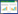中国科学院微生物研究所、中国微生物学会主办
文章信息
- 胡小露, 崔树梅, 柯崇榕, 陶勇, 黄建忠, 杨欣伟
- HU Xiaolu, CUI Shumei, KE Chongrong, TAO Yong, HUANG Jianzhong, YANG Xinwei
- 微生物合成L-脯氨酸和反式-4-羟基-L-脯氨酸的研究进展
- Advances on microbial synthesis of L-proline and trans-4-hydroxy-L-proline
- 生物工程学报, 2022, 38(12): 4498-4519
- Chinese Journal of Biotechnology, 2022, 38(12): 4498-4519
- 10.13345/j.cjb.220739
-
文章历史
- Received: September 15, 2022
- Accepted: November 18, 2022
- Published: November 21, 2022
2. 中国科学院微生物研究所, 北京 100101
2. Institute of Microbiology, Chinese Academy of Sciences, Beijing 100101, China
L-脯氨酸(L-proline, L-Pro),分子式为C5H9NO2,简称为脯氨酸,是20种常见氨基酸中唯一一种亚氨基氨基酸,具有香甜味,属于中性氨基酸,其侧链以环状形式(四氢吡咯环) 连接在α-碳原子上;反式-4-羟基-L-脯氨酸(trans-4-hydroxy-L-proline, T-4-Hyp),也可简称为羟脯氨酸(hydroxyproline, Hyp),其分子式为C5H9NO3,它不属于20种常见的氨基酸,是亚氨基酸L-Pro羟基化后最重要最普遍的一种产物,L-Pro和T-4-Hyp的理化性质见表 1。L-Pro和T-4-Hyp主要存在于胶原蛋白中,是结缔组织、皮肤、软骨等组织的主要胞外成分,在细胞生长、蛋白质折叠和稳定性中发挥着重要作用,尤其是在胶原蛋白三螺旋结构的稳定中作用显著,三螺旋结构的异常变化或不稳定都可能导致疾病[1-3],例如肌腱和韧带的结缔组织断裂[4]。随着对L-Pro和T-4-Hyp功能的深入挖掘,发现L-Pro和T-4-Hyp除了构成胶原蛋白外,在医药、农业、化工、食品和美容等方面都具有重要作用。
| Properties | L-proline (L-Pro) | trans-4-hydroxy-L-proline (T-4-Hyp) |
| Molecular formula | C5H9NO2 | C5H9NO3 |
| Molecular weight (g/mol) | 115.13 | 131.13 |
| Water-solubility (%, 25 ℃) | 162.30 | 36.10 |
| Boiling point (℃) | 252.20 | 355.20 |
| Melting point (℃) | 228.00 | 273.00 |
| Isoelectric point (pI) | 6.30 | 5.83 |
| Dissociation constant (pK) | pK1:1.99, pK2:10.60 | PK1:1.82, PK2:9.65 |
| Specific rotation | –84.50––86.00 | –74.00––77.00 |
| Refractive index | –85.00 | –75.50 |
| Density (g/cm3) | 1.35 | 1.39 |
L-Pro因其独特而广泛的生理生化功能,在医药、农业、食品和化工等领域具有广泛的作用,尤其是在生物医药领域功能众多。L-Pro可以制备氨基酸输液,作为蛋白质缺乏症和肠胃道疾病等的营养补充[5-8];L-Pro可以通过减轻氧化损伤和减少下游细胞凋亡来保护小鼠肾脏免受黄曲霉毒素的侵害[9];L-Pro可降低小鼠血液和组织(肺、肝和脾) 感染肺炎克雷伯菌的载量,提高其存活率[10];补充L-Pro可改善视网膜色素上皮(retinal pigment epithelium, RPE) 引发的视力丧失[11];L-Pro可以作为渗透保护剂,维持适当的渗透压并参与平衡氧化还原电位和pH值[6],以平衡膨胀压力并减轻水分胁迫[12-13],防止其在冷胁迫下变性、聚集或解离[12, 14],具有降低冰点和抑制冰晶生长的特点,可以与海藻糖联合使用,以冷冻保存红细胞,用于贫血、白血病以及创伤和外科手术中[15];L-Pro同样可用于冷冻保存哺乳动物卵母细胞和精母细胞[13, 16]。
L-Pro还可以作为原料合成多种药物用于医药领域。以L-Pro为原料可以合成依那普利用于预防心力衰竭[17];合成维格列汀用于2型糖尿病[18];合成美法仑(melphalan) 用于预防黑色素瘤[19]。L-Pro形成的多种氨基酸类衍生物也具有独特的生物医药价值[20]:L-硫代脯氨酸(L-thioproline) 具有控制药物毒性、抗肿瘤、抗实验性心律失常等功能,它还能延长机体寿命、抑制血小板凝结、阻止血栓形成[21];N-乙酰-L-脯氨酸(N-acetyl-L-proline) 用作药物中间体参与预防和治疗与神经、血管、肌肉骨骼或皮肤相关的疾病和综合征[22];甲琉丙脯酸(captopril) 作为药物卡托普利原料用于治疗顽固性高血压、充血性心力衰竭、甲亢、肾衰竭和糖尿病等[23-24]。L-硫代脯氨酸乙酯盐酸盐(ethyl L-thiazolidine-4-carboxylate hydrochloride) 可用于合成匹多莫德,该药物是一种免疫调节剂,可增强淋巴细胞的功能[25-27];Boc-L-脯氨酸(Boc-L-proline) 可作为药物中间体合成达卡他韦(daclatasvir)[28],该药物与索非布韦(sofosbuvir) 联合可用于治疗丙型肝炎(hepatitis C)[29]。L-Pro类似物:L-氮杂环丁烷-2-羧酸(L-azetidine-2-carboxylic acid, AZC) 可以抑制白色念珠菌菌丝的形成;AZC还可以与L-Pro衍生物α-N-甲基-L-脯氨酸(α-N-methyl-L-proline, mPro) 一起抑制胎牛血清中菌丝的形成[30]。由L-Pro衍生的三肽也具有特殊功能:Pro-Leu-甘氨酰胺、Gly-Pro-Glu、Pro-Gly-Pro等多肽类药物有望用于治疗帕金森氏症[23, 31-32];Ile-Pro-Pro和Ile-Ala-Pro等都具有抑制血管紧张的功能[23]。
此外L-Pro在其他领域也发挥着独特的作用,在农业上,L-Pro可以作为信号分子[33],参与植物细胞的渗透调节,对其抗逆性有重要作用[34],可抵御极端环境[35-36];在食品工业中,由于L-Pro的氨基能与糖发生反应,产物具有特殊香味,因而可作为食品级的香料,此外还可以作为食品添加剂和风味剂[23];在化工行业中,L-Pro作为结构简单、来源广泛的手性分子,能充当催化剂用于多种不对称反应,并且具有良好的对映选择性[37-40]。
1.1.2 反式-4-羟基-L-脯氨酸的应用T-4-Hyp在医药、化工、食品、美容等多个领域都具有独特而重要的作用,尤其在医药行业扮演着不可或缺的角色。T-4-Hyp具有抗氧化、抗超氧阴离子以及抗羟基自由基的能力[41],可通过芬顿反应抑制羟基自由基的产生[42],调整细胞氧化还原状态,从而保护细胞免受氧化损伤以延缓衰老[43-44]。此外,T-4-Hyp对治疗风湿性关节炎和各种皮肤性疾病有一定的疗效[45];T-4-Hyp还能够促进骨骼愈合[45]。口服T-4-Hyp可促进小鼠的胶原蛋白生物合成[46];T-4-Hyp可以作为白癜风胶原损伤以及肝纤维化、硬皮病和银屑病的重要生化指标[4, 47]。此外,T-4-Hyp还可以被氧化为活性氧簇(reactive oxygen species, ROS),用于抑制癌细胞的生长并促进其死亡[48],从而抑制肿瘤发生[49]。
T-4-Hyp还是合成多种药物的原料,如碳青霉烯类抗生素、抗肿瘤药物、抗高血压药物、消炎药和新型胃药等[50]。T-4-Hyp也可作为原料合成N-烷基吡咯[51]和N-芳香基吡咯,这些吡咯类药物具有显著的抗肿瘤、免疫抑制剂、抗艾滋病毒、抗炎、抗氧化等活性[52];T-4-Hyp还可以合成低聚大环化合物,用于抑制肺癌细胞[53]。T-4-Hyp可以作为原料合成聚碳酸酯等生物医学材料用作药物输送系统[54];作为原料合成具有胆碱能活性的生物碱[55]。T-4-Hyp类似物还有望用于治疗高草酸尿症[56]。
T-4-Hyp的衍生物也功能众多,其衍生物1, 4-二棕榈酰羟脯氨酸能刺激胶原纤维收缩、抑制弹性蛋白酶活性并清除氧自由基[57];衍生物N-乙酰-T-4-Hyp是无毒副作用药物奥沙西罗的有效成分,它对治疗骨关节炎等结缔组织疾病有一定的疗效[58-59]。由T-4-Hyp衍生的二肽三肽等具有特殊功能,例如脯氨酰羟脯氨酸(prolyl-hydroxyproline) 是一种胶原衍生的二肽,能抑制软骨细胞分化为矿化细胞,改善骨关节炎;同时也可促进小鼠海马细胞的增殖,从而产生抗抑郁药样作用[60]。其他二肽,如Leu-4-Hyp和Ile-4-Hyp具有清除羟基和超氧阴离子自由基的能力[44];Gly-X-Y胶原肽中T-4-Hyp可以降低中性粒细胞的趋化性并阻断细胞凋亡[49];Gly-Pro-Hyp可通过抑制核转录因子κB (nuclear transcription factor kappa B, NF-κB) 信号传导和氧化应激改善急性葡聚糖硫酸钠结肠炎小鼠的临床症状和组织病理学结肠变化[49, 61]。由于其多种多样的功能,膳食补充剂含有T-4-Hyp或其小肽可以改善哺乳动物和鱼类的肠道、关节、皮肤和骨骼健康,同时改善消化系统、心血管系统、免疫系统和骨骼系统的氧化应激[44, 49]。
此外,T-4-Hyp在其他领域的独特功能也不容忽视,在化工行业,T-4-Hyp是合成多种化合物必不可少的手性原料[51];在食品行业,可以弥补丙酮酸、甘氨酸和葡萄糖的摄入不足[1, 62];在美容行业,口服含T-4-Hyp的胶原蛋白肽可以改善面部皮肤的水分、弹性、粗糙度等状况[63]。
1.2 L-脯氨酸和反式-4-羟基-L-脯氨酸的生产方法L-Pro的生产方法主要包括化学合成法、生物提取法和微生物发酵法。由于L-Pro结构特殊是环亚氨基羧酸,因此化学合成路线长、效率低、成本高,难以投产[23, 64]。生物提取法工艺简单,其原料包括鱼皮、鸡毛以及明胶[65]等,但成本高、得率低,不适合工业化生产。微生物发酵法原料成本低,反应条件温和,不需要高温高压强酸强碱等极端条件,且产物单一,成为了大规模生产L-Pro的主要途径[66]。国际上微生物发酵法生产L-Pro始于1965年前后,我国相对较晚,始于20世纪80年代,源于中国科学院微生物研究所和上海市工业微生物研究所[23]。发酵法的生产菌株包括两类,一类是利用产谷氨酸的生产菌株,即在产生谷氨酸的基础上对其培养条件或者发酵途径进行改造,使其朝着有利于产生L-Pro的方向进行;另一类是在非谷氨酸生产菌的基础上,采用人工诱变或者代谢途径改造,选育营养缺陷型和/或抗反馈调节突变株,以糖质作为原料发酵生产L-Pro[67]。
