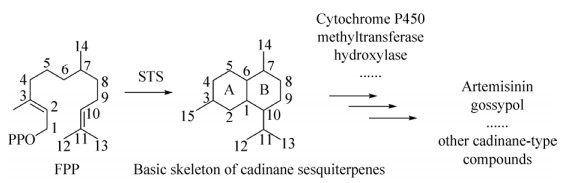
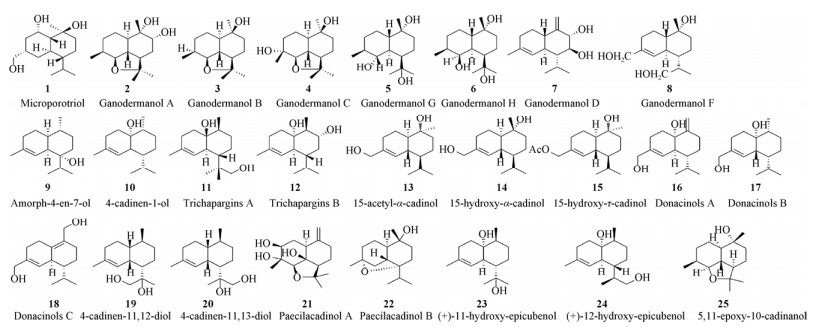
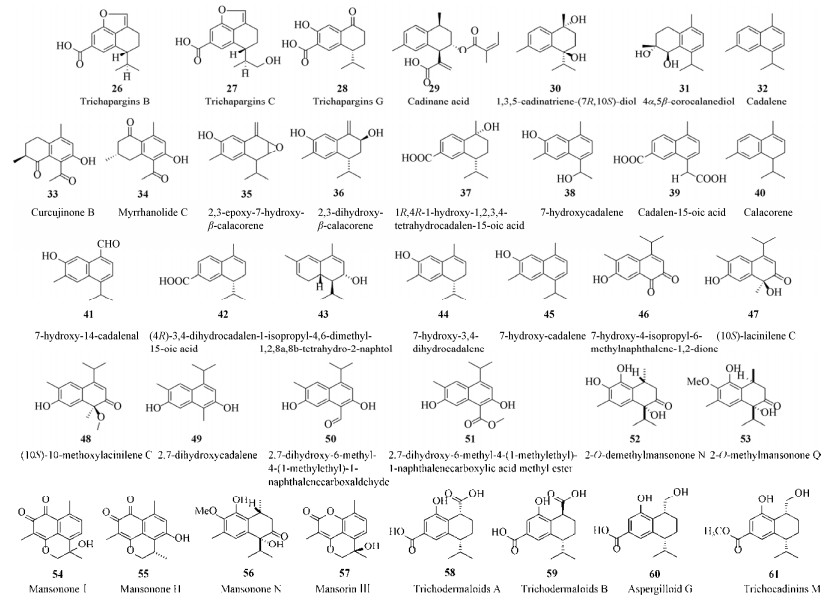
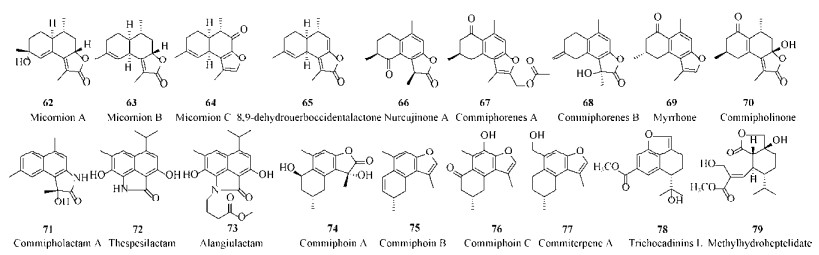
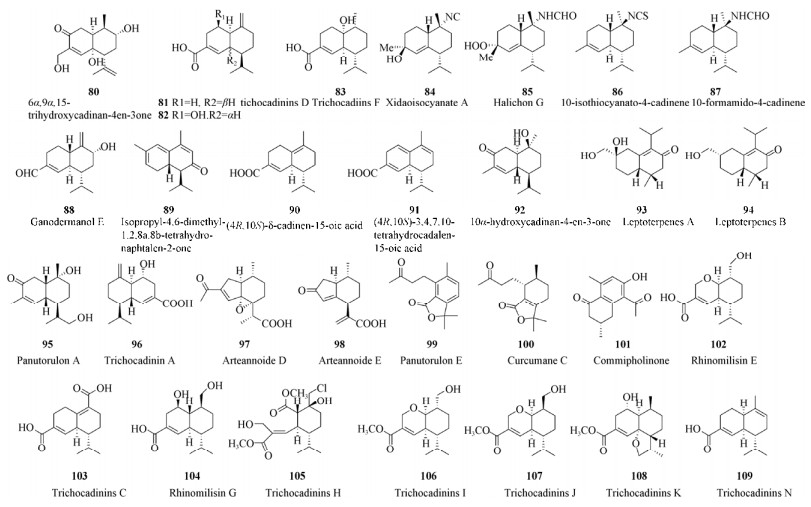
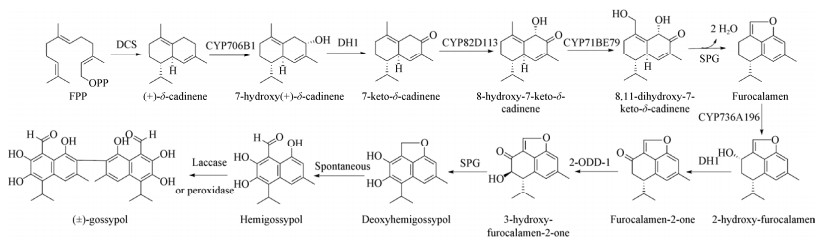
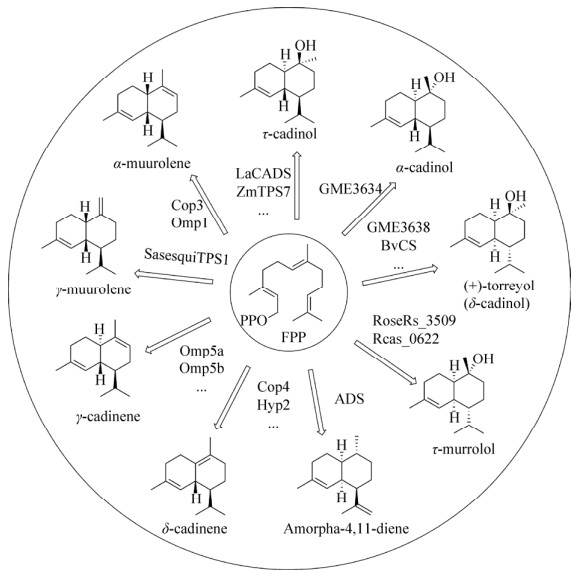
| [1] |
王飞生, 文媛, 龙高峰. 香根草杜松烷型倍半萜成分分析. 安徽农业科学, 2009, 37(12): 5636-5638. Wang FS, Wen Y, Long GF. Analysis on cadinane sesquiterpenoidnene composition in Vetiveria zizanioides. J Anhui Agric Sci, 2009, 37(12): 5636-5638 (in Chinese). DOI:10.3969/j.issn.0517-6611.2009.12.011
|
|
| [2] |
Shang ZC, Han C, Xu JL, et al. Twelve formyl phloroglucinol meroterpenoids from the leaves of Eucalyptus robusta. Phytochemistry, 2019, 163: 111-117. DOI:10.1016/j.phytochem.2019.04.008
|
|
| [3] |
Qin DP, Pan DB, Xiao W, et al. Dimeric cadinane sesquiterpenoid derivatives from Artemisia annua. Org Lett, 2018, 20(2): 453-456. DOI:10.1021/acs.orglett.7b03796
|
|
| [4] |
Zhou CX, Zhang LS, Chen FF, et al. Terpenoids from Curcuma wenyujin increased glucose consumption on HepG2 cells. Fitoterapia, 2017, 121: 141-145. DOI:10.1016/j.fitote.2017.06.011
|
|
| [5] |
Zhu SS, Qin DP, Wang SX, et al. Commipholactam A, a cytotoxic sesquiterpenoidal lactam from Resina commiphora. Fitoterapia, 2019, 134: 382-388. DOI:10.1016/j.fitote.2019.03.008
|
|
| [6] |
Paddon CJ, Westfall PJ, Pitera DJ, et al. High-level semi-synthetic production of the potent antimalarial artemisinin. Nature, 2013, 496(7446): 528-532. DOI:10.1038/nature12051
|
|
| [7] |
Czechowski T, Weathers PJ, Brodelius PE, et al. Editorial: artemisinin-from traditional Chinese medicine to artemisinin combination therapies; four decades of research on the biochemistry, physiology, and breeding of Artemisia annua. Front Plant Sci, 2020, 11: 594565. DOI:10.3389/fpls.2020.594565
|
|
| [8] |
Huang JQ, Fang X, Tian X, et al. Aromatization of natural products by a specialized detoxification enzyme. Nat Chem Biol, 2020, 16(3): 250-256. DOI:10.1038/s41589-019-0446-8
|
|
| [9] |
