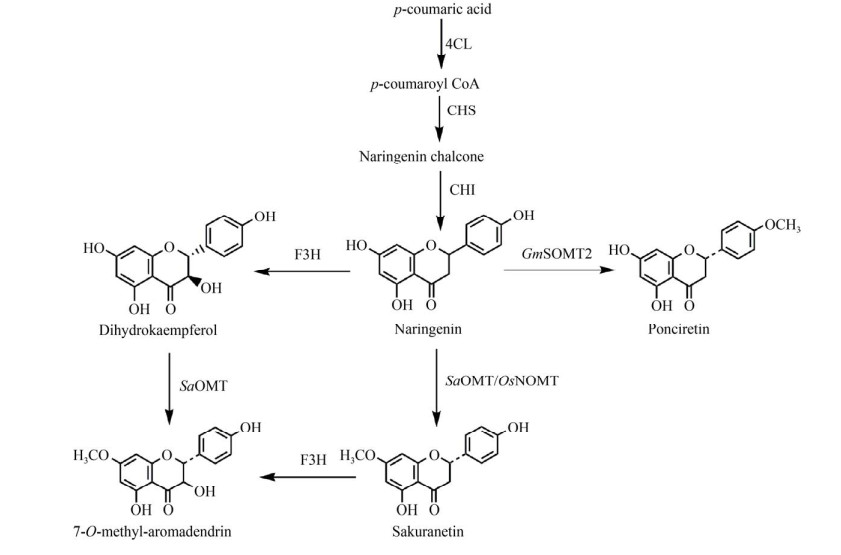
|
| 图 2 利用MTs生物合成黄酮类化合物 Fig. 2 Biosynthesis of flavonoids through MTs. 4CL: p-coumarate-CoA ligase; CHS: chalcone synthase; CHI: chalcone isomerase; F3H: flavanone-3-hydroxylase; SaOMT: Streptomyces avermitilis 7-O-methyltransferase; GmSOMT2: Glycine max O-methyltransferase; OsNOMT: Oryza sativa naringenin O-methyltransferase. |
| |
在微生物合成苯丙烷衍生物的途径中,甲基转移酶在增加产物多样性方面起了非常关键的催化作用。一方面,甲基转移酶具有宽泛的底物选择性,可以催化很多苯丙烷化合物的甲基化反应,进而增加其脂溶性,赋予其更多的生理药理活性;另一方面,大多数黄酮类甲基转移酶具有高区域选择性,不同的甲基转移酶可以将底物不同位点的羟基甲基化,通过选择合适的甲基转移酶,可以在提高目的产物产量的同时又避免了途径中副产物的生成。但是在目前的报道中,甲基转移酶的转化率还达不到人们的需求,针对这一问题,可以通过蛋白质工程构建甲基转移酶突变体,增强其与底物的结合能力,或者采用增加前体和辅助因子SAM的供应的方法,提高甲基转移酶的利用率,进一步促进产物的生成。
2 MTs在香料类化合物合成过程的应用香草醛(3-甲氧基-4-羟基苯甲醛,Vanillin) 是香精香料行业最常用的香味物质之一[45],广泛应用于食品、日化用品、医药、农业等领域[46],与人们的日常生活联系密切,市场规模也逐渐增长。目前,全球香草醛年消费量已超过20 000 t[47],市场价值超过1.8亿美元[48]。在香草醛的微生物合成途径中,通常需要MTs催化其氧甲基的形成。1958年,一种镁离子依赖型的MTs被发现具备催化邻苯二酚羟基甲基化的能力[49],随后被广泛应用于香草醛的合成途径中。Li等利用人儿茶酚-氧-甲基转移酶(Homo sapiens catechol-O- methyltransferase,HsCOMT) 在大肠杆菌中实现了香草醛的生物合成,该生物合成过程通过在大肠杆菌中转入3-脱氢莽草酸脱氢酶基因aroZ并敲除莽草酸脱氢酶基因aroE,将葡萄糖在3-脱氢莽草酸脱氢酶AroZ的作用下转化成原儿茶酸(Protocatechuic acid,PAC),后者在HsCOMT的催化下转化为香草酸,最终在芳醛脱氢酶的催化下生成香草醛。这项研究开拓了COMT在香草醛合成中的应用,但是当时技术水平有限,COMT的转化率很低,并且底物特异性不高,导致副产物异香草醛的生成[50]。2009年,Hansen等同时过表达HsCOMT和两个外源酶莽草酸脱水酶(3-dehydroshikimate dehydratase,3DSD)、芳香羧酸还原酶(Aromatic carboxylic acid reductase,ACAR),在粟酒裂殖酵母Schizosaccharomyces pombe中利用葡萄糖合成了香草醛(图 3)。但是由于菌株内源醛还原酶活性较强,香草醛被很快转化为香草醇,为了解决这一问题,他们敲除了编码乙醇脱氢酶(Alcohol dehydrogenase,ADH6) 的基因,最终在粟酒裂殖酵母中得到了65 mg/L的香草醛[51]。虽然香草醛的产量有所提升,但是由于甲基供体SAM的供应有限,COMT催化的原儿茶酸的甲基化依然是香草醛合成途径中的限速步骤。为了提高SAM的可用性,Kunjapur等敲除了编码抑制甲硫氨酸生物合成的转录调节因子metJ,同时将编码参与SAM合成途径的酶的基因metA和cysE过表达,使香草醛的产量从205 mg/L增加到272 mg/L,并通过过表达基因mtn和luxS在菌株中引入SAM再生系统,进一步将香草醛的产量提高至419 mg/L[52]。除了SAM供应不足之外,香草醛的细胞毒性是限制其高产的另一因素,为了解决这一问题,Brochado等在高产香草醛的酿酒酵母工程菌中引入了尿苷二磷酸葡萄糖醛酸转移酶(UDP-glucuronosyltransferase,UGT),将香草醛糖基化生成低毒且易溶的β-D-葡糖基香草醛(Vanillin β-D-glucoside,VG),最终通过分批发酵得到了400 mg/L的VG[53]。由于COMT具有广泛的底物谱,除了被用于生产香草醛之外,也常被用于其他香精香料的生物合成。2017年,Chen等在大肠杆菌中过表达了COMT及对羟基苯甲酸羟化酶和羧酸还原酶,实现了香草醇的从头合成,又通过对途径中基因的模块优化,最终在摇瓶中得到240.69 mg/L香草醇,这是迄今为止第一个微生物合成香草醇的研究[54]。然而,由于COMT的区域选择性不高,常常会导致途径中副产物的生成,这一特点也限制了其在香精香料化合物合成中的应用。大量的研究表明,对酶进行合理设计或定向进化,可显著改善或改变酶的对映选择性或区域选择性[55-56]。
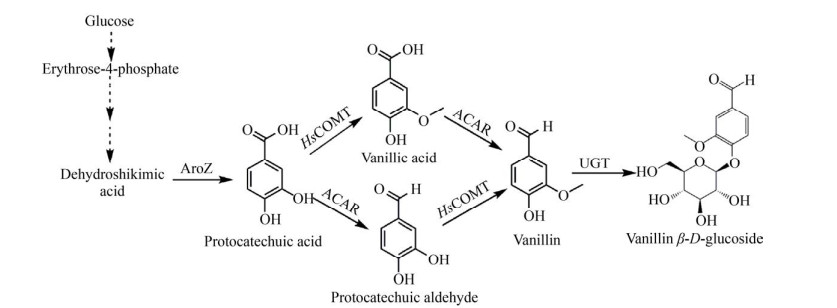
|
| 图 3 利用MTs生产β-D-葡糖基香草醛 Fig. 3 Biosynthesis of vanillin β-D-glucoside through MTs. AroZ: 3-dehydroshikimate dehydratase; ACAR: aromatic carboxylic acid reductase; HsCOMT: Homo sapiens catechol-O-methyltransferase; UGT: UDP-glucuronosyltransferase. |
| |
在香草醛的生物合成途径中,COMT除了能催化底物的间位甲基化生成香草醛之外,还会使其对位甲基化导致副产物异香草醛的生成。对COMT结构和催化机制的分析表明,COMT的区域选择性取决于其活性位点中疏水壁的协同作用[57-58]和底物特性[59-60]。含有极性和可电离基团的底物倾向于定位在酶的催化活性位点以外进入溶剂中,从而产生间甲基化产物,而具有中性基团和更多疏水性基团的底物更有可能朝向疏水性壁。根据这一机制,Law等构建了COMT Y200L突变体,该突变体表现出较高的区域选择性和催化活性,与野生型COMT相比,区域选择性从58%提高到90%,当以SAM类似物乙基腺苷甲硫氨酸为甲基供体时,Y200L以58%的转化率催化原儿茶醛生成了高价值的食品调味剂乙基香兰素[61]。