T-4-Hyp的生产方法有3类,分别是胶原蛋白提取法、化学合成法及微生物法。其中,微生物法包括以L-Pro为底物的生物转化法(酶法) 和以葡萄糖(glucose, Glc) 为底物的直接发酵法。哺乳动物胶原蛋白蕴含丰富的T-4-Hyp,采用酸水解获取T-4-Hyp工艺简单,技术门槛低,提取流程为:先获得胶原蛋白,依次用盐酸、亚硝酸盐、盐酸进行处理,脱色后利用离子交换层析进行纯化,最终得到精制的T-4-Hyp[23]。但其成品得率低,产量一般低于10%[68-70],无法满足当今急剧增加的需求。化学法合成T-4-Hyp相较于生物提取法,其转化率得到了很大的提高,Takano等和Mehlfuhrer等分别将T-4-Hyp的转化率提升至25.2%和67.5%[71-72]。但是化学合成T-4-Hyp步骤繁琐,需多次经过取代、还原、水解等化学反应才能完成,产物存在多种同分异构体,难以分离,废弃物污染严重,因而止步于实验室研究,无法用于工业化大规模生产。随着微生物发酵技术的兴起,在微生物体内可将L-Pro催化为T-4-Hyp,由于这种生物转化法(酶法) 具有绿色环保、产率高、产物专一、无同分异构体、杂质少利于后期的分离纯化等一系列的优点成为了研究热点。但是L-Pro作为底物成本较高,因此研究者逐渐采用以Glc为底物从头合成T-4-Hyp,极大地降低了生产成本。但以Glc为底物使得代谢路径变长,增加了能量损耗,导致转化率偏低,还存在较大的提升空间。
综上所述,以Glc为底物从头合成是目前生产L-Pro和T-4-Hyp的发展趋势,也最具有应用前景,但是由于从头合成L-Pro和T-4-Hyp代谢路径长、转化率低,因此利用代谢工程手段,在分子水平对代谢途径进行重新设计和定向改造,通过关键酶的筛选及代谢流的精细调控,精准地建构细胞工厂,高效专一性合成L-Pro和T-4-Hyp已成为研究热点。
2 微生物合成L-脯氨酸和反式-4-羟基-L-脯氨酸的代谢途径在以Glc为底物合成L-Pro的过程中,Glc首先经过糖酵解途径(glycolytic pathway, EMP) 和三羧酸循环(tricarboxylic acid cycle, TCA) 产生α-酮戊二酸(α-ketoglutarate, α-KG),α-KG在谷氨酸脱氢酶(glutamate dehydrogenase, GDH) 的作用下生成L-谷氨酸(L-glutamic acid, L-Glu),L-Glu再经过一系列酶的催化产生L-Pro[73]。从L-Glu生成L-Pro包括两大途径:最常见的是谷氨酸途径,它以L-Glu为底物通过3个酶促反应和一个自发环化过程形成L-Pro。另一类途径是精氨酸途径[74],即某些微生物能够以L-Glu合成精氨酸(arginine, Arg) 的代谢途径中间产物N-乙酰-γ-谷氨酸半醛(N-acetyl-γ-glutamyl semialdehyde, AGASA) 或鸟氨酸(ornithine, Orn) 为底物合成L-Pro,整个过程存在4条途径(见图 1,后面详细阐述)。
|
| 图 1 微生物产生L-脯氨酸和反式-4-羟基-L-脯氨酸的代谢途径 Fig. 1 Metabolic pathways of microbial production of L-Pro and T-4-Hyp. Red arrows indicate L-Pro and T-4-Hyp synthesis from glucose; yellow arrows indicate L-Glu to arginine and the urea cycle; blue arrows indicate the synthesis of L-Pro by the arginine pathway. 红色箭头表示葡萄糖合成L-脯氨酸和反式-4-羟基-L-脯氨酸途径;黄色箭头表示L-谷氨酸合成精氨酸和尿素循环;4条蓝色系列箭头表示精氨酸途径合成L-脯氨酸 |
| |
以Glc作为底物生产T-4-Hyp则需要先借助以上途径生成L-Pro,再利用脯氨酸羟化酶(proline hydroxylase, P4H) 一步催化产生T-4-Hyp。该催化过程需要以游离的L-Pro和α-KG为共底物,在有氧条件下,利用P4H的立体专一性,羟化L-Pro合成T-4-Hyp,同时将α-KG脱羧生成琥珀酸(succinate, Suc) (图 1)。
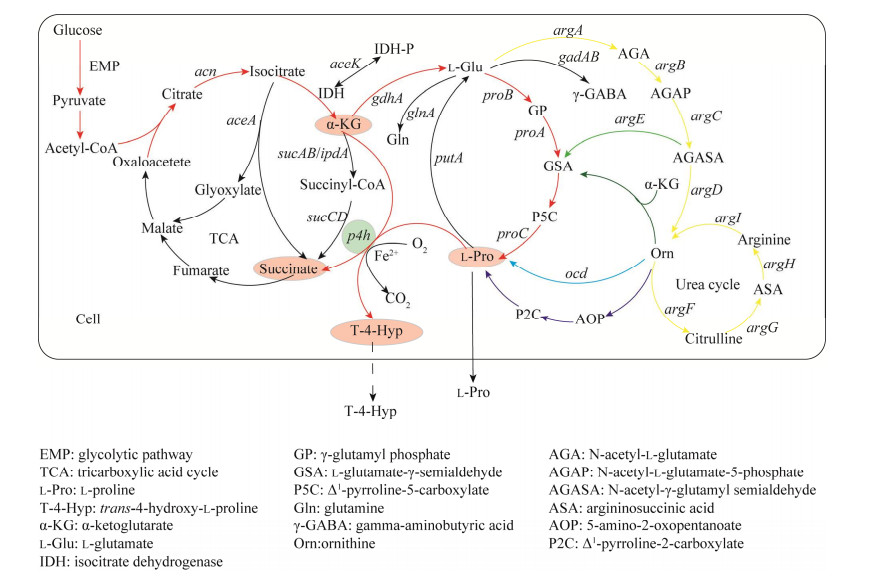
2.1 L-脯氨酸的代谢途径 2.1.1 谷氨酸途径中L-脯氨酸的合成与分解代谢谷氨酸途径是微生物合成L-Pro最重要的环节也是学者改造最多的关键途径。在谷氨酸途径中,L-Glu被proB编码的γ-谷氨酰激酶(γ-glutamyl kinase, GK) 磷酸化为γ-谷氨酰磷酸(γ-glutamyl phosphate, GP),消耗一分子ATP;随后,GP通过proA编码的γ-谷氨酰磷酸还原酶(γ-glutamyl phosphate reductase, GPR) 还原为L-谷氨酸-γ-半醛(L-glutamate-γ-semialdehyde, GSA),消耗一分子NADPH;GSA进而自发环化为Δ1-吡咯啉-5-羧酸(Δ1-pyrroline-5-carboxylate, P5C);最后,通过proC编码的Δ1-吡咯啉-5-羧酸还原酶(Δ1-pyrrolin-5-carboxylate reductase, P5CR) 将P5C还原形成L-Pro,消耗一分子NADPH[75],见图 2。其中,GK是谷氨酸途径中的第一个酶,它既是限速酶并且对最终产物L-Pro的反馈抑制也很敏感,是L-Pro合成的主要调控位点。
|
| 图 2 谷氨酸途径合成L-脯氨酸及其分解代谢 Fig. 2 Synthesis and catabolism of L-Pro by glutamate pathway. |
| |
L-Pro在相关酶的催化下可以分解成L-Glu,其合成和分解代谢过程的中间产物P5C/GSA相同,但参与催化反应的酶却不同,因此并非完全为合成过程的逆反应,见图 2。首先,脯氨酸脱氢酶(proline dehydrogenase, PRODH) 催化L-Pro生成P5C,同时FAD接受电子形成FADH2;随后P5C非酶水解生成GSA;GSA在L-谷氨酸-γ-半醛脱氢酶(L-glutamate-γ-semialdehyde dehydrogenase, GSADH) 的作用下被氧化成L-Glu,同时NAD+接受电子形成NADH[76-77]。上述途径中的2个重要酶PRODH和GSADH共同由putA基因编码,同时,putA在脯氨酸缺失的情况下会作为转录阻遏因子抑制putA和putP的表达[78]。putA、putP和putC共同组成L-Pro利用基因簇[79-80],putP编码的蛋白是一个Na+-Pro共转运蛋白[81],putC基因的产物则可调控PRODH的合成[78]。L-Glu除了在proBAC基因的指导下合成L-Pro,也可以作为底物合成Arg、γ-氨基丁酸(γ-aminobutyric acid, γ-GABA) 以及谷氨酰胺(glutamine, Gln)。在L-Glu合成L-Pro的代谢通路下,Arg、γ-GABA以及Gln作为竞争性旁路途径,会影响代谢流的走向,导致L-Pro合成减少,见图 1。
2.1.2 精氨酸途径合成L-脯氨酸的相关代谢除了最常见的谷氨酸途径合成L-Pro外,Fichman等和Adams等在其研究中提到某些生物可通过精氨酸的代谢途径合成L-Pro[82-83]。包括以下4种途径,见图 1。第1种途径是在proBA基因缺失的大肠杆菌(Escherichia coli) 和鼠伤寒沙门氏菌(Salmonella typhimurium) 中,argD编码的N-乙酰鸟氨酸氨基转移酶失活时会导致AGASA过量积累;过量的AGASA在N-乙酰鸟氨酸酶(N-acetylornithinase, NAO) 的催化下形成GSA,自发环化为P5C,在P5CR的催化下形成L-Pro[82, 84]。第2种途径是在枯草芽孢杆菌(Bacillus subtilis) 的proA缺失的突变体中,发现鸟氨酸-δ-转氨酶(ornithine δ-aminotransferase, δ-OAT) 使用辅因子吡哆醛-5-磷酸(pyridoxal-5-phosphate, PLP) 催化Orn和α-KG可逆地形成GSA/P5C,进而合成L-Pro[85-86]。第3种途径是在缺失精氨酸阻遏蛋白(argR) 和鸟氨酸转氨甲酰酶(argF) 的谷氨酸棒杆菌(Corynebacterium glutamicum) 中,由ocd编码的鸟氨酸环化脱氨酶(ornithine cyclodeaminase, OCD) 催化Orn一步形成L-Pro[82-83, 87]。第4种途径是鸟氨酸α-氨基转移酶(ornithine α-aminotransferase, α-OAT) 将Orn转化为5-氨基-2-氧戊酸(5-amino-2-oxopentanoate, AOP),自发环化成Δ1-吡咯啉-2-羧酸(Δ1-pyrroline-2-carboxylate, P2C),再由Δ1-吡咯啉-2-羧酸还原酶(Δ1-pyrroline-2-carboxylate reductase, P2CR) 还原成L-Pro[82]。
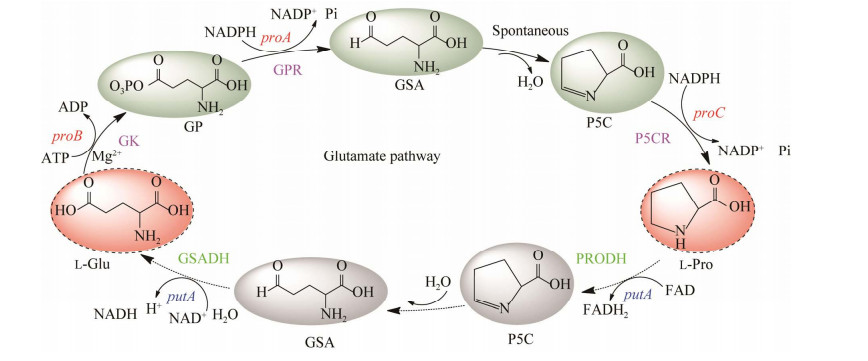
2.2 反式-4-羟基-L-脯氨酸的代谢途径微生物合成T-4-Hyp需要先合成L-Pro作为底物,并在脯氨酸羟化酶(proline hydroxylase, P4H) 的催化下完成。P4H也被称为Fe(Ⅱ)/α-KG依赖性双加氧酶,该反应的顺利进行除了需要L-Pro的供应外,还需要充足的α-KG和O2参与,见图 3。在微生物利用L-Pro合成T-4-Hyp的过程中,α-KG作为共底物发挥着重要作用[88-89],因此需了解相关代谢途径中对α-KG代谢流产生影响的因素。α-KG在TCA循环中会依次被sucAB和ipdA编码的α-酮戊二酸脱氢酶(α-ketoglutarate dehydrogenase, AKGDH) 复合体催化生成琥珀酰辅酶A (succinyl-coenzyme A, Suc-CoA) 随后被sucCD编码的琥珀酰辅酶A合成酶(succinyl-CoA synthase, SCS) 催化生成琥珀酸;而在微生物中导入p4h后,α-KG可直接被P4H催化生成琥珀酸[90],见图 1和图 3。在微生物中,还有两种情况会影响α-KG的代谢流:(1) 异柠檬酸通过乙醛酸途径在aceA编码的异柠檬酸裂解酶(isocitrate lyase, ICL) 的作用下直接产生琥珀酸,而越过α-KG的合成;(2) aceK编码的异柠檬酸脱氢酶激酶(isocitrate dehydrogenase kinase, IDHK) 使异柠檬酸脱氢酶(isocitrate dehydrogenase, IDH) 被磷酸化[91]而失去活性,不能催化异柠檬酸转化为α-KG,见图 1。
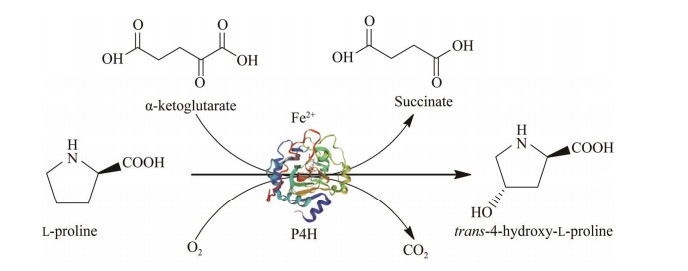
|
| 图 3 脯氨酸羟化酶催化L-脯氨酸合成反式-4-羟基-L-脯氨酸 Fig. 3 T-4-Hyp is synthesized by proline hydroxylase from L-Pro. |
| |
鉴于L-Pro需求量不断增长及其规模化生产水平还不能满足当今社会需要的现状,以及对L-Pro代谢途径及其关键酶的深入了解,研究者不断探索提高微生物生产L-Pro产量的策略,并取得了较大突破。目前普遍采用的是谷氨酸途径生产L-Pro,研究者大多选择以Glc为底物从头合成L-Pro,而早期需要外源添加L-Glu;微生物生产L-Pro的工程菌株主要是谷氨酸棒杆菌(Corynebacterium glutamicum),前期也有一部分研究者对其他几种菌株生产L-Pro的能力进行了研究。随着对精氨酸途径的了解,一些研究者也探索了精氨酸途径生产L-Pro的情况,并取得了显著的成果,见表 2。
| Metabolic pathways | Substrate | Microorganism | Metabolic engineering strategies | Culture system | L-Pro production | Reference | ||
| C (g/L) | P (g/(L·h)) | R (%) | ||||||
