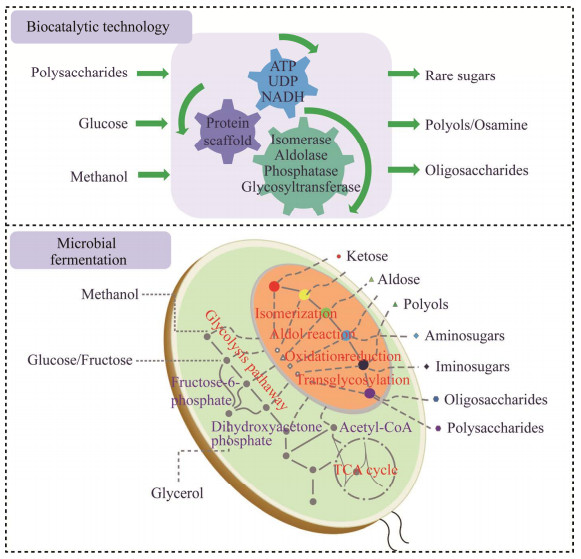
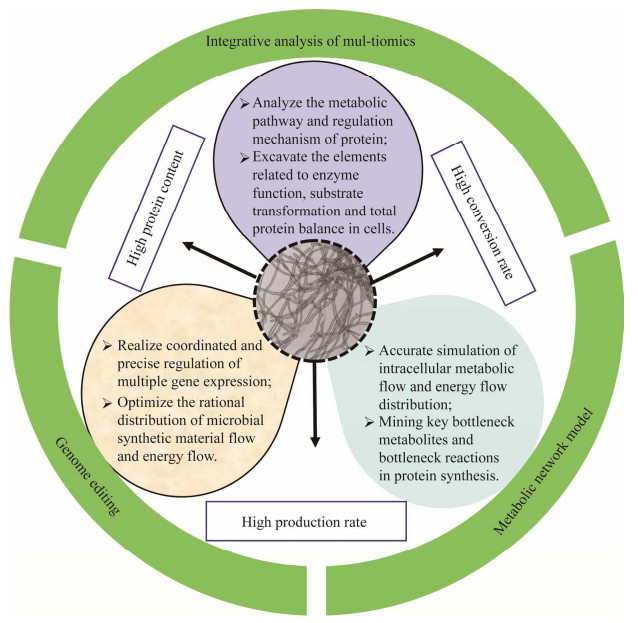
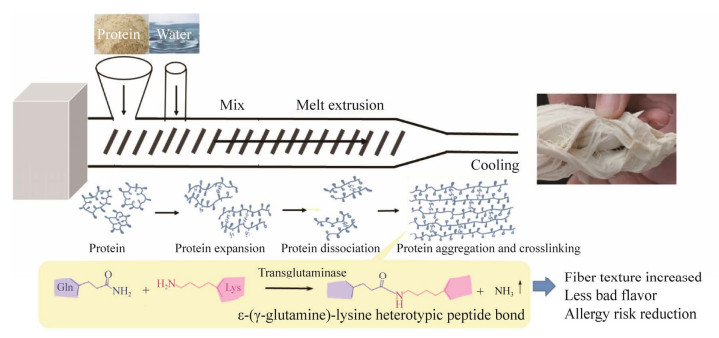
| [1] |
Malik VS, Hu FB. The role of sugar-sweetened beverages in the global epidemics of obesity and chronic diseases. Nat Rev Endocrinol, 2022, 18(4): 205-218. DOI:10.1038/s41574-021-00627-6
|
|
| [2] |
GBD 2015 Obesity Collaborators. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med, 2017, 377(1): 13-27. DOI:10.1056/NEJMoa1614362
|
|
| [3] |
Rasool S, Geetha T, Broderick TL, et al. High fat with high sucrose diet leads to obesity and induces myodegeneration. Front Physiol, 2018, 9: 1054. DOI:10.3389/fphys.2018.01054
|
|
| [4] |
Rippe JM, Angelopoulos TJ. Sucrose, high-fructose corn syrup, and fructose, their metabolism and potential health effects: what do we really know. Adv Nutr, 2013, 4(2): 236-245. DOI:10.3945/an.112.002824
|
|
| [5] |
柏玮, 朱玥明, 门燕, 等. 以D-果糖为原料利用新型异构酶转化生产D-阿洛糖. 生物工程学报, 2012, 28(4): 457-465. Bai W, Zhu YM, Men Y, et al. , Bioconversion of D-fructose to D-allose by novel isomerases. Chin J Biotech, 2012, 28(4): 457-465 (in Chinese). DOI:10.13345/j.cjb.2012.04.007
|
|
| [6] |
Men Y, Zhu YM, Zhang LL, et al. Enzymatic conversion of D-galactose to D-tagatose: cloning, overexpression and characterization of L-arabinose isomerase from Pediococcus pentosaceus PC-5. Microbiol Res, 2014, 169(2/3): 171-178.