甲酯类化合物如水杨酸甲酯、苯甲酸甲酯、茉莉酸甲酯和邻氨基苯甲酸甲酯是许多植物中常见的香味成分,可作为搽剂、食品和饮料中的香精香料[62-63]。羧甲基转移酶是催化这些香味物质合成的重要酶类,通过将甲基供体SAM的甲基转移到游离的羧酸基团上促进甲酯类物质的形成。Ross等在仙女扇Clarkia breweri中发现了以同型二聚体形式存在的水杨酸羧甲基转移酶(Salicylic acid carboxyl methyltransferase,CbSAMT),该酶能将水杨酸和苯甲酸转化为对应的甲酯化合物,尤其对水杨酸的亲和力极高,但对其他结构类似的化合物没有活性[64]。类似地,Negre等分离鉴定了金鱼草Antirrhinum majus中的羧甲基转移酶AmSAMT,蛋白序列比对结果显示,AmSAMT和CbSAMT有55%的氨基酸序列同源性,在大肠杆菌表达的AmSAMT蛋白对水杨酸和苯甲酸的Km值分别为83 μmol/L和1.72 mmol/L[65],说明AmSAMT对水杨酸也有较高的亲和力。有趣的是,除了AmSAMT外,金鱼草中还含有另一种羧甲基转移酶——苯甲酸羧甲基转移酶(Antirrhinum majus benzoic acid carboxyl methyltransferase,AmBAMT),AmBAMT在花瓣表皮细胞中的活性远高于其他细胞或组织,通过将苯甲酸甲基化生成金鱼草中含量最丰富的香气成分——苯甲酸甲酯。与AmSAMT不同的是,AmBAMT对苯甲酸具有严格的底物特异性,对水杨酸等结构类似的化合物没有活性[66]。还有报道称来自拟南芥的茉莉酸羧甲基转移酶(Arabidopsis thaliana jasmonic acid carboxyl methyltransferase,AtJMT) 也是一种羧甲基转移酶,可以催化茉莉酸甲基化生成茉莉酸甲酯,其对茉莉酸的Km值为38.5 μmol/L[67]。值得注意的是,SAMT、BAMT和JMT与之前鉴定的O-MTs的氨基酸序列相似性非常低,说明它们是一种新型的MTs[66],这类羧甲基转移酶通常具有较高的底物特异性和催化活性,在甲酯类化合物的生物合成中有很大的应用潜力。除了上述3种甲酯类香味物质外,邻氨基苯甲酸甲酯(Methyl anthranilate,MA) 也是一种重要的香味物质,存在于草莓[68]、葡萄[69]、苹果[70]、玉米叶片[71]等许多植物中,赋予水果独特的香气。Pillet等阐明了草莓Fragaria x ananassa中MA的生物合成途径,鉴定出一种邻氨基苯甲酸甲基转移酶(Fragaria x ananassa anthraniic acid methyl transferase,FanAAMT) 能够以邻氨基苯甲酸(Anthranilic acid,ANT) 为直接前体,催化草莓中邻氨基苯甲酸甲酯合成的最后一步。研究表明,过表达FanAAMT的大肠杆菌在邻氨基苯甲酸存在下成功地产生了MA[72]。最近,Luo等在玉米Zea mays中鉴定出了另一种邻氨基苯甲酸甲基转移酶(Zea mays anthranilic acid methyltransferase1,ZmAAMT1),并将其分别引入到大肠杆菌和谷氨酸棒状杆菌Corynebacterium glutamicum中,构建了以葡萄糖为碳源生产MA的生物合成途径,并通过优化ZmAAMT1的表达水平,增加SAM和直接前体ANT的供应,最终在重组大肠杆菌和谷氨酸棒状杆菌中通过分批补料发酵分别生产了4.47 g/L和5.74 g/L的MA[73]。
3 MTs在激素、抗生素合成过程的应用褪黑素(Melatonin,MT),又名褪黑激素、松果体素,存在于细菌、真菌、藻类、植物、昆虫、脊椎动物等多种生物体中[74],被广泛用于治疗失眠,同时也表现出良好的抗氧化、抗炎、清除自由基等生理活性[75]。MT的生物合成通常以5-羟色胺作为前体,需要两步酶促反应,首先,血清素N-乙酰基转移酶(Serotonin N-acetyltransferase,SNAT) 将5-羟色胺催化为N-乙酰血清素(N-acetylserotonin,NAS),然后由N-乙酰血清素氧甲基转移酶(N-acetylserotonin O-methyltransferase,ASMT) 将NAS甲基化生成MT。Lee等首次鉴定并表征了来自双子叶植物拟南芥的血清素N-乙酰基转移酶AtSNAT,发现血清素可以被AtSNAT转化为N-乙酰血清素,或者被AtCOMT转化为5-甲氧基色胺(5-methoxytryptamine,5-MT),然后分别通过AtCOMT或AtSNAT顺序代谢为MT,证明了AtCOMT具有ASMT的活性[76]。在另一项研究中,Byeon等鉴定并纯化来源于水稻的COMT (OsCOMT),发现OsCOMT具有与AtCOMT相似的ASMT活性,过表达了OsCOMT的转基因水稻中MT水平升高,表明OsCOMT在植物MT的生物合成中发挥重要作用[77]。基于这个结果,他将OsCOMT代替ASMT转入大肠杆菌中,同时过表达绵羊SNAT,从5-羟色胺生产了1.46 mg/L褪黑素[78]。近年来,通过在酿酒酵母中引入编码L-色氨酸羟化酶(L-tryptophan hydroxylase,TPH)、5-羟基-L-色氨酸脱羧酶(5-hydroxy-L- tryptophan decarboxylase,DDC)、5-羟色胺乙酰转移酶(Arylalkylamine-N-acetyltransferase,AANAT)、人乙酰羟色胺氧甲基转移酶(Homo sapiens acetylserotonin O-methyltransferase,HsASMT) 等基因,实现了从简单碳源葡萄糖到MT的生物合成,经改造的最佳工程菌可以生产14.50 mg/L MT (图 4)[79]。尽管微生物合成MT的方法已经被成功构建,但O-MTs的低催化活性被认为是该途径中的限速步骤。为了解决这个问题,Wang等通过对AtCOMT合理改造,增强酶的底物结合口袋与底物NAS末端结构之间的相互作用,最终,三重突变体(C296F-Q310L-V314T) 对NAS的O-甲基化活性提高了9.5倍[21],显示出了MTs在高效合成MT中巨大的应用潜力。

|
| 图 4 利用MTs合成褪黑素 Fig. 4 Biosynthesis of melatonin through MTs. DDC: 5-hydroxy-L-tryptophan decarboxylase; AANAT: arylalkylamine-N-acetyltransferase; HsASMT: Homo sapiens acetylserotonin O-methyltransferase; TPH: L-tryptophan hydroxylase; BH4: tetrahydrobiopterin; SAM: S-adenosyl-L-methionine; SAH: S-adenosyl-L-homocysteine. |
| |
芳香族聚酮化合物是由细菌、真菌或植物产生的结构和功能最多样化的天然产物之一,由于其重要的生物学活性在临床上常被用作抗生素,具有巨大的新药开发潜力和商业价值[80]。芳香族聚酮化合物的生物合成可以分为两个阶段:首先是聚酮链的合成,通常由数个低级脂肪酸经连续的缩合反应形成聚酮长碳链;然后是对聚酮长碳链的修饰,通过糖基化、羟基化、异戊二烯化以及由MTs催化的甲基化等反应使其转化为具有生物活性的化合物[81],因此MTs在芳香族聚酮抗生素的生物合成中发挥着重要的作用。雷帕霉素(Rapamycin) 是芳香族聚酮抗生素的一种,已被批准用于治疗多种疾病,如神经胶质和神经内分泌肿瘤[82]、肾血管平滑肌脂肪瘤和乳腺癌等[83-84]。Law等发现雷帕霉素的合成需要3个具有高度区域选择性的MTs:ShRapI、ShRapM和ShRapQ,它们分别将底物39、16、27位C原子的羟基甲基化(图 5)[85]。类似地,特曲霉素C (Tetracenomycin C,Tcm C) 在淡青链霉菌Streptomyces glaucescens中的生物合成途径也包含3种MTs,分别为SgTcmN、SgTcmO和SgTcmP。SgTcmN负责将Tcm D3的C-3位甲基化生成Tcm B3,Tcm B3在SgTcmO的催化下C-8位甲基化生成Tcm E,随后SgTcmP又将Tcm E的C-9位羧基甲基化生成Tcm A2,最终在羟化酶TcmG的作用下生成Tcm C。