Gennadios HA, Gonzalez V, Di Costanzo L, et al. Crystal structure of (+)-δ-cadinene synthase from Gossypium arboreum and evolutionary divergence of metal binding motifs for catalysis. Biochemistry, 2009, 48(26): 6175-6183. DOI:10.1021/bi900483b
|
|
| [10] | |
|
| [11] | |
|
| [12] | |
|
| [13] | |
|
| [14] | |
|
| [15] | |
|
| [16] | |
|
| [17] | |
|
| [18] | |
|
| [19] | |
|
| [20] | |
|
| [21] | |
|
| [22] | |
|
| [23] | |
|
| [24] | |
|
| [25] | |
|
| [26] | |
|
| [27] | |
|
| [28] | |
|
| [29] | |
|
| [30] |
Fraga BM. Natural sesquiterpenoids. Nat Prod Rep, 1994, 11(5): 533-554.
|
|
| [31] | |
|
| [32] | |
|
| [33] | |
|
| [34] | |
|
| [35] | |
|
| [36] | |
|
| [37] |
Fraga BM. Natural sesquiterpenoids. Nat Prod Rep, 1987, 4(5): 473-498.
|
|
| [38] |
Fraga BM. Natural sesquiterpenoids. Nat Prod Rep, 1986, 3(3): 273-296.
|
|
| [39] | |
|
| [40] | |
|
| [41] |
Zhao ZZ, Liu JK, Chen HP. Microporotriol, a new cadinane-type sesquiterpenoid from the cultures of the wood-decay fungus Microporus affinis HFG829. Nat Prod Res, 2020, 34(15): 2194-2201. DOI:10.1080/14786419.2019.1582038
|
|
| [42] | |
|
| [43] | |
|
| [44] |
Sun YY, Xing JZ, Zhang JS, et al. Sesquiterpenoids with antialgal activity against the common red tide microalgae from marine macroalga Porphyra yezoensis. Environ Sci Pollut Res Int, 2018, 25(8): 7844-7859. DOI:10.1007/s11356-017-0958-2
|
|
| [45] |
Tang B, Du X, Long HN, et al. Two new cadinane-type sesquiterpenes from cultures of the basidiomycete Trichaptum pargamenum. Nat Prod Res, 2017, 31(20): 2454-2458. DOI:10.1080/14786419.2017.1312390
|
|
| [46] |
Dibwe DF, Sun S, Ueda JY, et al. Discovery of potential antiausterity agents from the Japanese cypress Chamaecyparis obtusa. Bioorg Med Chem Lett, 2017, 27(21): 4898-4903. DOI:10.1016/j.bmcl.2017.09.034
|
|
| [47] | |
|
| [48] |
Song YP, Miao FP, Liang XR, et al. Harziane and cadinane terpenoids from the alga-endophytic fungus Trichoderma asperellum A-YMD-9-2. Phytochem Lett, 2019, 32: 38-41. DOI:10.1016/j.phytol.2019.05.001
|
|
| [49] |