| Glutamate pathway | Glucose; Ile | C. glutamicum | (1) Ilem; (2) 200 μg/mL Ile | NA | 14.80 | NA | NA | [95] |
| Glucose; Sugarcane molasses | C. glutamicum | (1) Nucleic acidsm; (2) POESA | Fed-batch; 5 L |
30.00 | 0.50 | NA | ||
| Glucose | C. glutamicum | (1) +proBo; (2) ΔputA, (3) acno |
Fed-batch; 7.5 L |
66.43 | 1.11 | 26.00 | [96] | |
| Glucose | C. glutamicum | (1) +proBm; (2) proBoe, gdhAoe; (3) ΔputA; ΔavtA; (4) odhAw; (5) zwfm, gndm |
Shake-flask | 19.68 | NA | NA | [97] | |
| Fed-batch; 3 L | 120.18 | 1.58 | 20.00 | |||||
| Glucose | C. glutamicum | (1) +proBm; (2) Fine-tuned: gdh, proBAC, pyc, gapN; (3) ΔputA; (4) Cgl2622oe; Δcgl1270 |
Fed-batch; 5 L |
142.40 | 2.90 | 31.00 | [98] | |
| Glucose | C. acetoacidophilum | (1) Mutagenesis; (2) Recombination; (3) Screening |
Shake-flask | 75.60 | 1.05 | NA | [99] | |
| Fed-batch; 50 L |
82.10 | 1.03 | NA | |||||
| Glucose | C. acetoacidophilum | Optimizing conditions | Fed-batch; 50 L |
112.00 | 1.56 | 42.20 | [100] | |
| Glucose; Ile | B. flavum | (1) Ilem; (2) Nucleic acidsm; | NA | 40.00 | 0.56 | NA | [101] | |
| Sucrose | S. marcescens | Optimizing conditions | Fed-batch | 100.00 | 1.04 | NA | [102] | |
| Glucose; Glutamate | C. acetoacidophilum | Optimizing conditions | Batch shake-flask |
52.00 | 0.54 | NA | [103] | |
| Glucose; Glutamate | C. acetoacidophilum | (1) Mutagenesis; (2) Screening; (3) Optimizing conditions | Fed-batch; 2 L |
108.3 | 2.58 | 30 | [104] | |
| Glucose; Glutamate | B. diminuta | (1) Screening; (2) Mutagenesis; (3) Optimizing conditions | Fed-batch; 5 L |
57.60 | 0.94 | NA | [105] | |
| Arginine pathway | Glucose; Arginine | C. pkinense | Optimizing conditions | Shake-flask | 25.00 | 0.21 | NA | [106] |
| Glucose; Arginine | C. glutamicum | (1) +ocdoe; (2) NAGK geneoe | Batch | 12.70 | 0.42 | 36 | [87] | |
| Glucose | C. crenatum | (1) ocdK205G/M86K/T162A; (2) argFw |
Fed-batch; 5 L |
38.40 | 0.64 | NA | [74] | |
| C: concentration; P: productivity; R: the conversion rate of substrate; NA: not available; o: optimization of the gene codon usage or promoters or expression vectors; oe: overexpression; m: mutation of gene; +: expresstion of the gene; w: weakening; POESA: polyoxyethylenestearylamine; NAGK: N-acetylglutamate kinase. | ||||||||
在微生物发酵生产L-Pro的研究中,C. glutamicum作为生产L-Pro的重要工程菌株被认为是安全无害的(generally recognized as safe, GRAS)[92-94]。起初研究者主要通过营养缺陷型或反馈型抑制的诱变选择获得高产L-Pro的C. glutamicum菌株,比如Araki等筛选到异亮氨酸(isoleucine, Ile) 营养缺陷菌株ATCC 19223和核酸碱基突变菌株R-412,其L-Pro产量均在15–30 g/L左右[95]。随着L-Pro代谢途径的深入解析,近些年研究者的改造焦点包括L-Pro的全合成途径、辅因子循环系统和转运蛋白的优化。Zhang等构建了C. glutamicum全基因组代谢模型,该模型仅需改造putA、proB和acn编码的顺乌头酸酶(aconitase, ACO) 基因就能实现碳代谢流流向L-Pro,最优菌株pro6发酵60 h合成L-Pro达66.43 g/L[96]。Zhang等在优化了C. glutamicum遗传改造系统(SacB-assisted CRISPR-Cpf1 system) 的基础上,过表达了proBG149K和gdhA以提高L-Pro的代谢流,弱化了α-KG脱氢酶基因(odhA) 以调控TCA循环,敲除了丙酮酸氨基转移酶基因avtA和putA以解除旁路途径和分解途径的影响,突变了磷酸戊糖途径中的葡萄糖-6-磷酸-脱氢酶基因zwf和6-磷酸葡萄糖酸脱氢酶基因gnd以提高NADPH的供应,最终高产菌株ZQJY-9以Glc为底物发酵76 h合成L-Pro 120.18 g/L[97]。Liu等进一步改造了C. glutamicum的CRISPR/Cas9介导的基因编辑系统,利用饱和突变筛选出了具有proBV150N的重组菌株,借助代谢流分析发现丙酮酸羧化酶基因pyc和甘油醛-3-磷酸脱氢酶基因gapN的加强有利于ATP和NADPH的供应,进一步采用启动子文库在基因组上强化了gdh、pyc和gapN的表达,同时在putA位点替换并加强了proBV150NAC的表达,最后在基因组上过表达了一个拷贝的L-Pro转运蛋白基因cgl2622并且敲除了L-Glu转运蛋白基因cgl1270;利用以上过程构建出不含质粒、不用抗生素和诱导剂的菌株PRO-19在5 L发酵罐中利用生物素限制法分批补料发酵49 h产生L-Pro达142.4 g/L,产率为2.90 g/(L·h)[98],是目前L-Pro发酵法的最高产量。
除了用C. glutamicum作为工程菌株发酵生产L-Pro,嗜醋酸棒杆菌(Corynebacterium acetoacidophilum)、黄色短杆菌(Brevibacterium flavum)、粘质沙雷氏菌(Serratia marcescens) 以及缺陷短波单胞菌(Brevendimonas diminuta) 也被用于微生物发酵生产L-Pro的研究中。卢伟宁等获得一株高产L-Pro的C. acetoacidophilum,50 L发酵罐中获得82.1 g/L的L-Pro[99]。全冰华同样以C. acetoacidophilum作为工程菌,通过优化培养条件、补料方式、底物添加实现在50 L发酵罐中75 h产生L-Pro 112 g/L[100],使得产量有了进一步的提高。Nakamori等以B. flavum 2247为出发菌株,经亚硝基胍处理获得异亮氨酸营养缺陷型和磺基鸟苷抗性突变株,在含有10%葡萄糖、6%硫酸铵、无机盐等组分的条件下,菌株B. flavum P-390产L-Pro 40 g/L[101]。Masuda等使用S. marcescens SP511生产L-Pro,在以铵作为氮源的培养基中,在连续添加蔗糖至终浓度32%条件下,培养4 d,L-Pro可达到100 g/L[102]。此外,在早期发酵生产L-Pro的研究中,许多研者会外源添加L-Glu,以提高L-Pro产量。张伟国等以C. acetoacidophilum XQ3作为工程菌,外源添加L-Glu,发酵96 h产L-Pro约为52 g/L[103]。Nakanishi等从C. acetoacidophilum ATCC 13870菌株中筛选到不依赖于生物素浓度或高盐浓度(超过4%) 的L-Pro产生菌,在2 L发酵罐中,L-Glu浓度为6%、硫酸铵浓度为2.5%,发酵42 h可积累L-Pro 108.3 g/L,Glc转化率达到30%以上。该研究中硫酸铵扮演着两个重要的角色:一是作为铵离子来源供给氮源;二是在调节渗透压的同时其硫酸根离子可作为钠离子中和剂,使调节pH的氨水顺利进入介质[104]。在外源添加L-Glu的研究中,工程菌株除了C. acetoacidophilum外,赵世杰还鉴定了一株B. diminuta,进行抗性筛选、生长优势筛选、耐盐筛选、复合诱变后,获得高产菌株在含Glc的5 L发酵罐中流加L-Glu,61 h可生产L-Pro 57.6 g/L,产率为0.944 g/(L·h)[105]。
3.2 精氨酸途径生产L-脯氨酸随着精氨酸代谢途径产L-Pro的发现及其深入研究,采用精氨酸途径生产L-Pro也逐渐引起研究者的重视。Csonka等发现在argD proB或argD proA双突变体中,L-Pro的生物合成受到Arg的调节而不再对L-Pro的反馈抑制作出反应,proBA argD argR突变体在缺乏Arg的培养基中分泌L-Pro[75],这激发了研究者探索精氨酸途径合成L-Pro的兴趣。方佩静等使用北京棒状杆菌(Corynebacterium pkinense) 的鸟氨酸缺陷型突变株在含精氨酸、生物素和高浓度铵离子的条件下,30 ℃培养5 d,可产生L-Pro 25 g/L[106],虽然产量不高,但却有力地证明了该途径的可行性。Jensen等将来自恶臭假单胞菌(Pseudomonas putida) 的基因ocd导入C. glutamicum中,并过表达编码N-乙酰谷氨酸激酶(N-acetylglutamate kinase, NAGK) 的基因,在含Arg的培养基中30 h内可由Glc合成L-Pro 12.7 g/L,转化率为0.36 g Pro/g Glc[87],极大地缩短了发酵时间,提高了转化率。龙梦飞进一步优化酶与代谢途径,利用定向进化技术(pEvolvR-rara codons selection) 筛选到P. putida来源的突变基因ocdK205G/M86K/T162A并导入高产L-Arg的C. crenatum (a subspecies of C. glutamicum) 中,使用sRNA干扰argF的表达使其下调到41.6%的同时结合核糖体结合位点(ribosome binding site, RBS)优化ocdK205G/M86K/T162A的表达量,以Glc为底物采用分批补料技术在5 L发酵罐中60 h产生38.4 g/L L-Pro,同时L-Arg和L-Orn的浓度均小于1 g/L[74]。精氨酸途径虽然代谢路径长、反应过程复杂,但整个过程可以同时合成多种代谢产物,且可以很好地避免产物抑制效应,因此这一途径可作为新的契机深入探究。此外,也有研究证明精氨酸途径在T-4-Hyp的合成中也具有重要意义[84]。