有趣的是,SgTcmO也可将Tcm D3作为底物,将其C-8位甲基化生成8-O-甲基-Tcm D3,随后由SgTcmN在8-O-甲基-Tcm D3的C-3羟基上添加一个甲基,从而形成Tcm E,而SgTcmP也可将8-O-甲基-Tcm D3的C-9羧基甲基化生成9-羧甲基-8-O-甲基-Tcm D3 (图 5)[86]。由于这3种MTs催化的顺序不同,可以衍生出多种Tcm类似物,展现出了MTs在扩大芳香族聚酮化合物多样性方面的潜力。道诺霉素(Daunorubicin)是由波赛链霉菌Streptomyces peucetius产生的另一种芳香族聚酮抗生素,其生物合成的最后一步需要甲基转移酶SpDnrK将洋红霉素(Carminomycin) 的4位羟基甲基化(图 5)[87]。Jansson等解析了SpDnrK蛋白和底物三元复合体的晶体结构[88],构建了同时具有甲基转移酶和单加氧酶活性的SpDnrK突变体,利用该突变体,生成了一种新的蒽环类化合物[89]。聚酮霉素(Polyketomycin,POK) 是由淀粉酶产色链霉菌Streptomyces diastatochromogenes产生的四环醌糖苷,据报道有两个MTs:SdpokMT1和SdpokMT2参与了POK的生物合成[90]。SdPokMT2能够将水杨酸的C-6位甲基化生成6-甲基水杨酸,然后被CoA连接酶PokM3进一步活化,再由SdPokMT1催化6-甲基水杨酰-CoA的甲基化生成POK生物合成的关键前体3, 6-二甲基水杨酰-CoA[91]。

|
| 图 5 雷帕霉素、四霉素和柔霉素的化学结构及MTs的催化位点 Fig. 5 Chemical structures of rapamycin, tetracenomycin C and daunorubicon, and the active sites recognized by MTs. |
| |
脂肪酸甲酯(Fatty acid methyl esters,FAMEs) 是脂肪酸与甲醇发生酯化反应生成的一类脂肪酸酯[92],是生物柴油的主要成分[93]。Nawabi等鉴定了一种来自海洋分枝杆菌Mycobacterium marinum的脂肪酸甲基转移酶MmFAMT,该酶能催化游离酸和SAM形成FAMEs和3-羟基脂肪酸甲酯[94]。通过分析MmFAMT的晶体结构和催化机理,发现MmFAMT只能甲基化最长12到14个碳原子的中链脂肪酸[95]。然而,在大肠杆菌中过表达MmFAMT和脂肪酸酰基酰基载体蛋白(ACP) 硫酯酶(FATs) 时,只产生了微量的FAME和3-羟基脂肪酸甲酯[94]。为了进一步提高微生物合成FAME的产量,Sherkhanov等采用了另一种广谱MTs,即黑腹果蝇保幼激素酸O-甲基转移酶(Drosophila melanogaster juvenile hormone acid O-methyltransferase,DmJHAMT),通过将DmJHAMT转入增强SAM供应的工程化生产中链脂肪酸的大肠杆菌中,生产了0.56 g/L的中链FAME,比之前的产量提高了35倍[96],显示出了FAMT在微生物合成生物柴油中的潜力。
生物碱是自然界中天然存在的一类含氮的碱性有机化合物,由动物、细菌、真菌和植物等多种生物产生[97]。链黑菌素(Streptonigrin,STN) 最早于1959年被报道,由Rao等在一株柔毛链霉菌Streptomyces flocculus中分离得到[98],STN是一种氨基醌类生物碱,具有抗肿瘤、抗菌等生物活性[99]。徐飞等发现柔毛链霉菌中STN的生物合成基因簇中含有48个基因,其中,stnQ2编码的SAM依赖的MTs负责将色氨酸转化为甲基色氨酸,这是生物合成SNT的第一步[100]。然而,SNT确切的生物合成机制尚不清楚,这也是SNT高产的瓶颈。诺司卡品(Noscapine) 是一种苯酞异喹啉类生物碱,具有潜在的抗癌疗效。最近在罂粟Papaver somniferum中发现了一个10基因的基因簇,参与了从S-金黄紫堇碱到诺司卡品的生物合成,其中,诺司卡品的4-O甲基化步骤由PsMT2和PsMT3两个甲基转移酶催化[101]。在此基础上,Li等在酵母中构建了一条从葡萄糖合成诺司卡品的生物合成途径[102],通过将来自哺乳动物、细菌、酵母和植物的29种途径酶转入到工程宿主菌中过表达,生产了约230 ng/L的诺司卡品。为了进一步提高其产量,采用了调节酶表达水平、工程改造限速步骤、增加NADPH供应和优化发酵条件等途径优化策略,得到的最佳工程菌株能生产2.2 mg/L的诺司卡品,比原菌株增加1.8万倍[102]。
5 讨论由SAM依赖性的MTs催化的甲基化反应,是天然产物生物合成途径中最基本的反应之一[103]。近年来,人们在MTs基因信息和催化机制等方面的研究取得了很大的进步,并在此基础上,将甲基转移酶应用到人工构建的微生物工厂中,实现了苯丙烷衍生物、香精香料类、激素、抗生素等多种高附加值天然产物的生物合成(表 1)。然而,MTs的低催化活性和较差的底物专一性,是限制这些天然产物高效合成的主要因素。虽然MTs较差的底物特异性通常会导致副产物的形成,但同时也为产生具有更高生物活性的新型天然产物提供了可能。随着基因组学研究的深入,越来越多的MTs的晶体结构被解析[104-105],研究者们可以通过合理的蛋白质工程将甲基转移酶进行改造,以提高甲氧基转移酶的稳定性和底物特异性,进一步拓展其在天然产物微生物合成中的应用。此外,利用代谢工程策略提高甲基转移酶辅因子SAM的供应,实现其在微生物体内的循环利用,可以一定程度上提高甲基转移酶的转化效率。更有研究表明,除了SAM以外,许多甲基转移酶对SAM类似物也显示了良好的催化活性,可以促进一系列官能团如乙基、烯丙基等向受体底物的转移[106-107],这将极大地拓展甲基转移酶在天然产物微生物合成中的应用。
| Name | Substrate | Research content | Product | References |
| AtCOMT | Caffeic acid (alcohol) | Biosynthesis of lignin monomers | Coniferyl alcohol (124.90 mg/L) | [30] |
| N-acetylserotonin | Heterologous expression and functional identification of proteins | Melatonin | [77] | |
| N-acetylserotonin | Rational modification and heterologous expression of proteins | Melatonin | [21] | |
| AtCCoAOMT | Caffeoyl-CoA | Biosynthesis of lignin monomers | Coniferyl alcohol (124.90 mg/L) | [30] |
| MpOMTs | Flavonols | Increase the diversity of flavonoids through biological fermentation | Rhamnetin compounds | [39] |
| POMT-7 | Flavonols | Protein regioselectivity | 7-O-methyl compounds | [40] |
| Quercetin | Protein modification and biosynthesis of rhamnetin | Rhamnetin (111.00 mg/L) | [41] | |