Xu K, Zhou Q, Li XQ, et al. Cadinane- and drimane-type sesquiterpenoids produced by Paecilomyces sp. TE-540, an endophyte from Nicotiana tabacum L., are acetylcholinesterase inhibitors. Bioorg Chem, 2020, 104: 104252. DOI:10.1016/j.bioorg.2020.104252
|
|
| [50] | |
|
| [51] |
Shi XS, Meng LH, Li XM, et al. Trichocadinins B-G: antimicrobial cadinane sesquiterpenes from Trichoderma virens QA-8, an endophytic fungus obtained from the medicinal plant Artemisia argyi. J Nat Prod, 2019, 82(9): 2470-2476. DOI:10.1021/acs.jnatprod.9b00139
|
|
| [52] |
De Mieri M, Monteleone G, Ismajili I, et al. Antiprotozoal activity-based profiling of a dichloromethane extract from Anthemis nobilis flowers. J Nat Prod, 2017, 80(2): 459-470. DOI:10.1021/acs.jnatprod.6b00980
|
|
| [53] | |
|
| [54] |
Hu BY, Qin DP, Wang SX, et al. Novel terpenoids with potent cytotoxic activities from Resina commiphora. Molecules, 2018, 23(12): E3239. DOI:10.3390/molecules23123239
|
|
| [55] |
Rodríguez-Chávez JL, Egas V, Linares E, et al. Mexican Arnica ( Heterotheca inuloides Cass. Asteraceae: Astereae): Ethnomedical uses, chemical constituents and biological properties. J Ethnopharmacol, 2017, 195: 39-63. DOI:10.1016/j.jep.2016.11.021
|
|
| [56] |
Egas V, Millán E, Collado JA, et al. Effect of natural and semi-synthetic cadinanes from Heterotheca inuloides on NF-κB, Nrf2 and STAT3 signaling pathways and evaluation of their in vitro cytotoxicity in human cancer cell lines. Bioorg Med Chem, 2017, 25(12): 3135-3147. DOI:10.1016/j.bmc.2017.03.069
|
|
| [57] |
Ren J, Xie YG, Guo YG, et al. Unusual metal complex of cadinane sesquiterpene alkaloid and new neolignan glycosides from Alangium alpinum. Fitoterapia, 2018, 125: 18-23. DOI:10.1016/j.fitote.2017.12.008
|
|
| [58] |
Sato T, Arai MA, Yixizhuoma, et al. Cadinane sesquiterpenoids isolated from Santalum album using a screening program for Wnt signal inhibitory activity. J Nat Med, 2020, 74(2): 476-481. DOI:10.1007/s11418-019-01380-x
|
|
| [59] |
Cui J, Shang RY, Sun M, et al. Trichodermaloids A-C, cadinane sesquiterpenes from a marine sponge symbiotic Trichoderma sp. SM16 fungus. Chem Biodivers, 2020, 17(4): e2000036.
|