4 微生物生产反式-4-羟基-L-脯氨酸的代谢改造策略与研究进展T-4-Hyp的生产主要以大肠杆菌(Escherichia coli) 作为工程菌株,此外也有以C. glutamicum作为工程菌株的报道。研究者重点从筛选羟化酶、优化L-Pro的合成和降解途径、提高α-KG供应和增加溶解氧等方面进行了深入探究和优化。在底物选择上,研究者起初是在Glc的基础上外源添加L-Pro或者利用L-Pro作为全细胞催化的底物直接产生T-4-Hyp,随后进一步将代谢途径延伸至Glc,从头合成T-4-Hyp。此外,还有用甘油或玉米芯替代Glc作为底物或者以自养形式合成T-4-Hyp的研究,见表 3。
| Optimizing strategies | Substrate | Microorganism | Source of p4h | Metabolic engineering strategies | Culture system | T-4-Hyp production | Reference | ||
| C (g/L) | P (g/(L·h)) | R (%) | |||||||
| Hydroxylase | Glucose; L-proline | E. coli | Dactylosporangium sp. | (1) +Dsp4ho; (2) Promoterso |
Batch; 5 L | 41.00 | 0.41 | 87.00a | [108] |
| Glucose; L-proline | E. coli | Dactylosporangium sp. | (1) +Dsp4ho; (2) Fermentationo |
Fed-batch | 42.50 | 0.97 | 81.10a | [109] | |
| Glucose; Isoleucine | C. glutamicum | Dactylosporangium sp. | (1) +Dsp4h; (2) Ilem; (3) Glc: Ile (46:1) |
Fed-batch; | 7.10 | 0.31 | 98.50a | [110] | |
| Hydroxylase; pathways of L-Pro |
Glucose | E. coli | Dactylosporangium sp | (1) +Dsp4ho; (2) +proB74o, (3) + proAo; (4) Δput A |
Batch; 5 L | 25.00 | 0.26 | NA | [111] |
| Glucose | E. coli | A. mediterranea | (1) ΔputA; (2) +alp4hgm; (3) +proB74oeAco+ alp4hco |
Fed-batch; 5 L |
45.83 | 1.27 | NA | [112] | |
| Glucose | E. coli | Micromonospora sp. | (1) +PT7-mp4hgm; (2) +Ptrc-pro; (3) operono; (4) NADPHo; (5) Δput A |
Fed-batch; 5 L |
48.60 | 1.22 | 21.60 | [113] | |
| Hydroxylase; pathways of L-Pro; supply of α-KG |
Glucose | C. glutamicum | Dactylosporangium sp | (1) ΔsucCD; (2) +RBSo-Dsp4h; (3) RBS+proBm; (4) +Dsp4hco-proBco |
Shake-flask | 21.72 | 0.36 | 27.00 | [114] |
| Glucose | E. coli | Dactylosporangium sp. | (1) ΔputA; (2) ΔsucAB; (3) ΔaceAK; (4) +Trc-Trp; (5) +p4hco-proBAco |
Fed-batch; 5 L |
31.00 | 0.60 | NA | [115] | |
| Glucose | E. coli | B. cereus | (1) +bp4h; (2) Ptrc99a+ proBAco+bp4hco; (3) +E. coli 3ΔW3110 |
Fed-batch; 5 L |
46.20 | 0.96 | NA | [116] | |
| Glucose | E. coli | Uncultured bacterium | (1) +Ubp4hm; gm; (2) +proBm; (3) ΔputA; ΔputP; ΔproP; (4) ΔaceA; (5) PesaSre+sucA |
Fed-batch; 7.5 L |
54.80 | 0.91 | 23.60 | [117] | |
| Glucose; α-KG | E. coli | Micromonospora sp. | Fed-batch α-KG and glucose | Fed-batch; 30 L |
62.14 | 1.64 | 22.37 | [118] | |
| 14.40 | 0.25 | NA | [122] | ||||||
| Glucose; L-proline | E. coli | Dactylosporangium sp. | (1) +p4hco+vgbco; (2) Δput A; (3) Fermentationo |
Fed-batch; 7 L |
45.23 | 0.57 | NA | [81] | |
| Glucose; L-proline | E. coli | Dactylosporangium sp. | (1) +Dsp4hoe; (2) ΔputA; (3) ΔsucCD; (4) +vgb; (5) RBSo |
Batch; 5 L | 49.80 | 1.38 | 87.40a | [124] | |
| Glucose; L-proline | E. coli | Dactylosporangium sp. | (1) +Dsp4hoe; (2) Kinetic parameterso; (3) Fermentationo; (4) Substrateo |
Whole-cell catalysis; 5 L |
99.90 | 2.77 | 99.90a | [125] | |
| Glucose | E. coli | NA | Optimal protease, concentration, addition time |
Fed-batch; 5 L | 45.30 | 1.19 | 18.10 | [126] | |
| Glucose | E. coli | NA | Dissolved oxygen stage control strategy | Fed-batch; 30 L | 45.30 | 1.42 | 20.70 | [127] | |
| New exploration | Glycerin | E. coli | Dactylosporangium sp. | (1) +Dsp4ho; (2) +Dsp4hm; (3) Fermentationo; |
Fed-batch; 5 L |
25.40 | 0.53 | NA | [130] |
| Corncob | C. glutamicum | Uncultured bacterium | (1) +Ubp4hgm; (2) +ocd; (3) ΔputA |
Shake-flask | 0.33 | NA | NA | [131] | |
| Photoautotrophy | Synechocystis sp. | Dactylosporangium sp. | NA | NA | NA | NA | NA | [132] | |
| C: concentration; P: productivity; R: conversion rate of substrate; a: conversion rate of L-Pro to T-4-Hyp; NA: not available; o: optimization of the gene codon usage or promoters or expression vectors; oe: overexpression; m: mutation of gene; w: weakening; +: expresstion of the gene; Δ: deletion of the gene; co: coexpression; gm: genome mining; re: replacement of the gene. | |||||||||
采用以L-Pro为底物的生物转化法生产T-4-Hyp的核心是找到合适的羟化酶使其在微生物体内催化L-Pro生成T-4-Hyp。由于常用的工程菌株不存在脯氨酸羟化酶基因p4h或者表达较弱,一般需要外源引入高活性的P4H。Shibasaki等对8株不同种类的菌株进行全细胞酶活测定,发现来源于指孢囊菌RH1 (Dactylosporangium sp. RH1) 的P4H酶活最高,将该基因Dsp4h在E. coli中重组表达,其酶活提高了约12.6倍[107]。Shibasaki等随后利用色氨酸串联启动子在E. coli中过表达密码子优化后的Dsp4h,以L-Pro和Glc作为共底物,在5 L发酵罐中发酵100 h,获得T-4-Hyp 41 g/L[108]。刘合栋在E. coli中进一步改进发酵条件,以Glc和L-Pro作为底物并流加Glc,44 h后T-4-Hyp产量达到42.5 g/L,产率为0.966 g/(L·h),极大地缩短了发酵时间[109]。Falcioni等将Dsp4h引入异亮氨酸(isoleucine, Ile) 营养突变型菌株C. glutamicum中,发现Glc/Ile摩尔比为46︰1时,23 h内可以产生7.1 g/L T-4-Hyp,转化率为98.5%[110]。此外研究者也通过不断探索和筛选发现来源于小单孢菌属(Micromonospora sp. CNB394)、地中海交替单胞菌(Alteromonas mediterranea)、蜡样芽孢杆菌(Bacillus cereus) 以及未培养细菌(uncultured bacterium) 等的P4H也都能有效地将L-Pro转化为T-4-Hyp。
4.1.2 优化L-Pro的合成和降解途径若采用以Glc为底物的直接发酵法,则还需在优化羟化酶相关基因的基础上进一步优化Glc合成L-Pro的途径,尤其是谷氨酸合成途径中的proB和分解代谢中的putA两种基因。Shibasaki等将定点突变优化后的proB74A导入含有Dsp4h的质粒中,同时无义突变了E. coli基因组中L-Pro降解途径的关键基因putA,以Glc为单一底物,在5 L发酵罐中96 h合成T-4-Hyp 25 g/L[111]。Wang等同样优化了谷氨酸合成与分解代谢途径,并筛选到来自A. mediterranea的p4h,将其与优化后的proB74A串联表达,导入高产L-Pro的E. coli/∆putA中,以Glc为底物36 h积累45.83 g/L T-4-Hyp[112],产量提高了1.8倍,时间缩短至原来的37.5%。李强等同样敲除putA、基因组整合表达proBA并在此基础上进一步增加NADPH供应,这些措施强化了L-Pro的合成,加上导入从Micromonospora sp.中获得的p4h,使得优化后的E. coli培养40 h后T-4-Hyp达到48.6 g/L[113]。