| SaOMT | Naringenin | Biosynthesis of 7-O-methyl-aromadendrin | 7-O-methyl-aromadendrin (30.00 mg/L) |
[42] |
| GmSOMT2 | Naringenin | Biosynthesis of O-methyl flavonoids | Ponciretin (40.10 mg/L) | [43] |
| OsNOMT | Naringenin | Biosynthesis of O-methyl flavonoids | Sakuranetin (42.50 mg/L) | [43] |
| VvAOMT1 | Anthocyanidin 3-O-glucoside | Biosynthesis of peonidin 3-O-glucoside | Peonidin 3-O-glucoside (56.00 mg/L) |
[44] |
| CkmOMT2 | Anthocyanidin 3-O-glucoside | Biosynthesis of peonidin 3-O-glucoside | Peonidin 3-O-glucoside (2.70 mg/L) |
[44] |
| HsCOMT | Protocatechuic acid | Biosynthesis of vanillin | Vanillin | [50] |
| Protocatechuic aldehyde | Biosynthesis of vanillin | Vanillin (65.00 mg/L and 45.00 mg/L) | [51] | |
| Protocatechuic aldehyde | Biosynthesis of vanillin | Vanillin β-D-glucoside, (400.00 mg/L) |
[53] | |
| Protocatechuic aldehyde | Optimization of synthesis route of vanillin | Vanillin (419.00 mg/L) | [52] | |
| Protocatechuic aldehyde | Protein regioselectivity | Ethyl vanillin | [61] | |
| 3, 4-dihydroxybenzyl alcohol | Biosynthesis of vanillyl alcohol | Vanillyl alcohol (240.69 mg/L) | [54] | |
| CbSAMT | Salicylic acid and benzoic acid | Heterologous expression and functional identification of proteins | Methyl salicylate and methyl benzoate | [64] |
| AmBAMT | Benzoic acid | Heterologous expression and functional identification of proteins | Methyl benzoate | [66] |
| AtJMT | Jasmonic acid | Gene cloning and functional identification | Methyl jasmonate | [67] |
| FanAAMT | Anthranilic acid | Gene cloning and functional identification | Methyl anthranilate | [72] |
| ZmAAMT1 | Anthranilic acid | Biosynthesis of methyl anthranilate | Methyl anthranilate (4.47 g/L and 5.74 g/L) | [73] |
| OsCOMT | N-acetylserotonin | Biosynthesis of melatonin | Melatonin (1.46 mg/L) | [78] |
| HsASMT | N-acetylserotonin | Biosynthesis of melatonin | Melatonin (14.50 mg/L) | [79] |
| ShRapI ShRapM ShRapQ | Rapamycin precursor | Protein purification characterization and substrate specificity | Rapamycin | [85] |
| SgTcmN SgTcmO SgTcmP | Tetracenomycin C precursor | Protein gene expression and substrate specificity | Tetracenomycin C and its derivatives | [86] |
| SpDnrK | Carminomycin | Protein crystal structure and catalytic mechanism | Daunorubicin | [88] |
| SdPokMT1 | 6-methylsalicyloyl-CoA | Protein crystal structure and catalytic mechanism | Polyketomycin | [91] |
| MmFAMT | Free fatty acid | Biosynthesis of biodiesel | Fatty acid methyl ester | [94] |
| DmJHAMT | Free fatty acid | Biosynthesis of biodiesel | Medium chain FAME (0.56 g/L) | [96] |
| PsMT | Noscapine precursor | Heterologous expression of proteins and biosynthesis of Noscapine | Noscapine | [101] |
| [1] |
Butler MS. Natural products to drugs: natural product-derived compounds in clinical trials. Nat Product Reports, 2008, 25(3): 475-516. DOI:10.1039/b514294f
|
| [2] |
Wang J, Shen XL, Rey J, et al. Recent advances in microbial production of aromatic natural products and their derivatives. Appl Microbiol Biotechnol, 2018, 102(1): 47-61. DOI:10.1007/s00253-017-8599-4
|
| [3] |
Parajuli P, Pandey RP, Nguyen THT, et al. Substrate scope of O-methyltransferase from Streptomyces peucetius for biosynthesis of diverse natural products methoxides. Appl Biochem Biotechnol, 2017, 184(4): 1404-1420.