|
|
| [7] |
Zhu YM, Men Y, Bai W, et al. Overexpression of D-psicose 3-epimerase from Ruminococcus sp. in Escherichia coli and its potential application in D-psicose production. Biotechnol Lett, 2012, 34(10): 1901-1906.
|
|
| [8] |
Yang JG, Tian CY, Zhang T, et al. Development of food-grade expression system for D-allulose 3-epimerase preparation with tandem isoenzyme genes in Corynebacterium glutamicum and its application in conversion of cane molasses to D-allulose. Biotechnol Bioeng, 2019, 116(4): 745-756. DOI:10.1002/bit.26909
|
|
| [9] |
Yang JG, Zhang T, Tian CY, et al. Multi-enzyme systems and recombinant cells for synthesis of valuable saccharides: advances and perspectives. Biotechnol Adv, 2019, 37(7): 107406. DOI:10.1016/j.biotechadv.2019.06.005
|
|
| [10] |
Tian CY, Yang JG, Liu C, et al. Engineering substrate specificity of HAD phosphatases and multienzyme systems development for the thermodynamic-driven manufacturing sugars. Nat Commun, 2022, 13(1): 3582. DOI:10.1038/s41467-022-31371-8
|
|
| [11] |
Li YJ, Shi T, Han PP, et al. Thermodynamics-driven production of value-added D-allulose from inexpensive starch by an in vitro enzymatic synthetic biosystem. ACS Catalysis, 2021, 11(9): 5088-5099. DOI:10.1021/acscatal.0c05718
|
|
| [12] |
You C, Shi T, Li YJ, et al. An in vitro synthetic biology platform for the industrial biomanufacturing of myo-inositol from starch. Biotechnol Bioeng, 2017, 114(8): 1855-1864. DOI:10.1002/bit.26314
|
|
| [13] |
Yang JG, Zhu YM, Li JT, et al. Biosynthesis of rare ketoses through constructing a recombination pathway in an engineered Corynebacterium glutamicum. Biotechnol Bioeng, 2015, 112(1): 168-180. DOI:10.1002/bit.25345
|
|
| [14] |
Yang JG, Li JT, Men Y, et al. Biosynthesis of L-sorbose and L-psicose based on C–C bond formation catalyzed by aldolases in an engineered Corynebacterium glutamicum strain. Appl Environ Microbiol, 2015, 81(13): 4284-4294. DOI:10.1128/AEM.00208-15
|
|
| [15] |
Li JT, Yang JG, Men Y, et al. Biosynthesis of 2-deoxysugars using whole-cell catalyst expressing 2-deoxy-d-ribose 5-phosphate aldolase. Appl Microbiol Biotechnol, 2015, 99(19): 7963-7972. DOI:10.1007/s00253-015-6740-9
|
|
| [16] | |
|
| [17] |
Zhu Y, Chen P, Bao Y, et al. Complete genome sequence and transcriptomic analysis of a novel marine strain Bacillus weihaiensis reveals the mechanism of brown algae degradation. Sci Reports, 2016, 6(1): 38248.