4.1.3 优化α-KG的供应在优化羟化酶和谷氨酸途径的基础上,研究者进一步发现TCA循环中α-KG的代谢流也会影响T-4-Hyp的合成,具体表现为α-KG作为共底物被大量消耗或其供应不足,都会降低T-4-Hyp的产量。考虑到P4H的双加氧酶特性,当在微生物中导入p4h后,P4H催化L-Pro生成T-4-Hyp的同时,也会催化α-KG直接生成琥珀酸[90],切断TCA循环中由α-KG到琥珀酸的代谢流而通过P4H连接,既联通了TCA循环,保证其完整性,又可以促进T-4-Hyp的合成,以提高其产量,见图 1。Zhang等以实验室已有的高产L-Pro的C. glutamicum作为基础菌株[96],敲除sucCD以减少α-KG的消耗,并结合羟化酶和谷氨酸途径的优化,即利用Ptac启动子串联表达RBS微调后的Dsp4h和proB,得到的工程菌株Hyp-7在摇瓶中以Glc为底物补料分批发酵60 h,T-4-Hyp产量可达21.72 g/L,容积产率为0.36 g/(L·h)[114]。此外乙醛酸途径的存在和IDH的磷酸化会导致α-KG的供应减少,相应地可以敲除aceA和aceK以增加α-KG的供应。Zhang等在敲除putA的基础上,进而敲除了TCA途径中的关键基因sucAB和aceAK,并导入利用串联杂交启动子(Trc-Trp) 共表达Dsp4h和proBA的质粒,最优菌株52 h产T-4-Hyp 31 g/L[115]。Wang等分离到来源于B. cereus的p4h基因,合成pTrc99a-proBA-bp4h串联表达质粒,导入到E. coli/ΔputAΔsucAB ΔaceAK菌株中,48 h后T-4-Hyp产量为46.2 g/L,产率为1.0 g/(L·h)[116],和前者相比其羟化酶的来源不同,结果缩短了发酵时间,增加了T-4-Hyp产量,说明新筛选到的B. cereus来源的羟化酶活性优于Dsp4h。Long等利用稀有密码子体内进化技术(rare-codon selection-based in vivo evolution) 获得了一株L-Pro高产菌株,对其进行转录组分析后继续敲除putA、putP、proP (L-Pro转运系统) 和aceA以获得L-Pro合成代谢流的最大化;引入3-氧代己酰基高丝氨酸内酯(3-oxohexanoylhomoserine lactone, AHL) 调控系统,以PeaS替换sucA原有启动子动态控制TCA循环保证a-KG的供应,最后利用基因组挖掘技术(genome mining) 得到未培养细菌的p4hL170A/P172N基因提高羟化酶酶活,在7.5 L发酵罐中60 h可产T-4-Hyp 54.8 g/L[84, 117]。蔡萌萌等发现α-KG对菌体生长有一定的抑制作用,在以Glc为底物的发酵过程中随糖流加5 g/L α-KG,使T-4-Hyp的产量在30 L发酵罐中达到62.14 g/L[118]。
4.1.4 提高溶解氧,增加反式-4-羟基-L-脯氨酸产量进一步的研究发现,由于P4H是依赖α-KG的双加氧酶这一特性,整个羟基化过程除了保障α-KG的供应外,还需要充足的氧气。此外,微生物在培养过程中形成的高黏度也阻碍了氧气转移[110]。发酵液溶解氧降低还会导致副产物乙酸积累,抑制细胞生长和产物积累[119-120]。研究者发现了一种由vgb编码的透明颤血红蛋白(vitreoscilla hemoglobin, VHB),它具有极高的氧解离速率,能很好地解决细胞溶解氧的问题[121]。Zhao等将vgb整合到表达启动子优化后的Dsp4h的E. coli染色体中,以避免质粒在发酵过程中丢失,在1.4 L反应器培养57 h后可产T-4-Hyp 14.4 g/L,同时也发现VHB的表达使乙酸浓度降低77%[122]。张胜利等进一步将优化溶解氧与优化羟化酶和谷氨酸途径相结合,即在E. coli/ΔputA中引入Dsp4h和vgb共表达质粒pUHVT4以提高溶解氧,在Glc︰甘油︰玉米浆为8︰10︰8的GC培养基中以1.6 g/(L·h)补糖,外源添加L-Pro,80 h后T-4-Hyp产量为45.23 g/L[81, 123]。Chen等在E. coli中过表达了Dsp4h和RBS微调后的vgb以实现细胞呼吸和T-4-Hyp合成的平衡,敲除了putA和sucCD以提高L-Pro和α-KG的供应,最终在以L-Pro和Glc作为共同底物的5 L发酵罐中T-4-Hyp产量达到49.8 g/L[124]。Chen等进一步利用全细胞催化法,以L-pro作为底物生产T-4-Hyp,在对温度、pH、培养基成分、诱导时间、诱导剂浓度以及催化体系等一系列因素进行优化后,5 L反应器中36 h可生产T-4-Hyp 99.9 g/L,L-pro合成T-4-Hyp的转化率为99.9%,产率为2.77 g/(L·h)[125]。此外,也有研究者采用了其他的策略以提高溶解氧,比如Liu提出在T-4-Hyp发酵过程中加入蛋白酶水解可溶性蛋白以增加溶解氧,且加入的蛋白酶不会破坏T-4-Hyp,最佳条件下,5 L发酵罐中发酵液可溶性蛋白含量降低43.5%,T-4-Hyp产量达到45.3 g/L,Glc转化率为18.1%[126]。蔡萌萌等采用分阶段控制溶氧,30 L发酵罐中T-4-Hyp的产量为45.3 g/L,Glc转化率为20.7%[127]。
4.2 微生物以其他底物生产反式-4-羟基-L-脯氨酸除了以Glc或L-Pro为底物,一些学者也探究了使用廉价副产物或者可再生资源作为碳源生产T-4-Hyp,既可减少环境污染,又能避免资源浪费,体现了绿色环保的理念。甘油可作为天然碳源,用于微生物生产T-4-Hyp,而且以甘油为碳源还能避免乙酸的积累[128-129]。Wang等将密码子优化后的Dsp4h导入E. coli BL21(DE3) 中,通过诱变处理筛选到以甘油为唯一碳源的菌株NA45,优化培养基后在5 L发酵罐中48 h可产25.4 g/L T-4-Hyp[130]。李浩以玉米芯酶解液作为底物,以导入ocd、p4h,同时敲除putA的C. glutamicum作为工程菌株,摇瓶发酵92 h后,T-4-Hyp产量为327 mg/L[131]。Brandenburg等将Dsp4h导入集胞蓝藻(Synechocystis sp. PCC 6803) 中,首次引入光能作为能源物质生产T-4-Hyp[132],但由于人们对光自养细菌的理解和设计能力有限,以及实验所需反应器生产技术的限制,其研究尚不够成熟,产量仍有待进一步提高。
5 总结与展望近年来,合成生物学的飞速发展为氨基酸及其衍生物的工业化生产打开了新的局面。通过对微生物代谢网络的深入解析,L-Pro和T-4-Hyp的合成和降解途径、旁路途径及其与三羧酸循环的关联都已经研究透彻,对代谢途径中关键酶的结构与功能也已经基本阐明。由于L-Pro和T-4-Hyp独特而广泛的生理生化功能,将现有微生物细胞工厂的成果用于工业化大规模生产仍然无法满足其日益增长的需求。这就需要我们在前人的基础上继续采用“绿色生物制造”的理念,结合基因组学、蛋白组学、代谢组学与合成生物学等先进手段,对代谢途径进行重新设计和定向改造,筛选出具有更高催化效率的关键酶,精准地控制L-Pro和T-4-Hyp生产过程中的碳通量,建构出更加高效专一地合成L-Pro和T-4-Hyp的细胞工厂。
| [1] |
Wu G, Bazer FW, Burghardt RC, et al. Proline and hydroxyproline metabolism: implications for animal and human nutrition. Amino Acids, 2011, 40(4): 1053-1063. DOI:10.1007/s00726-010-0715-z
|
| [2] |
Taga Y, Kusubata M, Mizuno K. Quantitative analysis of the positional distribution of hydroxyproline in collagenous Gly-Xaa-Yaa sequences by LC-MS with partial acid hydrolysis and precolumn derivatization. Anal Chem, 2020, 92(12): 8427-8434. DOI:10.1021/acs.analchem.0c01098
|
| [3] |
Berisio R, Granata V, Vitagliano L, et al. Characterization of collagen-like heterotrimers: implications for triple-helix stability. Biopolymers, 2004, 73(6): 682-688. DOI:10.1002/bip.20017
|
| [4] |
Srivastava AK, Khare P, Nagar HK, et al. Hydroxyproline: a potential biochemical marker and its role in the pathogenesis of different diseases. Curr Protein Pept Sci, 2016, 17(6): 596-602. DOI:10.2174/1389203717666151201192247
|
| [5] |
Christgen SL, Becker DF. Role of proline in pathogen and host interactions. Antioxid Redox Sign, 2019, 30(4): 683-709. DOI:10.1089/ars.2017.7335
|
| [6] |
Hu CA, Phang JM, Valle D. Proline metabolism in health and disease. Amino Acids, 2008, 35(4): 651-652. DOI:10.1007/s00726-008-0102-1
|
| [7] |
Cohen SM, Nadler JV. Proline-induced potentiation of glutamate transmission. Brain Res, 1997, 761(2): 271-282. DOI:10.1016/S0006-8993(97)00352-1
|
| [8] |
Nam SY, Yoou MS, Kim HM, et al. Efficacy of proline in the treatment of menopause. Exp Biol Med, 2016, 241(6): 611-619. DOI:10.1177/1535370216629011
|
| [9] |
Li H, Li S, Yang H, et al. L-proline alleviates kidney injury caused by AFB1 and AFM1 through regulating excessive apoptosis of kidney cells. Toxins, 2019, 11(4): 226. DOI:10.3390/toxins11040226
|
| [10] |
Chen X, Qin S, Zhao X, et al. L-proline protects mice challenged by Klebsiella pneumoniae bacteremia. J Microbiol Immunol, 2021, 54(2): 213-220.