|
| [4] |
Yang H, Ahn JH, Ibrahim RK, et al. The three-dimensional structure of Arabidopsis thaliana O-methyltransferase predicted by homology-based modelling. J Mol Graph Modell, 2004, 23(1): 77-87. DOI:10.1016/j.jmgm.2004.02.001
|
| [5] |
Liscombe DK, Louie GV, Noel JP. Architectures, mechanisms and molecular evolution of natural product methyltransferases. Nat Prod Rep, 2012, 29(10): 1238-1250. DOI:10.1039/c2np20029e
|
| [6] |
Lee SG, Kim Y, Alpert TD, et al. Structure and reaction mechanism of phosphoethanolamine methyltransferase from the malaria parasite Plasmodium falciparum: an antiparasitic drug target. J Biol Chem, 2012, 287(2): 1426-1434. DOI:10.1074/jbc.M111.315267
|
| [7] |
Yue Y, Yuan JS, Ross J, et al. An Arabidopsis thaliana methyltransferase capable of methylating farnesoic acid. Arch Biochem Biophys, 2006, 448(1/2): 123-132.
|
| [8] |
王志刚, 吴建新. DNA甲基转移酶分类、功能及其研究进展. 遗传, 2009, 31(9): 903-912. Wang ZG, Wu JX. DNA methyltransferases: classification, functions and research progress. Hereditas, 2009, 31(9): 903-912 (in Chinese). |
| [9] |
Männistö PT, Kaakkola S. Catechol-O- methyltransferase (COMT): biochemistry, molecular biology, pharmacology, and clinical efficacy of the new selective COMT inhibitors. Pharmacol Rev, 1999, 51(4): 593-628.
|
| [10] |
Zhu BT, Ezell EL, Liehr JG. Catechol-O- methyltransferase-catalyzed rapid O-methylation of mutagenic flavonoids. Metabolic inactivation as a possible reason for their lack of carcinogenicity in vivo. J Biol Chem, 1994, 269(1): 292-299. DOI:10.1016/S0021-9258(17)42348-9
|
| [11] |
He XZ, Dixon RA. Genetic manipulation of isoflavone 7-O-methyltransferase enhances biosynthesis of 4'-O-methylated isoflavonoid phytoalexins and disease resistance in alfalfa. Plant Cell, 2000, 12(9): 1689-1702.
|
| [12] |
徐晚枫, 厉平, 李玲. 烟酰胺N-甲基转移酶的研究进展. 中国医科大学学报, 2021, 50(3): 254-257. Xu WF, Li P, Li L. Research progress on nicotinamide N-methyltransferase. J China Med Univ, 2021, 50(3): 254-257 (in Chinese). |
| [13] |
晏嫦妤, 任秋婧, 陈小芳, 等. 咖啡碱合成N-甲基转移酶研究进展. 茶叶科学, 2014, 34(6): 531-540. Yan CY, Ren QQ, Chen XF, et al. Research progress of N-methyltransferases involved in caffeine biosynthesis. J Tea Sci, 2014, 34(6): 531-540 (in Chinese). DOI:10.3969/j.issn.1000-369X.2014.06.002 |
| [14] |
刘治民, 杨芷怡, 冀凤丹, 等. 非生物胁迫下植物DNA甲基化研究进展. 生物技术通报, 2020, 36(11): 122-132. Liu ZM, Yang ZY, Ji FD, et al. Research progress of plant DNA methylation under abiotic stress. Biotechnol Bull, 2020, 36(11): 122-132 (in Chinese). |
| [15] |
张旭, 秦文, 刘佳, 等. m6A RNA甲基化修饰参与干细胞多向分化调控的研究进展. 中华口腔医学研究杂志(电子版), 2020, 14(4): 201-206. Zhang X, Qin W, Liu J, et al. Research progress of m6A RNA methylation modification involved in the regulation of stem cell multidirectional differentiation. Chin J Stomatol Res (Electr Ed), 2020, 14(4): 201-206 (in Chinese). |
| [16] |
文君, 闵雪洁, 赵丽, 等. 蛋白质精氨酸甲基转移酶在肿瘤中的作用及机制研究进展. 上海交通大学学报(医学版), 2017, 37(6): 842-846. Wen J, Min XJ, Zhao L, et al. Research progress of effect and mechanism of protein arginine methyltransferase in tumors. J Shanghai Jiaotong Univ (Med Sci), 2017, 37(6): 842-846 (in Chinese). |
| [17] |
赵敏, 肖辉, 潘之, 等. 组蛋白甲基转移酶NSD家族的研究进展. 医学综述, 2015, 21(8): 1370-1372. Zhao M, Xiao H, Pan Z, et al. Research progress of histone methyltransferase NSD. Med Recapitul, 2015, 21(8): 1370-1372 (in Chinese). DOI:10.3969/j.issn.1006-2084.2015.08.009 |
| [18] |
江芮, 吕柯孬, 潘学峰, 等. 表观遗传药物研发的现状与挑战. 生物技术通报, 2019, 35(8): 213-225. Jiang R, Lv KN, Pan XF, et al. Current status and challenges of epigenetic drug research and development. Biotechnol Bull, 2019, 35(8): 213-225 (in Chinese). |
| [19] |
Jia B, Li BZ, Yuan YJ. Prospects of synthetic biology. Sci Sin Chim, 2014, 44(9): 1455-1461. DOI:10.1360/N032014-00160
|
| [20] |
Sun XX, Shen XL, Jain R, et al. Synthesis of chemicals by metabolic engineering of microbes. Chem Soc Rew, 2015, 46(30): 3760-3785.
|
| [21] |
Wang WY, Su SS, Wang SZ, et al. Significantly improved catalytic efficiency of caffeic acid O-methyltransferase towards N-acetylserotonin by strengthening its interactions with the unnatural substrate's terminal structure. Enzyme Microb Technol, 2019, 125: 1-5. DOI:10.1016/j.enzmictec.2019.02.005
|
| [22] |
Lee D, Park HL, Lee SW, et al. Biotechnological production of dimethoxyflavonoids using a fusion flavonoid O-methyltransferase possessing both 3'- and 7-O-methyltransferase activities. J Nat Prod, 2017, 80(5): 1467-1474. DOI:10.1021/acs.jnatprod.6b01164
|
| [23] |
Berim A, Gang DR. Production of methoxylated flavonoids in yeast using ring a hydroxylases and flavonoid O-methyltransferases from sweet basil. Appl Microbiol Biotechnol, 2018, 102(13): 5585-5598. DOI:10.1007/s00253-018-9043-0
|
| [24] |
Camm EL, Towers GHN. Phenylalanine ammonia lyase. Phytochemistry, 1973, 12(5): 961-973. DOI:10.1016/0031-9422(73)85001-0
|
| [25] |
Cao H, Jing XH, Wu DH, et al. Methylation of genistein and kaempferol improves their affinities for proteins. Int J Food Sci Nutrit, 2013, 64(4): 437-443. DOI:10.3109/09637486.2012.759186
|
| [26] |
Weisshaar B, Jenkins GI. Phenylpropanoid biosynthesis and its regulation. Curr Opin Plant Biol, 1998, 1(3): 251-257. DOI:10.1016/S1369-5266(98)80113-1
|
| [27] |
Quideau S, Ralph J. Facile large-scale synthesis of coniferyl, sinapyl, and p-coumaryl alcohol. J Agric Food Chem, 1992, 40(7): 1108-1110. DOI:10.1021/jf00019a003
|
| [28] |
Gažák R, Walterová D, Křen V. Silybin and silymarin——new and emerging applications in medicine. Curr Med Chem, 2007, 14(3): 315-338. DOI:10.2174/092986707779941159
|
| [29] |
Cefarelli G, D'Abrosca B, Fiorentino A, et al. Isolation, characterization, and antioxidant activity of E- And Z-p-coumaryl fatty acid esters from cv. Annurca apple fruits. J Agric Food Chem, 2005, 53(9): 3525-3529. DOI:10.1021/jf047838g
|
| [30] |
Chen ZY, Sun XX, Li Y, et al. Metabolic engineering of Escherichia coli for microbial synthesis of monolignols. Metab Eng, 2017, 39: 102-109. DOI:10.1016/j.ymben.2016.10.021
|
| [31] |
李波, 倪志勇, 王娟, 等. 木质素生物合成关键酶咖啡酸-O-甲基转移酶基因(COMT)的研究进展. 分子植物育种, 2010, 8(1): 117-124. Li B, Ni ZY, Wang J, et al. Advances on key enzyme gene (COMT) involved in lignin biosynthesis. Mol Plant Breed, 2010, 8(1): 117-124 (in Chinese). |
| [32] |
Ververidis F, Trantas E, Douglas C, et al. Biotechnology of flavonoids and other phenylpropanoid-derived natural products. Part Ⅱ: Reconstruction of multienzyme pathways in plants and microbes. Biotechnol J, 2007, 2(10): 1235-1249. DOI:10.1002/biot.200700184
|
| [33] |
Hillis WE. Biosynthesis of flavonoids. Nature, 1960, 186(4725): 635.