|
|
| [18] |
Chen P, Zhu YM, Men Y, et al. Purification and characterization of a novel alginate lyase from the marine bacterium Bacillus sp. Alg07. Mar Drugs, 2018, 16(3): 86. DOI:10.3390/md16030086
|
|
| [19] |
Yan JJ, Chen P, Zeng Y, et al. The characterization and modification of a novel bifunctional and robust alginate lyase derived from Marinimicrobium sp. H1. Mar Drugs, 2019, 17(10): 545. DOI:10.3390/md17100545
|
|
| [20] |
Yan JJ, Chen P, Zeng Y, et al. Production of neoagarobiose from agar through a dual-enzyme and two-stage hydrolysis strategy. Int J Biol Macromol, 2020, 160: 288-295. DOI:10.1016/j.ijbiomac.2020.05.206
|
|
| [21] |
Tian CY, Yang JG, Zeng Y, et al. Biosynthesis of raffinose and stachyose from sucrose via an in vitro multienzyme system. Appl Environ Microbiol, 2019, 85(2): e02306-e02318.
|
|
| [22] |
Sun SS, Wei XL, Zhou XG, et al. Construction of an artificial in vitro synthetic enzymatic platform for upgrading low-cost starch to value-added disaccharides. J Agric Food Chem, 2021, 69(1): 302-314. DOI:10.1021/acs.jafc.0c06936
|
|
| [23] |
Schwaiger KN, Cserjan-Puschmann M, Striedner G, et al. Whole cell-based catalyst for enzymatic production of the osmolyte 2-O-α-glucosylglycerol. Microb Cell Fact, 2021, 20(1): 79. DOI:10.1186/s12934-021-01569-4
|
|
| [24] |
Kruschitz A, Nidetzky B. Reactive extraction of fructose for efficient separation of sucrose-derived glucosides produced by enzymatic glycosylation. Green Chem, 2020, 22(15): 4985-4994. DOI:10.1039/D0GC01408G
|
|
| [25] |
Zhang T, Yang JG, Tian CY, et al. High-yield biosynthesis of glucosylglycerol through coupling phosphorolysis and transglycosylation reactions. J Agric Food Chem, 2020, 68(51): 15249-15256. DOI:10.1021/acs.jafc.0c04851
|
|
| [26] |
Tian YQ, Xu W, Zhang WL, et al. Amylosucrase as a transglucosylation tool: from molecular features to bioengineering applications. Biotechnol Adv, 2018, 36(5): 1540-1552. DOI:10.1016/j.biotechadv.2018.06.010
|
|
| [27] |
Qi P, You C, Zhang YHP. One-pot enzymatic conversion of sucrose to synthetic amylose by using enzyme cascades. ACS Catal, 2014, 4(5): 1311-1317. DOI:10.1021/cs400961a
|
|
| [28] |
Zhang XW, Leemhuis H, Van Der Maarel MJEC. Synthesis of highly branched α-glucans with different structures using GH13 and GH57 glycogen branching enzymes. Carbohydr Polym, 2019, 216: 231-237. DOI:10.1016/j.carbpol.2019.04.038
|
|
| [29] |
Zhang XW, Leemhuis H, Van Der Maarel MJEC. Digestion kinetics of low, intermediate and highly branched maltodextrins produced from gelatinized starches with various microbial glycogen branching enzymes. Carbohydr Polym, 2020, 247: 116729. DOI:10.1016/j.carbpol.2020.116729
|
|
| [30] |
Qiu XL, Gu Y, Du GC, et al. Conferring thermotolerant phenotype to wild-type Yarrowia lipolytica improves cell growth and erythritol production. Biotechnol Bioeng, 2021, 118(8): 3117-3127. DOI:10.1002/bit.27835
|
|
| [31] |
Yang JG, Zhu YM, Men Y, et al. Pathway construction in Corynebacterium glutamicum and strain engineering to produce rare sugars from glycerol. J Agric Food Chem, 2016, 64(50): 9497-9505. DOI:10.1021/acs.jafc.6b03423
|
|
| [32] |
Yang JG, Sun SS, Men Y, et al. Transformation of formaldehyde into functional sugars via multi-enzyme stepwise cascade catalysis. Catal Sci Technol, 2017, 7(16): 3459-3463. DOI:10.1039/C7CY01062A
|
|
| [33] |
Yang JG, Zhu YM, Qu G, et al. Biosynthesis of dendroketose from different carbon sources using in vitro and in vivo metabolic engineering strategies. Biotechnol Biofuels, 2018, 11: 290. DOI:10.1186/s13068-018-1293-7
|
|
| [34] |
Deng C, Lv XQ, Li JH, et al. Synergistic improvement of N-acetylglucosamine production by engineering transcription factors and balancing redox cofactors. Metab Eng, 2021, 67: 330-346. DOI:10.1016/j.ymben.2021.07.012
|
|
| [35] |
Zhang XL, Wang CY, Lv XQ, et al. Engineering of synthetic multiplexed pathways for high-level N-acetylneuraminic acid bioproduction. J Agric Food Chem, 2021, 69(49): 14868-14877. DOI:10.1021/acs.jafc.1c06017
|
|
| [36] |
Bych K, Mikš MH, Johanson T, et al. Production of HMOs using microbial hosts—from cell engineering to large scale production. Curr Opin Biotechnol, 2019, 56: 130-137.