|
| [11] |
Du J, Zhu S, Lim RR, et al. Proline metabolism and transport in retinal health and disease. Amino Acids, 2021, 53(12): 1789-1806. DOI:10.1007/s00726-021-02981-1
|
| [12] |
Liang X, Zhang L, Natarajan SK, et al. Proline mechanisms of stress survival. Antioxid Redox Sign, 2013, 19(9): 998-1011. DOI:10.1089/ars.2012.5074
|
| [13] |
Zhang L, Xue X, Yan J, et al. Cryobiological characteristics of L-proline in mammalian oocyte cryopreservation. Chin Med J, 2016, 129(16): 1963-1968. DOI:10.4103/0366-6999.187846
|
| [14] |
Koštál V, Korbelová J, Poupardin R, et al. Arginine and proline applied as food additives stimulate high freeze tolerance in larvae of Drosophila melanogaster. J Exp Biol, 2016, 219(15): 2358-2367. DOI:10.1242/jeb.142158
|
| [15] |
Dou M, Lu C, Sun Z, et al. Natural cryoprotectants combinations of L-proline and trehalose for red blood cells cryopreservation. Cryobiology, 2019, 91: 23-29. DOI:10.1016/j.cryobiol.2019.11.002
|
| [16] |
Moradi B, Faramarzi A, Ghasemi-Esmailabad S, et al. L-proline as a novel additive to cryopreservation media improved post-thaw quality of human spermatozoon via reducing oxidative stress. Andrologia, 2022, 54(1): 1-8.
|
| [17] |
Packer M, Lee WH, Yushak M, et al. Comparison of captopril and enalapril in patients with severe chronic heart failure. N Engl J Med, 1986, 315(14): 847-853. DOI:10.1056/NEJM198610023151402
|
| [18] |
江澜, 李毓飞, 张立, 等. 维格列汀中间体的合成工艺改进. 中国药物化学杂志, 2021, 31(4): 280-285. Jiang L, Li YF, Zhang L, et al. Improved synthesis process of a vildagliptin intermediate. Chin J Med Chem, 2021, 31(4): 280-285 (in Chinese). |
| [19] |
Mittal S, Tsume Y, Landowski CP, et al. Proline prodrug of melphalan, prophalan-l, demonstrates high therapeutic index in a murine melanoma model. Eur J Pharm Biopharm, 2007, 67(3): 752-758. DOI:10.1016/j.ejpb.2007.03.024
|
| [20] |
邱靖萱. L-脯氨酸及其衍生物的溶解行为与溶剂化效应研究[D]. 长春: 长春工业大学, 2022. Qiu JX. Solubility behavior and solvation effects of L-proline and its derivatives[D]. Changchun: Changchun University of Technology, 2022 (in Chinese). |
| [21] |
郝一彬, 周尔凤, 汤允昭, 等. 硫代脯氨酸对血栓形成和血小板功能的影响. 中国药理学通报, 1990(4): 249-251. Hao YB, Zhou EF, Tang YZ, et al. Effect of thioproline on thrombosis and platelet function. Chin Pharmacol Bull, 1990(4): 249-251 (in Chinese). DOI:10.3321/j.issn:1001-1978.1990.04.024 |
| [22] |
Qiu J, Wang P, Hu S, et al. Solubility behavior and polymorphism of N-acetyl-L-proline in 16 individual solvents from 283.15 to 323.15 K. J Chem Eng Data, 2021, 66(3): 1533-1542. DOI:10.1021/acs.jced.1c00032
|
| [23] |
缪正兴, 张仲明, 李宝忠. L-脯氨酸的生产及其应用. 发酵科技通讯, 2004(2): 21-22. Miao ZX, Zhang ZM, Li BZ. Production and application of L-proline. Bull Ferm Sci Tech, 2004(2): 21-22 (in Chinese). DOI:10.3969/j.issn.1674-2214.2004.02.012 |
| [24] |
林水美. 卡托普利临床新用途. 海峡药学, 2006(5): 136-137. Lin SM. New clinical use of captopril. Strait Pharm J, 2006(5): 136-137 (in Chinese). DOI:10.3969/j.issn.1006-3765.2006.05.076 |
| [25] |
Pugliese A, Marinelli L, Forno B, et al. Ex vivo evaluation of pidotimod effect on immune response. Pharmacol Res, 1992, 26 Suppl 2: 178.
|
| [26] |
Migliorati G, D'Adamio L, Coppi G, et al. Pidotimod stimulates natural killer cell activity and inhibits thymocyte cell death. Immunopharm Immunot, 1992, 14(4): 737. DOI:10.3109/08923979209009231
|
| [27] |
Giagulli C, Noerder M, Avolio M, et al. Pidotimod promotes functional maturation of dendritic cells and displays adjuvant properties at the nasal mucosa level. Int Immunopharmacol, 2009, 9(12): 1366-1373. DOI:10.1016/j.intimp.2009.08.010
|
| [28] |
Moore TO, Paradowski M, Ward SE. An atom-efficient and convergent approach to the preparation of NS5A inhibitors by C-H activation. Org Biomol Chem, 2016, 14(12): 3307-3313. DOI:10.1039/C6OB00340K
|
| [29] |
Sulkowski MS, Gardiner DF, Marbel RT, et al. Daclatasvir plus sofosbuvir for previously treated or untreated chronic HCV infection. NEJM, 2014, 3(370): 211-221.
|
| [30] |
Sato T, Hoshida H, Akada R. Inhibition of distinct proline- or N-acetylglucosamine-induced hyphal formation pathways by proline analogs in Candida albicans. Biomed Res Int, 2020, 2020: 1-10.
|
| [31] |
Peter Gluckman, Krishnamurthi R, Waldvogel HJ, et al. N-terminal tripeptide of IGF-1 (GPE) prevents the loss of TH positive neurons after 6-OHDA induced nigral lesion in rats. Brain Res, 2000, 859(2): 286-292. DOI:10.1016/S0006-8993(00)01988-0
|
| [32] |
Alexey VV, Maxim DE, Lyudmila AA, et al. Phosphine modification of proline-glycine-proline tripeptide and study of its neuroprotective properties. Biochem Bioph Res Co, 2021, 539: 15-19. DOI:10.1016/j.bbrc.2020.12.087
|
| [33] |
Saibi W, Feki K, Yacoubi I, et al. Bridging between proline structure, functions, metabolism, and involvement in organism physiology. Appl Biochem Biotech, 2015, 176(8): 2107-2119. DOI:10.1007/s12010-015-1713-0
|
| [34] |
Mansour MMF, Ali EF. Evaluation of proline functions in saline conditions. Phytochemistry, 2017, 140: 52-68. DOI:10.1016/j.phytochem.2017.04.016
|
| [35] |
Hoffmann T, Bleisteiner M, Sappa PK, et al. Synthesis of the compatible solute proline by Bacillus subtilis: point mutations rendering the osmotically controlled proHJ promoter hyperactive. Environ Microbiol, 2017, 19(9): 3700-3720. DOI:10.1111/1462-2920.13870
|
| [36] |
Mahipant G, Paemanee A, Roytrakul S, et al. The significance of proline and glutamate on butanol chaotropic stress in Bacillus subtilis 168. Biotechnol Biofuels, 2017, 10(1): 1-14.