|
| [34] |
Tahara S. A journey of twenty-five years through the ecological biochemistry of flavonoids. Biosci Biotechnol Biochem, 2007, 71(6): 1387-1404. DOI:10.1271/bbb.70028
|
| [35] |
Harborne JB, Williams CA. Advances in flavonoid research since 1992. Phytochemistry, 2000, 55(6): 481-504. DOI:10.1016/S0031-9422(00)00235-1
|
| [36] |
Di Carlo G, Mascolo N, Izzo AA, et al. Flavonoids: old and new aspects of a class of natural therapeutic drugs. Life Sci, 1999, 65(4): 337-353. DOI:10.1016/S0024-3205(99)00120-4
|
| [37] |
Hollman PCH, Arts ICW. Flavonols, flavones and flavanols——nature, occurrence and dietary burden. J Sci Food Agric, 2000, 80(7): 1081-1093. DOI:10.1002/(SICI)1097-0010(20000515)80:7<1081::AID-JSFA566>3.0.CO;2-G
|
| [38] |
Xu P, Marsafari M, Zha J, et al. Microbial coculture for flavonoid synthesis. Trends Biotechnol, 2020, 38(7): 686-688. DOI:10.1016/j.tibtech.2020.01.008
|
| [39] |
Willits MG, Giovanni M, Prata RTN, et al. Bio-fermentation of modified flavonoids: an example of in vivo diversification of secondary metabolites. Phytochemistry, 2004, 65(1): 31-41. DOI:10.1016/j.phytochem.2003.10.005
|
| [40] |
Kim BG, Kim H, Hur HG, et al. Regioselectivity of 7-O-methyltransferase of poplar to flavones. J Biotechnol, 2006, 126(2): 241-247. DOI:10.1016/j.jbiotec.2006.04.019
|
| [41] |
Lee S, Shin SY, Lee Y, et al. Rhamnetin production based on the rational design of the poplar O-methyltransferase enzyme and its biological activities. Bioorgan Med Chem Lett, 2011, 21(13): 3866-3870. DOI:10.1016/j.bmcl.2011.05.043
|
| [42] |
Malla S, Koffas MAG, Kazlauskas RJ, et al. Production of 7-O-methyl aromadendrin, a medicinally valuable flavonoid, in Escherichia coli. Appl Environ Microbiol, 2012, 78(3): 684-694. DOI:10.1128/AEM.06274-11
|
| [43] |
Kim MJ, Kim BG, Ahn JH. Biosynthesis of bioactive O-methylated flavonoids in Escherichia coli. Appl Microbiol Biotechnol, 2013, 97(16): 7195-7204. DOI:10.1007/s00253-013-5020-9
|
| [44] |
Cress BF, Leitz QD, Kim DC, et al. CRISPRi-mediated metabolic engineering of E. coli for O-methylated anthocyanin production. Microb Cell Factor, 2017, 16: 10. DOI:10.1186/s12934-016-0623-3
|
| [45] |
Priefert H, Rabenhorst J, Steinbüchel A. Biotechnological production of vanillin. Appl Microbiol Biotechnol, 2001, 56(3/4): 296-314.
|
| [46] |
Benz I, Muheim A. Biotechnological production of vanillin. Flavour Science, 1996, 111-117.
|
| [47] |
Krings U, Berger RG. Biotechnological production of flavours and fragrances. Appl Microbiol Biotechnol, 1998, 49(1): 1-8. DOI:10.1007/s002530051129
|
| [48] |
Li T, Rosazza JPN. Biocatalytic synthesis of vanillin. Appl Environ Microbiol, 2000, 66(2): 684-687. DOI:10.1128/AEM.66.2.684-687.2000
|
| [49] |
Axelrod J, Senoh S, Witkop B. O-methylation of catechol amines in vivo. J Biol Chem, 1958, 233(3): 697-701. DOI:10.1016/S0021-9258(18)64730-1
|
| [50] |
Li K, Frost JW. Synthesis of vanillin from glucose. J Am Chem Soc, 1998, 120(40): 10545-10546. DOI:10.1021/ja9817747
|
| [51] |
Hansen EH, Møller BL, Kock GR, et al. De novo biosynthesis of vanillin in Fission Yeast (Schizosaccharomyces pombe) and Baker's Yeast (Saccharomyces cerevisiae). Appl Environ Microbiol, 2009, 75(9): 2765-2774. DOI:10.1128/AEM.02681-08
|
| [52] |
Kunjapur AM, Hyun JC, Prather KLJ. Deregulation of S-adenosylmethionine biosynthesis and regeneration improves methylation in the E. coli de novo vanillin biosynthesis pathway. Microb Cell Factor, 2016, 15: 61. DOI:10.1186/s12934-016-0459-x
|
| [53] |
Brochado AR, Patil KR. Overexpression of O-methyltransferase leads to improved vanillin production in baker's yeast only when complemented with model-guided network engineering. Biotechnol Bioeng, 2013, 110(2): 656-659. DOI:10.1002/bit.24731
|
| [54] |
Chen ZY, Shen XL, Wang J, et al. Establishing an artificial pathway for de novo biosynthesis of vanillyl alcohol in Escherichia coli. ACS Synth Biol, 2017, 6(9): 1784-1792. DOI:10.1021/acssynbio.7b00129
|
| [55] |
Bornscheuer UT, Huisman GW, Kazlauskas RJ, et al. Engineering the third wave of biocatalysis. Nature, 2012, 485(7397): 185-194. DOI:10.1038/nature11117
|
| [56] |
Jaeger KE, Eggert T. Enantioselective biocatalysis optimized by directed evolution. Curr Opin Biotechnol, 2004, 15(4): 305-313. DOI:10.1016/j.copbio.2004.06.007
|
| [57] |
Vidgren J, Svensson LA, Liljas A. Crystal structure of catechol O-methyltransferase. Nature, 1994, 368(6469): 354-358. DOI:10.1038/368354a0
|
| [58] |
Palma PN, Rodrigues ML, Archer M, et al. Comparative study of ortho- and meta-nitrated inhibitors of catechol-O-methyltransferase: interactions with the active site and regioselectivity of O-methylation. Mol Pharmacol, 2006, 70(1): 143-153. DOI:10.1124/mol.106.023119
|
| [59] |
Creveling CR, Dalgard N, Shimizu HE, et al. Catechol O-methyltransferase. 3. M- and p-O-methylation of catecholamines and their metabolites. Mol Pharmacol, 1970, 6(6): 691-696.