|
|
| [37] |
Wan L, Zhu YY, Chen G, et al. Efficient production of 2′-fucosyllactose from L-fucose via self-assembling multienzyme complexes in engineered Escherichia coli. ACS Synth Biol, 2021, 10(10): 2488-2498. DOI:10.1021/acssynbio.1c00102
|
|
| [38] |
Yu WW, Jin K, Wu YK, et al. A pathway independent multi-modular ordered control system based on thermosensors and CRISPRi improves bioproduction in Bacillus subtilis. Nucleic Acids Res, 2022, 50(11): 6587-6600. DOI:10.1093/nar/gkac476
|
|
| [39] |
Tian CY, Yang JG, Li YJ, et al. Artificially designed routes for the conversion of starch to value-added mannosyl compounds through coupling in vitro and in vivo metabolic engineering strategies. Metab Eng, 2020, 61: 215-224. DOI:10.1016/j.ymben.2020.06.008
|
|
| [40] |
舒芹, 李凯凯, 全拓, 等. 微生物蛋白作为优质替代蛋白资源的应用研究. 未来食品科学, 2022, 2: 96-106.
Shu Q, Li KK, Quan T, et al. Application of microbial proteins as high-quality alternative protein resources. Future Food Sci, 2022, 2: 96-106 (in Chinese).
|
|
| [41] |
Ritala A, Häkkinen ST, Toivari M, et al. Single cell protein-state-of-the-art, industrial landscape and patents 2001–2016. Front Microbiol, 2017, 8: 2009. DOI:10.3389/fmicb.2017.02009
|
|
| [42] |
De Vree JH, Bosma R, Wieggers R, et al. Turbidostat operation of outdoor pilot-scale photobioreactors. Algal Res, 2016, 18: 198-208. DOI:10.1016/j.algal.2016.06.006
|
|
| [43] |
Pereira H, Páramo J, Silva J, et al. Scale-up and large-scale production of Tetraselmis sp. CTP4 (Chlorophyta) for CO2 mitigation: from an agar plate to 100-m3 industrial photobioreactors. Sci Reports, 2018, 8: 5112.
|
|
| [44] | |
|
| [45] |
Ahmad MI, Farooq S, Alhamoud Y, et al. A review on mycoprotein: history, nutritional composition, production methods, and health benefits. Trends Food Sci Technol, 2022, 121(1): 14-29.
|
|
| [46] |
谷孚. 新蛋白发酵行业报告: 发酵技术驱动中国未来食品发展. 2022.