|
| [37] |
Hayashi Y, Yamaguchi J, Hibino K, et al. Direct proline catalyzed asymmetric α-aminooxylation of aldehydes. Tetrahedron Lett, 2003, 44(45): 8293-8296. DOI:10.1016/j.tetlet.2003.09.057
|
| [38] |
List B. Proline-catalyzed asymmetric reactions. Tetrahedron, 2002, 58(28): 5573-5590. DOI:10.1016/S0040-4020(02)00516-1
|
| [39] |
Córdova A, Sundén H, Bøgevig A, et al. The direct catalytic asymmetric α-aminooxylation reaction: development of stereoselective routes to 1, 2-diols and 1, 2-amino alcohols and density functional calculations. Chem Eur J, 2004, 10(15): 3673-3684. DOI:10.1002/chem.200400137
|
| [40] |
Ahuja BB, Sudalai A. A concise enantioselective synthesis of (2S, 3S)-3-hydroxypipecolic acid via proline catalyzed α-aminooxylation of aldehydes and Pd-catalyzed ether directed aza-claisen rearrangements. Tetrahedron: Asymmetry, 2015, 26(1): 24-28. DOI:10.1016/j.tetasy.2014.11.010
|
| [41] |
左瑞雅, 周小华, 杜首英. L-羟脯氨酸-Zn(Ⅱ)的配合机制及其抗氧化性研究. 生物工程学报, 2007(4): 704-709. Zuo RY, Zhou XH, Du SY. Coordination mechanism and antioxidant activity of L-hydroxyproline zinc(Ⅱ). Chin J Biotech, 2007(4): 704-709 (in Chinese). DOI:10.3321/j.issn:1000-3061.2007.04.027 |
| [42] |
Milić S, Bogdanović Pristov J, Mutavdžić D, et al. The relationship of physicochemical properties to the antioxidative activity of free amino acids in fenton system. Environ Sci Technol, 2015, 49(7): 4245-4254. DOI:10.1021/es5053396
|
| [43] |
Phang JM, Donald SP, Pandhare J, et al. The metabolism of proline, a stress substrate, modulates carcinogenic pathways. Amino Acids, 2008, 35(4): 681-690. DOI:10.1007/s00726-008-0063-4
|
| [44] |
Hu S, He W, Wu G. Hydroxyproline in animal metabolism, nutrition, and cell signaling. Amino Acids, 2022, 54(4): 513-528. DOI:10.1007/s00726-021-03056-x
|
| [45] |
衣玉兰. 反式-4-羟基-L-脯氨酸的生物合成[D]. 上海: 华东理工大学, 2014. Yi YL. Biosynthesis of trans-4-hydroxy-L-proine[D]. Shanghai: East China University of Science and Technology, 2014 (in Chinese). |
| [46] |
Aoki M, Suto K, Komatsu M, et al. Increasing effect of an oral intake of L-hydroxyproline on the soluble collagen content of skin and collagen fragments in rat serum. Biosci Biotechnol Biochem, 2012, 76(6): 1242-1244. DOI:10.1271/bbb.120058
|
| [47] |
Gabr SA, Alghadir AH. Prediction of fibrosis in hepatitis C patients: assessment using hydroxyproline and oxidative stress biomarkers. VirusDis, 2014, 25(1): 91-100. DOI:10.1007/s13337-013-0182-8
|
| [48] |
Cooper SK, Pandhare J, Donald SP, et al. A novel function for hydroxyproline oxidase in apoptosis through generation of reactive oxygen species. J Biol Chem, 2008, 283(16): 10485-10492. DOI:10.1074/jbc.M702181200
|
| [49] |
Wu G. Important roles of dietary taurine, creatine, carnosine, anserine and 4-hydroxyproline in human nutrition and health. Amino Acids, 2020, 52(3): 329-360. DOI:10.1007/s00726-020-02823-6
|
| [50] |
Brands KMJ, Jobson RB, Conrad KM, et al. Efficient one-pot synthesis of the 2-aminocarbonylpyrrolidin-4-ylthio-containing side chain of the new broad-spectrum carbapenem antibiotic ertapenem. The Journal of Organic Chemistry, 2002, 67(14): 4771-4776. DOI:10.1021/jo011170c
|
| [51] |
Vijay Kumar A, Rama Rao K. trans-4-Hydroxy-L-proline: a novel starting material for N-alkylpyrroles synthesis. Tetrahedron Lett, 2011, 52(25): 3237-3239. DOI:10.1016/j.tetlet.2011.04.045
|
| [52] |
Reddy VP, Kumar AV, Rao KR. New strategy for the synthesis of N-aryl pyrroles: Cu-catalyzed C-N cross-coupling reaction of trans-4-hydroxy-L-proline with aryl halides. Tetrahedron Lett, 2011, 52(7): 777-780. DOI:10.1016/j.tetlet.2010.12.016
|
| [53] |
Cao G, Yang K, Li Y, et al. Synthetic strategy and anti-tumor activities of macrocyclic scaffolds based on 4-hydroxyproline. Molecules, 2016, 21(2): 212. DOI:10.3390/molecules21020212
|
| [54] |
Lee RS, Yang JM, Lin TF. Novel, biodegradable, functional poly(ester-carbonate)s by copolymerization of trans-4-hydroxy-L-proline with cyclic carbonate bearing a pendent carboxylic group. Journal of Polymer Science, 2004, 42: 2303-2312.
|
| [55] |
Nagumo S, Matoba A, Ishii Y, et al. Synthesis of (-)-TAN1251A using 4-hydroxy-L-proline as a chiral source. Tetrahedron, 2002, 58: 9871-9877. DOI:10.1016/S0040-4020(02)01292-9
|
| [56] |
Yang H, Male M, Li Y, et al. Efficacy of hydroxy-L-proline (HYP) analogs in the treatment of primary hyperoxaluria in Drosophila Melanogaster. Bmc Nephrol, 2018, 19(1): 1-12. DOI:10.1186/s12882-017-0808-y
|
| [57] |
蒋惠亮, 俞晓峰, 方银军. 1, 4-二棕榈酰羟脯氨酸的合成. 化学世界, 2008(9): 546-552. Jiang HL, Yu XF, Jun FY. Synthesis of 1, 4-dipalmitoyl hydroxyproline. Chem World, 2008(9): 546-552 (in Chinese). DOI:10.3969/j.issn.0367-6358.2008.09.011 |
| [58] |
Bach TMH, Takagi H. Properties, metabolisms, and applications of L-proline analogues. Appl Microbiol Biot, 2013, 97(15): 6623-6634. DOI:10.1007/s00253-013-5022-7
|
| [59] |
Yi Y, Sheng H, Li Z, et al. Biosynthesis of trans-4-hydroxyproline by recombinant strains of Corynebacterium glutamicum and Escherichia coli. Bmc Biotechnol, 2014, 14(1): 44. DOI:10.1186/1472-6750-14-44
|
| [60] |
Nogimura D, Mizushige T, Taga Y, et al. Prolyl-hydroxyproline, a collagen-derived dipeptide, enhances hippocampal cell proliferation, which leads to antidepressant-like effects in mice. The FASEB Journal, 2020, 34(4): 5715-5723. DOI:10.1096/fj.201902871R
|
| [61] |
Heimesaat MM, Heilmann K, Kühl AA, et al. The synthetic hydroxyproline-containing collagen analogue (Gly-Pro-Hyp)10 ameliorates acute DSS colitis. Eur J Microbiol Immu, 2012, 2(3): 192-200. DOI:10.1556/EuJMI.2.2012.3.4
|
| [62] |
Wu G, Bazer FW, Davis TA, et al. Arginine metabolism and nutrition in growth, health and disease. Amino Acids, 2009, 37(1): 153-168. DOI:10.1007/s00726-008-0210-y
|
| [63] |
Inoue N, Sugihara F, Wang X. Ingestion of bioactive collagen hydrolysates enhance facial skin moisture and elasticity and reduce facial ageing signs in a randomised double-blind placebo-controlled clinical study. J Sci Food Agr, 2016, 96(12): 4077-4081. DOI:10.1002/jsfa.7606
|
| [64] |
马政生, 李平, 陈开勋. 以味精为原料制备L-脯氨酸. 西北大学学报(自然科学版), 2000(5): 403-405. Ma ZS, Li P, Chen KX. The synthsis of L-proline from sodium glutamate. J Northwest Uni: (Nat Sci Ed), 2000(5): 403-405 (in Chinese). |
| [65] |
郑素慧, 李文军, 娄恺. L-脯氨酸发酵生产的研究进展. 新疆农业科学, 2007(S2): 6-10. Zheng SH, Li WJ, Lou K. Research progress of L-proline production by fermentation. Xinjiang Agric Sci, 2007(S2): 6-10 (in Chinese). |
| [66] |
郭明, 胡昌华. 生物转化—从全细胞催化到代谢工程. 中国生物工程杂志, 2010, 30(4): 110-115. Guo M, Hu CH. Biotransformation-from whole cell catalysis to metabolic engineering. Chin Biotech, 2010, 30(4): 110-115 (in Chinese). |
| [67] |
张克旭, 高年发, 李冰, 等. 关于谷氨酸发酵向脯氨酸发酵转换的研究. 食品与发酵工业, 1982(5): 1-7. Zhang KX, Gao NF, Li B, et al. Study on the conversion of glutamate fermentation to proline fermentation. Food Ferment Ind, 1982(5): 1-7 (in Chinese). |
| [68] |
杜英华, 陈保国, 贾洪斌. 由骨胶水解液中分离提取L-羟脯氨酸和L-脯氨酸的实验研究. 内蒙古石油化工, 2002(3): 16-18. Du YH, Chen BG, Jia HB. Experimental study on the separation and extraction of L-hydroxyproline and L-proline from bone glue hydrolysate. Inner Mongolia Pe Ind, 2002(3): 16-18 (in Chinese). |
| [69] |
高焕春, 李文英. 从骨胶水解液中分离L-羟脯氨酸和L-脯氨酸. 天津轻工业学院学报, 1997(2): 35-39. Gao HC, Ying LW. Isolating L-hydroxyproline and L-proline from hydrolytic-liquor of bone glue. J Tianjin Inst Light Ind, 1997(2): 35-39 (in Chinese). |
| [70] |
李娟, 陈舜胜. 从明胶水解液中分离制备L-羟脯氨酸和L-脯氨酸. 现代食品科技, 2006(4): 151-152. Li J, Chen SS. Isolating L-hydroxyproline and L-proline from hydrolytic-liquor of glutin. Mod Food Sci Technol, 2006(4): 151-152 (in Chinese). |
| [71] |
Takano S, Iwabuchi Y, Ogasawara K. Concise stereoselective synthesis of (2S, 4R)-4-hydroxyproline from (S)-0-benzylglycidol by a novel cyclization. J Chem Soc Chem Commun, 1988, 1988(23): 1527-1528.
|
| [72] |
Mehlfuhrer M, Berner H, Thirring K. A Short stereoselective synthesis of cis- and trans-4-hydroxy-L-proline. J Chem Soc Chem Commun, 1994, 1994(11): 1291.
|
| [73] |
Zhang Z, Su W, Bao Y, et al. Modular reconstruction and optimization of the trans-4-hydroxy-L-proline synthesis pathway in Escherichia coli. Microb Cell Fact, 2022((2022) 21: 159.
|
| [74] |
Long M, Xu M, Qiao Z, et al. Directed evolution of ornithine cyclodeaminase using an evolvR-based growth-coupling strategy for efficient biosynthesis of L-proline. Acs Synth Biol, 2020, 9(7): 1855-1863. DOI:10.1021/acssynbio.0c00198
|
| [75] |
Csonka LN, Leisinger T, Stewart V. Biosynthesis of proline. Ecosal plus, 2007, 2(2): 1-20.