|
| [60] |
Song YP, Shi XS, Wang BG, et al. Cadinane and carotane derivatives from the marine algicolous fungus Trichoderma virens RR-dl-6-8. Fitoterapia, 2020, 146: 104715. DOI:10.1016/j.fitote.2020.104715
|
|
| [61] |
Yu YF, Liu YH, Chen XH, et al. Cadinane-type sesquiterpenes from the resinous exudates of Commiphora myrrha and their anti-Alzheimer's disease bioactivities. Fitoterapia, 2020, 142: 104536. DOI:10.1016/j.fitote.2020.104536
|
|
| [62] |
Cao L, Shehla N, Tasneem S, et al. New cadinane sesquiterpenes from the stems of Kadsura heteroclita. Molecules, 2019, 24(9): 1664. DOI:10.3390/molecules24091664
|
|
| [63] |
Wu QH, Chen WT, Li SW, et al. Cytotoxic nitrogenous terpenoids from two South China sea nudibranchs Phyllidiella pustulosa, Phyllidia coelestis, and their sponge-prey Acanthella cavernosa. Mar Drugs, 2019, 17(1): 56. DOI:10.3390/md17010056
|
|
| [64] |
Zhang P, Li J, Lang J, et al. Two new sesquiterpenes derivatives from marine fungus Leptosphaerulina Chartarum sp. 3608. Nat Prod Res, 2018, 32(19): 2297-2303. DOI:10.1080/14786419.2017.1408102
|
|
| [65] |
Ding JH, Li ZH, Feng T, et al. A new cadinane sesquiterpenoid from cultures of the Basidiomycete Panus conchatus. Nat Prod Res, 2018, 32(19): 2333-2337. DOI:10.1080/14786419.2017.1413559
|
|
| [66] |
Shi ZZ, Fang ST, Miao FP, et al. Trichocarotins A-H and trichocadinin A, nine sesquiterpenes from the marine-alga-epiphytic fungus Trichoderma virens. Bioorg Chem, 2018, 81: 319-325. DOI:10.1016/j.bioorg.2018.08.027
|
|
| [67] |
Xu J, Zhu HL, Zhang J, et al. Sesquiterpenoids from Chloranthus anhuiensis with neuroprotective effects in PC12 cells. J Nat Prod, 2018, 81(6): 1391-1398. DOI:10.1021/acs.jnatprod.7b01076
|
|
| [68] |
Qiao MM, Liu F, Liu Y, et al. Curcumane C and (±)-curcumane D, an unusual seco-cadinane sesquiterpenoid and a pair of unusual nor-bisabolane enantiomers with significant vasorelaxant activity from Curcuma longa. Bioorg Chem, 2019, 92: 103275. DOI:10.1016/j.bioorg.2019.103275
|
|
| [69] |