|
| [60] |
Creveling CR, Morris N, Shimizu H, et al. Catechol O-methyltransferase. Ⅳ. Factors affecting m- and p-methylation of substituted catechols. Mol Pharmacol, 1972, 8(4): 398-409.
|
| [61] |
Law BJC, Bennett MR, Thompson ML, et al. Effects of active-site modification and quaternary structure on the regioselectivity of catechol-O-methyltransferase. Angew Chem, 2016, 128(8): 2733-2737. DOI:10.1002/ange.201508287
|
| [62] |
Raguso RA, Light DM. Electroantennogram responses of male Sphinx perelegans hawkmoths to floral and 'green-leaf volatiles. Entomol Exp Appl, 1998, 86(3): 287-293. DOI:10.1046/j.1570-7458.1998.00291.x
|
| [63] |
Raguso RA, Light DM, Pickersky E. Electroantennogram responses of Hyles lineata (Sphingidae: Lepidoptera) to volatile compounds from Clarkia breweri (Onagraceae) and other moth-pollinated flowers. J Chem Ecol, 1996, 22(10): 1735-1766. DOI:10.1007/BF02028502
|
| [64] |
Ross JR, Nam KH, D'auria JC, et al. S-adenosyl-L-methionine: salicylic acid carboxyl methyltransferase, an enzyme involved in floral scent production and plant defense, represents a new class of plant methyltransferases. Arch Biochem Biophys, 1999, 367(1): 9-16. DOI:10.1006/abbi.1999.1255
|
| [65] |
Negre F, Kolosova N, Knoll J, et al. Novel S-adenosyl-L-methionine: salicylic acid carboxyl methyltransferase, an enzyme responsible for biosynthesis of methyl salicylate and methyl benzoate, is not involved in floral scent production in snapdragon flowers. Arch Biochem Biophys, 2002, 406(2): 261-270. DOI:10.1016/S0003-9861(02)00458-7
|
| [66] |
Murfitt LM, Kolosova N, Mann CJ, et al. Purification and characterization of S-adenosyl-l- methionine: benzoic acid carboxyl methyltransferase, the enzyme responsible for biosynthesis of the volatile ester methyl benzoate in flowers of Antirrhinum majus. Arch Biochem Biophys, 2000, 382(1): 145-151. DOI:10.1006/abbi.2000.2008
|
| [67] |
Seo HS, Song JT, Cheong JJ, et al. Jasmonic acid carboxyl methyltransferase: a key enzyme for jasmonate-regulated plant responses. Proc Natl Acad Sci USA, 2001, 98(8): 4788-4793. DOI:10.1073/pnas.081557298
|
| [68] |
Pyysalo T, Honkanen E, Hirvi T. Volatiles of wild strawberries, Fragaria Vesca L., compared to those of cultivated berries, Fragaria .times. Ananassa cv senga sengana. Agric Food Chem, 1979, 27(1): 19-22. DOI:10.1021/jf60221a042
|
| [69] |
Wang JH, De Luca V. The biosynthesis and regulation of biosynthesis of concord grape fruit esters, including 'foxy' methylanthranilate. Plant J, 2005, 44(4): 606-619. DOI:10.1111/j.1365-313X.2005.02552.x
|
| [70] |
Krokida MK, Philippopoulos C. Volatility of apples during air and freeze drying. J Food Eng, 2005, 73(2): 135-141.
|
| [71] |
Köllner TG, Lenk C, Zhao N, et al. Herbivore-induced SABATH methyltransferases of maize that methylate anthranilic acid using S-adenosyl-L-methionine. Plant Physiol, 2010, 153(4): 1795-1807. DOI:10.1104/pp.110.158360
|
| [72] |
Pillet J, Chambers AH, Barbey C, et al. Identification of a methyltransferase catalyzing the final step of methyl anthranilate synthesis in cultivated strawberry. BMC Plant Biol, 2017, 17: 147. DOI:10.1186/s12870-017-1088-1
|
| [73] |
Luo ZW, Cho JS, Lee SY. Microbial production of methyl anthranilate, a grape flavor compound. Proc Natl Acad Sci USA, 2019, 116(22): 10749-10756. DOI:10.1073/pnas.1903875116
|
| [74] |
Tan DX, Manchester LC, Hardeland R, et al. Melatonin: a hormone, a tissue factor, an autocoid, a paracoid, and an antioxidant vitamin. J Pineal Res, 2010, 34(1): 75-78.
|
| [75] |
李晓艳, 张卯年. 褪黑素的抗氧化作用及其在眼部疾病应用的研究进展. 国际眼科杂志, 2008, 8(8): 1657-1660. Li XY, Zhang MN. Antioxidation of melatonin and its research advance in ocular disease. Int J Ophthalmol, 2008, 8(8): 1657-1660 (in Chinese). |
| [76] |
Lee HY, Byeon Y, Lee K, et al. Cloning of Arabidopsis serotonin N-acetyltransferase and its role with caffeic acid O-methyltransferase in the biosynthesis of melatonin in vitro despite their different subcellular localizations. J Pineal Res, 2014, 57(4): 418-426. DOI:10.1111/jpi.12181
|
| [77] |
Byeon Y, Choi GH, Lee HY, et al. Melatonin biosynthesis requires N-acetylserotonin methyltransferase activity of caffeic acid O-methyltransferase in rice. J Exp Bot, 2015, 66(21): 6917-6925. DOI:10.1093/jxb/erv396
|
| [78] |
Byeon Y, Back K. Melatonin production in Escherichia coli by dual expression of serotonin N-acetyltransferase and caffeic acid O-methyltransferase. Appl Microbiol Biotechnol, 2016, 100(15): 6683-6691. DOI:10.1007/s00253-016-7458-z
|
| [79] |
Germann SM, Jacobsen SAB, Schneider K, et al. Glucose-based microbial production of the hormone melatonin in yeast Saccharomyces cerevisiae. Biotechnol J Healthcare Nutr Technol, 2016, 11(5): 717-724.