Gu Fu. New protein fermentation industry report: fermentation technology drives China's future food development. 2022 (in Chinese).
|
|
| [47] |
Tong S, An KX, Zhou WY, et al. Establishment of high-efficiency screening system for gene deletion in Fusarium venenatum TB01. J Fungi, 2022, 8(2): 169. DOI:10.3390/jof8020169
|
|
| [48] |
Tong S, An KX, Chen WX, et al. Evasion of Cas9 toxicity to develop an efficient genome editing system and its application to increase ethanol yield in Fusarium venenatum TB01. Appl Microbiol Biotechnol, 2022.
|
|
| [49] |
李秋燕, 朱文学. 食品添加剂在改善肉制品色泽中的应用. 肉类工业, 2009(1): 44-46. Li QY, Zhu WX. Application of additive in improving color of meat products. Meat Ind, 2009(1): 44-46 (in Chinese). DOI:10.3969/j.issn.1008-5467.2009.01.019
|
|
| [50] |
赵鑫锐, 张国强, 李雪良, 等. 人造肉大规模生产的商品化技术. 食品与发酵工业, 2019, 45(11): 248-253. Zhao XR, Zhang GQ, Li XL, et al. Commercial production of artificial meat. Food Ferment Ind, 2019, 45(11): 248-253 (in Chinese). DOI:10.13995/j.cnki.11-1802/ts.020859
|
|
| [51] |
Natarajan C, Jiang XB, Fago A, et al. Expression and purification of recombinant hemoglobin in Escherichia coli. PLoS One, 2011, 6(5): e20176. DOI:10.1371/journal.pone.0020176
|
|
| [52] |
Liu LF, Martínez JL, Liu ZH, et al. Balanced globin protein expression and heme biosynthesis improve production of human hemoglobin in Saccharomyces cerevisiae. Metab Eng, 2014, 21: 9-16. DOI:10.1016/j.ymben.2013.10.010
|
|
| [53] |
Martínez JL, Liu LF, Petranovic D, et al. Engineering the oxygen sensing regulation results in an enhanced recombinant human hemoglobin production by Saccharomyces cerevisiae. Biotechnol Bioeng, 2015, 112(1): 181-188. DOI:10.1002/bit.25347
|
|
| [54] |
Fraser R, Brown, PO, Karr J, et al. Methods and compositions for affecting the flavor and aroma profile of consumables: US, 15/398479. 2017-01-04.
|
|
| [55] |
Wei L, Wang Q, Xu N, et al. Combining protein and metabolic engineering strategies for high-level production of O-acetylhomoserine in Escherichia coli. ACS Synth Biol, 2019, 8(5): 1153-1167. DOI:10.1021/acssynbio.9b00042
|
|
| [56] |
Wei L, Wang H, Xu N, et al. Metabolic engineering of Corynebacterium glutamicum for L-cysteine production. Appl Microbiol Biotechnol, 2019, 103(3): 1325-1338. DOI:10.1007/s00253-018-9547-7
|
|
| [57] |
Ceresino EB, De Melo RR, Kuktaite R, et al. Transglutaminase from newly isolated Streptomyces sp. CBMAI 1617: production optimization, characterization and evaluation in wheat protein and dough systems. Food Chem, 2018, 241: 403-410.
|
|
| [58] |
Fuchsbauer HL. Approaching transglutaminase from Streptomyces bacteria over three decades. FEBS J, 2022, 289(16): 4680-4703. DOI:10.1111/febs.16060
|
|
| [59] |
Huang YM, Jin MF, Yan WJ, et al. A point mutant in the promoter of transglutaminase gene dramatically increased yield of microbial transglutaminase from Streptomyces mobaraensis TX1. Process Biochem, 2022, 112: 92-97. DOI:10.1016/j.procbio.2021.11.021
|
|
| [60] |
杜建辉, 刘松, 陆信曜, 等. 构建分子内二硫键提升谷氨酰胺转氨酶热稳定性. 食品与发酵工业, 2021, 47(15): 1-8. Du JH, Liu S, Lu XY, et al. Improving thermostability of transglutaminase by introducing intramolecular disulfide bonds. Food Ferment Ind, 2021, 47(15): 1-8 (in Chinese).
|
|
| [61] |
Suzuki M, Date M, Kashiwagi T, et al. Rational design of a disulfide bridge increases the thermostability of microbial transglutaminase. Appl Microbiol Biotechnol, 2022, 106(12): 4553-4562. DOI:10.1007/s00253-022-12024-8
|
|
| [62] |
Moreno HM, Pedrosa MM, Tovar CA, et al. Effect of microbial transglutaminase on the production of fish myofibrillar and vegetable protein-based products. Value-Addition in Food Products and Processing Through Enzyme Technology. Amsterdam: Elsevier, 2022: 427-436.