Chen DL, Li G, Liu YY, et al. A new cadinane sesquiterpenoid glucoside with cytotoxicity from Abelmoschus sagittifolius. Nat Prod Res, 2019, 33(12): 1699-1704. DOI:10.1080/14786419.2018.1431635
|
|
| [70] |
Jiang H, Zhang GJ, Liao HB, et al. New terpenoid and phenylpropanoid glycosides from Tinospora sinensis. Fitoterapia, 2018, 131: 127-133. DOI:10.1016/j.fitote.2018.10.018
|
|
| [71] |
He J, Xu JK, Pan XG, et al. Unusual cadinane-type sesquiterpene glycosides with α-glucosidase inhibitory activities from the fruit of Cornus officinalis Sieb. et Zuuc. Bioorg Chem, 2019, 82: 1-5. DOI:10.1016/j.bioorg.2018.09.026
|
|
| [72] |
Song DH, Jo YH, Ahn JH, et al. Sesquiterpenes from fruits of Torilis japonica with inhibitory activity on melanin synthesis in B16 cells. J Nat Med, 2018, 72(1): 155-160. DOI:10.1007/s11418-017-1123-4
|
|
| [73] |
Nyandoro SS, Maeda G, Munissi JJE, et al. A new benzopyranyl cadenane sesquiterpene and other antiplasmodial and cytotoxic metabolites from Cleistochlamys kirkii. Molecules, 2019, 24(15): 2746. DOI:10.3390/molecules24152746
|
|
| [74] |
Wu CL, Chien SC, Wang SY, et al. Structure-activity relationships of cadinane-type sesquiterpene derivatives against wood-decay fungi. Holzforschung, 2005, 59(6): 620-627. DOI:10.1515/HF.2005.100
|
|
| [75] |
Chang ST, Wang SY, Wu CL, et al. Comparison of the antifungal activity of cadinane skeletal sesquiterpenoids from Taiwania ( Taiwania cryptomerioides Hayata) heartwood. Holzforschung, 2000, 54(3): 241-245. DOI:10.1515/HF.2000.041
|
|
| [76] |
Egas V, Salazar-Cervantes G, Romero I, et al. Anti- Helicobacter pylori metabolites from Heterotheca inuloides (Mexican Arnica). Fitoterapia, 2018, 127: 314-321. DOI:10.1016/j.fitote.2018.03.001
|
|
| [77] |
Zhu H, Hua XX, Gong T, et al. Hypocreaterpenes A and B, cadinane-type sesquiterpenes from a marine-derived fungus, Hypocreales sp. Phytochem Lett, 2013, 6(3): 392-396. DOI:10.1016/j.phytol.2013.04.008
|
|
| [78] |
方欣, 卢山, 于宗霞, 等. 青蒿素的生物合成研究. 科技导报, 2015, 33(20): 31-35. Fang X, Lu S, Yu ZX, et al. Study on the biosynthesis of artemisinin. Sci Technol Rev, 2015, 33(20): 31-35 (in Chinese).