|
| [80] |
Bentley R, Bennett JW. Constructing polyketides: from collie to combinatorial biosynthesis. Ann Rev Microbiol, 1999, 53: 411-446. DOI:10.1146/annurev.micro.53.1.411
|
| [81] |
黄惠娟, 乔建军. 聚酮类抗生素组合生物合成. 细胞生物学杂志, 2007, 29(5): 692-696. Huang HJ, Qiao JJ. The combinatorial biosynthesis of polyketide. Chin J Cell Biol, 2007, 29(5): 692-696 (in Chinese). |
| [82] |
Luan FL, Ding RC, Sharma VK, et al. Rapamycin is an effective inhibitor of human renal cancer metastasis. Kidney Int, 2003, 63(3): 917-926. DOI:10.1046/j.1523-1755.2003.00805.x
|
| [83] |
Smolewski P. Recent developments in targeting the mammalian target of rapamycin (mTOR) kinase pathway. Anti-Cancer Drugs, 2006, 17(5): 487-494. DOI:10.1097/00001813-200606000-00001
|
| [84] |
Récher C, Beyne-Rauzy O, Demur C, et al. Antileukemic activity of rapamycin in acute myeloid leukemia. Blood, 2005, 105(6): 2527-2534. DOI:10.1182/blood-2004-06-2494
|
| [85] |
Law BJC, Struck AW, Bennett MR, et al. Site-specific bioalkylation of rapamycin by the RapM 16-O-methyltransferase. Chem Sci, 2015, 6(5): 2885-2892. DOI:10.1039/C5SC00164A
|
| [86] |
Decker H, Motamedi H, Hutchinson CR. Nucleotide sequences and heterologous expression of tcmG and tcmP, biosynthetic genes for tetracenomycin C in Streptomyces glaucescens. J Bacteriol, 1993, 175(12): 3876-3886. DOI:10.1128/jb.175.12.3876-3886.1993
|
| [87] |
Madduri K, Torti F, Colombo AL, et al. Cloning and sequencing of a gene encoding carminomycin 4-O-methyltransferase from Streptomyces peucetius and its expression in Escherichia coli. J Bacteriol, 1993, 175(12): 3900-3904. DOI:10.1128/jb.175.12.3900-3904.1993
|
| [88] |
Jansson A, Koskiniemi H, Mäntsälä P, et al. Crystal structure of a ternary complex of DnrK, a methyltransferase in daunorubicin biosynthesis, with bound products. J Biol Chem, 2004, 279(39): 41149-41156. DOI:10.1074/jbc.M407081200
|
| [89] |
Metsä-Ketelä M. Evolution inspired engineering of antibiotic biosynthesis enzymes. Organ Biomol Chem, 2017, 15(19): 4036-4041. DOI:10.1039/C7OB00189D
|
| [90] |
Daum M, Daum I, Linnenbrink A, et al. Organisation of the biosynthetic gene cluster and tailoring enzymes in the biosynthesis of the tetracyclic quinone glycoside antibiotic polyketomycin. Chem Bio Chem, 2009, 10(6): 1073-1083. DOI:10.1002/cbic.200800823
|
| [91] |
Guo X, Crnovcic I, Chang CY, et al. PokMT1 from the polyketomycin biosynthetic machinery of Streptomyces diastatochromogenes Tü6028 belongs to the emerging family of C-methyltransferases that act on CoA-activated aromatic substrates. Biochemistry, 2018, 57(6): 1003-1011. DOI:10.1021/acs.biochem.7b01219
|
| [92] |
Atadashi IM, Aroua MK, Aziz AA. High quality biodiesel and its diesel engine application: A review. Renew Sustain Energy Rev, 2010, 14(7): 1999-2008. DOI:10.1016/j.rser.2010.03.020
|
| [93] |
Tong DM, Hu CW, Jiang KH, et al. Cetane number prediction of biodiesel from the composition of the fatty acid methyl esters. J Am Oil Chem Soc, 2011, 88(3): 415-423. DOI:10.1007/s11746-010-1672-0
|
| [94] |
Nawabi P, Bauer S, Kyrpides N, et al. Engineering Escherichia coli for biodiesel production utilizing a bacterial fatty acid methyltransferase. Appl Environ Microbiol, 2011, 77(22): 8052-8061. DOI:10.1128/AEM.05046-11
|
| [95] |
Petronikolou N, Nair SK. Biochemical studies of mycobacterial fatty acid methyltransferase: a catalyst for the enzymatic production of biodiesel. Chem Biol, 2015, 22(11): 1480-1490. DOI:10.1016/j.chembiol.2015.09.011
|
| [96] |
Sherkhanov S, Korman TP, Clarke SG, et al. Production of FAME biodiesel in E. coli by direct methylation with an insect enzyme. Sci Rep, 2016, 6: 24239. DOI:10.1038/srep24239
|
| [97] |
李杨, 左国营. 生物碱类化合物抗菌活性研究进展. 中草药, 2010, 41(6): 1006-1014. Li Y, Zuo GY. Advances in studies on antimicrobial activities of alkaloids. Chin Tradit Herbal Drugs, 2010, 41(6): 1006-1014 (in Chinese). |
| [98] |
Rao KV, Cullen WP. Streptonigrin, an antitumor substance. Ⅰ. Isolation and characterization. Antibiot Annu, 1959, 7: 950-953.
|
| [99] |
Cone R, Hasan SK, Lown JW, et al. The mechanism of the degradation of DNA by streptonigrin. Canad J Biochem, 1976, 54(3): 219-223. DOI:10.1139/o76-034
|
| [100] |
徐飞. 抗肿瘤抗生素链黑菌素生物合成机制研究[D]. 上海: 上海交通大学, 2013. Xu F. Research on the biosynthetic mechanism of streptonigrin, an antitumor antibiotc[D]. Shanghai: Shanghai Jiao Tong University, 2013 (in Chinese). |
| [101] |
Li YR, Smolke CD. Engineering biosynthesis of the anticancer alkaloid noscapine in yeast. Nat Commun, 2016, 7: 12137. DOI:10.1038/ncomms12137
|
| [102] |
Li YR, Li SJ, Thodey K, et al. Complete biosynthesis of noscapine and halogenated alkaloids in yeast. Proc Natl Acad Sci USA, 2018, 115(17): E3922-E3931. DOI:10.1073/pnas.1721469115
|
| [103] |
Martin JL, McMillan FM. SAM (dependent) I AM: the S-adenosylmethionine-dependent methyltransferase fold. Curr Opin Struct Biol, 2002, 12(6): 783-793. DOI:10.1016/S0959-440X(02)00391-3
|
| [104] |
Zubieta C, Ross JR, Koscheski P, et al. Structural basis for substrate recognition in the salicylic acid carboxyl methyltransferase family. Plant Cell, 2003, 15(8): 1704-1716. DOI:10.1105/tpc.014548
|
| [105] |
Dinet V, Girard-Naud N, Voisin P, et al. Melatoninergic differentiation of retinal photoreceptors: Activation of the chicken hydroxyindole-O- methyltransferase promoter requires a homeodomain- binding element that interacts with Otx2. Exp Eye Res, 2006, 83(2): 276-290. DOI:10.1016/j.exer.2005.12.011
|
| [106] |
Singh S, Zhang JJ, Huber TD, et al. Facile chemoenzymatic strategies for the synthesis and utilization of S-adenosyl-L-methionine analogues. Angew Chem Int Ed, 2014, 53(15): 3965-3969. DOI:10.1002/anie.201308272
|
| [107] |
Bennett MR, Shepherd SA, Cronin VA, et al. Recent advances in methyltransferase biocatalysis. Curr Opin Chem Biol, 2017, 37: 97-106. DOI:10.1016/j.cbpa.2017.01.020
|
 2021, Vol. 37
2021, Vol. 37