|
|
| [63] |
Fatima SW, Khare SK. Effect of key regulators in augmenting transcriptional expression of transglutaminase in Streptomyces mobaraensis. Bioresour Technol, 2021, 340: 125627. DOI:10.1016/j.biortech.2021.125627
|
|
| [64] |
Wang HB, Ji Y, Yuan ZT, et al. Insights into the mechanism on the high-temperature activity of transglutaminase from Bacillus clausii and its crosslinked mode at protein level. Biochem Eng J, 2022, 185: 108544. DOI:10.1016/j.bej.2022.108544
|
|
| [65] |
Wang XL, Zhao BC, Du JH, et al. Active secretion of a thermostable transglutaminase variant in Escherichia coli. Microb Cell Fact, 2022, 21(1): 74. DOI:10.1186/s12934-022-01801-9
|
|
| [66] |
Hirono-Hara Y, Yui M, Hara KY. Active transglutaminase production from synthetic whey using engineered Saccharomyces cerevisiae. Bioresour Technol Rep, 2022, 19: 101154. DOI:10.1016/j.biteb.2022.101154
|
|
| [67] |
Wang XL, Du JH, Zhao BC, et al. Significantly improving the thermostability and catalytic efficiency of Streptomyces mobaraenesis transglutaminase through combined rational design. J Agric Food Chem, 2021, 69(50): 15268-15278.
|
|
| [68] |
Yin XQ, Li YY, Zhou JW, et al. Enhanced production of transglutaminase in Streptomyces mobaraensis through random mutagenesis and site-directed genetic modification. J Agric Food Chem, 2021, 69(10): 3144-3153. DOI:10.1021/acs.jafc.1c00645
|
|
| [69] |
Zhang ZY, Zhang LJ, He SD, et al. High-moisture extrusion technology application in the processing of textured plant protein meat analogues: a review. Food Rev Int, 2022. DOI:10.1080/87559129.201.2024223
|
|
| [70] |
Zhang JC, Chen QL, Liu L, et al. High-moisture extrusion process of transglutaminase-modified peanut protein: effect of transglutaminase on the mechanics of the process forming a fibrous structure. Food Hydrocoll, 2021, 112: 106346. DOI:10.1016/j.foodhyd.2020.106346
|
|
| [71] |
Zhang ZY, Kobata K, Pham H, et al. Production of plant-based seafood: scallop analogs formed by enzymatic gelation of pea protein-pectin mixtures. Foods, 2022, 11(6): 851.
|
|
| [72] |
Ramachandraiah K. Potential development of sustainable 3D-printed meat analogues: a review. Sustainability, 2021, 13(2): 938.
|
|
| [73] |
Yu NN, Yang F, Gong H, et al. Gel & three-dimensional printing properties of sheep plasma protein-surimi induced by transglutaminase. J Food Eng, 2022, 323: 111006.
|
|
| [74] |
Sakai K, Sato Y, Okada M, et al. Improved functional properties of meat analogs by laccase catalyzed protein and pectin crosslinks. Sci Rep, 2021, 11(1): 16631.
|
|
| [75] |
Zhu D, Damodaran S. Removal of off-flavour-causing precursors in soy protein by concurrent treatment with phospholipase A2 and cyclodextrins. Food Chem, 2018, 264: 319-325.
|
|
| [76] |
Faisal S, Zhang JC, Meng S, et al. Effect of high-moisture extrusion and addition of transglutaminase on major peanut allergens content extracted by three step sequential method. Food Chem, 2022, 385: 132569.
|
|
| [77] |
李德茂, 曾艳, 周桔, 等. 生物制造食品原料市场准入政策比较及对我国的建议. 中国科学院院刊, 2020, 35(8): 1041-1052. Li DM, Zeng Y, Zhou J, et al. Comparison of market access policies for foods and its raw materials made from biomanufacturing and suggestions for China. Bull Chin Acad Sci, 2020, 35(8): 1041-1052 (in Chinese).
|
|
 2022, Vol. 38
2022, Vol. 38