|
|
| [79] |
Faraldos JA, Miller DJ, González V, et al. A 1, 6-ring closure mechanism for (+)-δ-cadinene synthase?. J Am Chem Soc, 2012, 134(13): 5900-5908. DOI:10.1021/ja211820p
|
|
| [80] |
González V, Grundy DJ, Faraldos JA, et al. The amino-terminal segment in the β-domain of δ-cadinene synthase is essential for catalysis. Org Biomol Chem, 2016, 14(31): 7451-7454. DOI:10.1039/C6OB01398H
|
|
| [81] |
Loizzi M, González V, Miller DJ, et al. Nucleophilic water capture or proton loss: single amino acid switch converts δ-cadinene synthase into germacradien-4-ol synthase. Chembiochem, 2018, 19(1): 100-105. DOI:10.1002/cbic.201700531
|
|
| [82] |
Loizzi M, Miller DJ, Allemann RK. Silent catalytic promiscuity in the high-fidelity terpene cyclase δ-cadinene synthase. Org Biomol Chem, 2019, 17(5): 1206-1214. DOI:10.1039/C8OB02821D
|
|
| [83] |
Jullien F, Moja S, Bony A, et al. Isolation and functional characterization of a τ-cadinol synthase, a new sesquiterpene synthase from Lavandula angustifolia. Plant Mol Biol, 2014, 84(1/2): 227-241.
|
|
| [84] |
Townsend BJ, Poole A, Blake CJ, et al. Antisense suppression of a (+)-δ-cadinene synthase gene in cotton prevents the induction of this defense response gene during bacterial blight infection but not its constitutive expression. Plant Physiol, 2005, 138(1): 516-528. DOI:10.1104/pp.104.056010
|
|
| [85] |
Chen XY, Wang MS, Chen Y, et al. Cloning and heterologous expression of a second (+)-δ-cadinene synthase from Gossypium arboreum. J Nat Prod, 1996, 59(10): 944-951. DOI:10.1021/np960344w
|
|
| [86] |
Tan XP, Liang WQ, Liu CJ, et al. Expression pattern of (+)-δ-cadinene synthase genes and biosynthesis of sesquiterpene aldehydes in plants of Gossypium arboreum L. Planta, 2000, 210(4): 644-651. DOI:10.1007/s004250050055
|
|
| [87] |
Ren F, Mao HJ, Liang J, et al. Functional characterization of ZmTPS7 reveals a maize τ-cadinol synthase involved in stress response. Planta, 2016, 244(5): 1065-1074. DOI:10.1007/s00425-016-2570-y
|
|
| [88] |
Jin Z, Kwon M, Lee AR, et al. Molecular cloning and functional characterization of three terpene synthases from unripe fruit of black pepper ( Piper nigrum). Arch Biochem Biophys, 2018, 638: 35-40. DOI:10.1016/j.abb.2017.12.011
|
|
| [89] |
Xu YH, Wang JW, Wang S, et al. Characterization of GaWRKY1, a cotton transcription factor that regulates the sesquiterpene synthase gene (+)-δ-cadinene synthase-A. Plant Physiol, 2004, 135(1): 507-515. DOI:10.1104/pp.104.038612
|
|
| [90] |
Benedict CR, Lu JL, Pettigrew DW, et al. The cyclization of farnesyl diphosphate and nerolidyl diphosphate by a purified recombinant δ-cadinene synthase. Plant Physiol, 2001, 125(4): 1754-1765. DOI:10.1104/pp.125.4.1754
|
|
| [91] |
Göpfert JC, Macnevin G, Ro DK, et al. Identification, functional characterization and developmental regulation of sesquiterpene synthases from sunflower capitate glandular trichomes. BMC Plant Biol, 2009, 9: 86. DOI:10.1186/1471-2229-9-86
|
|
| [92] |
Jones CG, Keeling CI, Ghisalberti EL, et al. Isolation of cDNAs and functional characterisation of two multi-product terpene synthase enzymes from sandalwood, Santalum album L. Arch Biochem Biophys, 2008, 477(1): 121-130. DOI:10.1016/j.abb.2008.05.008
|
|
| [93] |
Bouwmeester HJ, Wallaart TE, Janssen MHA, et al. Amorpha-4, 11-diene synthase catalyses the first probable step in artemisinin biosynthesis. Phytochemistry, 1999, 52(5): 843-854. DOI:10.1016/S0031-9422(99)00206-X
|
|
| [94] |
Zhou H, Yang YL, Zeng J, et al. Identification and characterization of a δ-cadinol synthase potentially involved in the formation of boreovibrins in Boreostereum vibrans of basidiomycota. Nat Prod Bioprospecting, 2016, 6(3): 167-171. DOI:10.1007/s13659-016-0096-4
|
|
| [95] |
Agger S, Lopez-Gallego F, Schmidt-Dannert C. Diversity of sesquiterpene synthases in the basidiomycete Coprinus cinereus. Mol Microbiol, 2009, 72(5): 1181-1195. DOI:10.1111/j.1365-2958.2009.06717.x
|
|
| [96] |
Wawrzyn GT, Quin MB, Choudhary S, et al. Draft genome of Omphalotus olearius provides a predictive framework for sesquiterpenoid natural product biosynthesis in Basidiomycota. Chem Biol, 2012, 19(6): 772-783. DOI:10.1016/j.chembiol.2012.05.012
|
|
| [97] |
Quin MB, Flynn CM, Wawrzyn GT, et al. Mushroom hunting by using bioinformatics: application of a predictive framework facilitates the selective identification of sesquiterpene synthases in basidiomycota. Chembiochem, 2013, 14(18): 2480-2491. DOI:10.1002/cbic.201300349
|
|
| [98] |
Yap HY, Muria-Gonzalez MJ, Kong BH, et al. Heterologous expression of cytotoxic sesquiterpenoids from the medicinal mushroom Lignosus rhinocerotis in yeast. Microb Cell Fact, 2017, 16(1): 103. DOI:10.1186/s12934-017-0713-x
|
|
| [99] |
Lin YL, Ma LT, Lee YR, et al. Differential gene expression network in terpenoid synthesis of Antrodia cinnamomea in mycelia and fruiting bodies. J Agric Food Chem, 2017, 65(9): 1874-1886. DOI:10.1021/acs.jafc.6b05386
|
|
| [100] |
Shaw JJ, Berbasova T, Sasaki T, et al. Identification of a fungal 1, 8-cineole synthase from Hypoxylon sp. with specificity determinants in common with the plant synthases. J Biol Chem, 2015, 290(13): 8511-8526. DOI:10.1074/jbc.M114.636159
|
|
| [101] |
Hu Y, Chou WK, Hopson R, et al. Genome mining in Streptomyces clavuligerus: expression and biochemical characterization of two new cryptic sesquiterpene synthases. Chem Biol, 2011, 18(1): 32-37. DOI:10.1016/j.chembiol.2010.11.008
|
|
| [102] |
Rabe P, Dickschat JS. Rapid chemical characterization of bacterial terpene synthases. Angew Chem Int Ed Engl, 2013, 52(6): 1810-1812. DOI:10.1002/anie.201209103
|
|
| [103] |
Styles MQ, Nesbitt EA, Marr S, et al. Characterization of the first naturally thermostable terpene synthases and development of strategies to improve thermostability in this family of enzymes. Febs J, 2017, 284(11): 1700-1711. DOI:10.1111/febs.14072
|
|
| [104] |
Yoshikuni Y, Martin VJJ, Ferrin TE, et al. Engineering cotton (+)-δ-cadinene synthase to an altered function: germacrene D-4-ol synthase. Chem Biol, 2006, 13(1): 91-98. DOI:10.1016/j.chembiol.2005.10.016
|
|
 2021, Vol. 37
2021, Vol. 37