|
| [76] |
Campbell AC, Bogner AN, Mao Y, et al. Structural analysis of prolines and hydroxyprolines binding to the L-glutamate-γ-semialdehyde dehydrogenase active site of bifunctional proline utilization A. Arch Biochem Biophys, 2021, 698: 108727. DOI:10.1016/j.abb.2020.108727
|
| [77] |
Nadaraia S, Lee YH, Becker DF, et al. Crystallization and preliminary crystallographic analysis of the proline dehydrogenase domain of the multifunctional putA flavoprotein from Escherichia coli. Acta Crystallogr D Biol Crystallogr, 2001, 57(Pt 12): 1925-1927.
|
| [78] |
林凡. 产反式-4-羟脯氨酸putA/sucA敲除型大肠杆菌的构建与发酵优化[D]. 无锡: 江南大学, 2016. Lin F. Construction of putA/sucA-knockout Escherichia coli strains producing trans-4- hydroxyproline and optimization of fermentation conditions[D]. Wuxi: Jiangnan University, 2016 (in Chinese). |
| [79] |
Mogi T, Yamamoto H, Nakao T, et al. Genetic and physical characterization of putP, the proline carrier gene of Escherichia coli K12. Mol Gen Genet, 1986, 202(1): 35-41. DOI:10.1007/BF00330513
|
| [80] |
Nakao T, Yamato I, Anraku Y. Mapping of the multiple regulatory sites for putP and putA expression in the putC region of Escherichia coli. Molecular general genetics, 1988, 214(3): 379-388. DOI:10.1007/BF00330470
|
| [81] |
张胜利. 产反式-4-羟基-L-脯氨酸大肠杆菌菌株的改造及发酵条件初步研究[D]. 无锡: 江南大学, 2015. Zhang SL. The improvement of trans-4-hydroxy-L-proline producing Escherichia coli and priliminary research of its fermentation condition[D]. Wuxi: Jiangnan University, 2015 (in Chinese). |
| [82] |
Fichman Y, Gerdes SY, Kovács H, et al. Evolution of proline biosynthesis: enzymology, bioinformatics, genetics, and transcriptional regulation. Biol Rev, 2015, 90(4): 1065-1099. DOI:10.1111/brv.12146
|
| [83] |
Adams E, Frank L. Metabolism of proline and the hydroxyprolines. Ann Rev Biochem, 1980, 49: 1005-1061. DOI:10.1146/annurev.bi.49.070180.005041
|
| [84] |
龙梦飞. 系统代谢改造大肠杆菌高效合成反式-4-羟基-L-脯氨酸[D]. 无锡: 江南大学, 2021. Long MF. Systematic metabolic engineering for efficient synthesis of trans-4-hydroxy-L-proline in Escherichia coli[D]. Wuxi: Jiangnan University, 2021 (in Chinese). |
| [85] |
Zaprasis A, Hoffmann T, Wünsche G, et al. Mutational activation of the RocR activator and of a cryptic rocDEF promoter bypass loss of the initial steps of proline biosynthesis in Bacillus subtilis. Environ Microbiol, 2014, 16(3): 701-717. DOI:10.1111/1462-2920.12193
|
| [86] |
Belitsky BR, Brill J, Bremer E, et al. Multiple genes for the last step of proline biosynthesis in Bacillus subtilis. J Bacteriol, 2001, 183(14): 4389-4392. DOI:10.1128/JB.183.14.4389-4392.2001
|
| [87] |
Jensen JV, Wendisch VF. Ornithine cyclodeaminase-based proline production by Corynebacterium glutamicum. Microb Cell Fact, 2013, 12: 63. DOI:10.1186/1475-2859-12-63
|
| [88] |
Kal S, Que L. Dioxygen activation by nonheme iron enzymes with the 2-His-1-carboxylate facial triad that generate high-valent oxoiron oxidants. J Biol Inorg Chem, 2017, 22(2-3): 339-365. DOI:10.1007/s00775-016-1431-2
|
| [89] |
Hausinger RP. Fe(Ⅱ)/α-ketoglutarate-dependent hydroxylases and related enzymes. Crit Rev Biochem Mol, 2008, 39(1): 21-68.
|
| [90] |
Lawrence CC, Sobey WJ, Field RA, et al. Purification and initial characterization of proline 4-hydroxylase from Streptomyces griseoviridus P8648: a 2-oxoacid, ferrous-dependent dioxygenase involved in etamycin biosynthesis. Biochem J, 1996, 313(Pt 1): 185-191.
|
| [91] |
Smirnov SV, Kodera T, Samsonova NN, et al. Metabolic engineering of Escherichia coli to produce (2S, 3R, 4S)-4-hydroxyisoleucine. Appl Microbiol Biot, 2010, 88(3): 719-726. DOI:10.1007/s00253-010-2772-3
|
| [92] |
Becker J, Giesselmann G, Hoffmann SL, et al. Corynebacterium glutamicum for sustainable bioproduction: from metabolic physiology to systems metabolic engineering. Adv Biochem Eng Biotechnol, 2018, 162: 217-263.
|
| [93] |
Becker J, Rohles CM, Wittmann C. Metabolically engineered Corynebacterium glutamicum for bio-based production of chemicals, fuels, materials, and healthcare products. Metab Eng, 2018, 50: 122-141. DOI:10.1016/j.ymben.2018.07.008
|
| [94] |
Wolf S, Becker J, Tsuge Y, et al. Advances in metabolic engineering of Corynebacterium glutamicum to produce high-value active ingredients for food, feed, human health, and well-being. Essays Biochem, 2021, 65(2): 197-212. DOI:10.1042/EBC20200134
|
| [95] |
Araki K, Takasawa Y, Nakajima J. Fermentative production of L-proline with auxotrophs of Corynebacterium glutamicum. Agric Biol Chem, 1975, 39: 1193-1200.
|
| [96] |
Zhang Y, Cai J, Shang X, et al. A new genome-scale metabolic model of Corynebacterium glutamicum and its application. Biotechnol Biofuels, 2017, 10(1): 1-16.
|
| [97] |
Zhang J, Qian F, Dong F, et al. De novo engineering of Corynebacterium glutamicum for L-proline production. Acs Synth Biol, 2020, 9(7): 1897-1906. DOI:10.1021/acssynbio.0c00249
|
| [98] |
Liu J, Liu M, Shi T, et al. CRISPR-assisted rational flux-tuning and arrayed CRISPRi screening of an L-proline exporter for L-proline hyperproduction. Nat Commun, 2022, 13(1): 1-16. DOI:10.1038/s41467-021-27699-2
|
| [99] |
卢伟宁, 廖瑜玲. 一株高产脯氨酸的嗜醋酸棒杆菌的选育及发酵条件优化. 中国生物工程杂志, 2010, 30(11): 70-74. Lu WN, Liao YL. Screening and fermentation optimization of Corynebacterium acetoacidophilum mutant for high-level production of L-proline. China Biotechnol, 2010, 30(11): 70-74 (in Chinese). |
| [100] |
全冰华. 嗜醋酸棒杆菌发酵生产L-脯氨酸的优化研究[D]. 广州: 华南理工大学, 2017. Quan BH. Optimization of fermentation producing L-proline by Corynebacterium acetoacidophilum[D]. Guangzhou: South China University of Technology, 2017 (in Chinese). |
| [101] |
Nakamori S, Morioka H, Yoshinaga F. Fermentative production of L-proline by DL-3, 4-dehydroproline resistant mutants of L-glutamate producing bacteria. Agric Biol Chem, 1982, 46: 487-491.
|
| [102] |
Masuda M, Takamatu S, Nishimura N, et al. Improvement of culture conditions for L-proline production by a recombinant strain of Serratia marcescens. Appl Biochem Biotechnol, 1993, 43(3): 189-197. DOI:10.1007/BF02916452
|
| [103] |
张伟国, 顾正华, 唐鸿. L-脯氨酸发酵条件的研究. 江苏食品与发酵, 2000(4): 1-4. Zhang WG, Gu ZH, Tang H. Study on fermentation conditions of L-proline. Jiangsu Food Fermentation, 2000(4): 1-4 (in Chinese). |
| [104] |
Nakanishi T, Hirao T, Azuma T, et al. Application of L-glutamate to L-proline fermentation by Corynebacterium acetoacidophilum. J Ferment Technol, 1987, 2(65): 139-144.
|
| [105] |
赵世杰. 高产L-脯氨酸缺陷短波单胞菌的选育及其发酵条件优化[D]. 无锡: 江南大学, 2012. Zhao SJ. Breeding of Brevundimonas diminuta for L-proline and optimization of fermentation[D]. Wuxi: Jiangnan University, 2012 (in Chinese). |
| [106] |
方佩静, 毛维颖, 陈琦. L-脯氨酸发酵研究. 微生物学报, 1982, 22(4): 353-360. Fang PJ, Mao WY, Chen Q. Study on L-proline fermentation. Acta Microbiol Sin, 1982, 22(4): 353-360 (in Chinese). |
| [107] |
Shibasaki T, Mori H, Chiba S, et al. Microbial proline 4-hydroxylase screening and gene cloning. Appl Environ Microb, 1999, 65(9): 4028-4031. DOI:10.1128/AEM.65.9.4028-4031.1999
|
| [108] |
Shibasaki T, Mori H, Ozaki A. Enzymatic production of trans-4-hydroxy-L-proline by regio- and stereospecific hydroxylation of L-proline. Biosci Biotechnol Biochem, 2000, 64(4): 746-750. DOI:10.1271/bbb.64.746
|
| [109] |
刘合栋. 高产反式-4-羟脯氨酸重组大肠杆菌的构建和发酵优化[D]. 无锡: 江南大学, 2013. Liu HD. Construction and fermentation of recombinant Escherichia coli producing trans-4-hydroxyproline[D]. Wuxi: Jiangnan University, 2013 (in Chinese). |
| [110] |
Falcioni F, Bühler B, Schmid A. Efficient hydroxyproline production from glucose in minimal media by Corynebacterium glutamicum. Biotechnol Bioeng, 2015, 112(2): 322-330. DOI:10.1002/bit.25442
|
| [111] |
Shibasaki T, Hashimoto S, Mori H, et al. Construction of a novel hydroxyproline-producing recombinant Escherichia coli by introducing a proline 4-hydroxylase gene. J Biosci Bioeng, 2000, 90(5): 522-525. DOI:10.1016/S1389-1723(01)80033-5
|
| [112] |
Wang X, Liu J, Zhao J, et al. Efficient production of trans-4-hydroxy-L-proline from glucose using a new trans-proline 4-hydroxylase in Escherichia coli. J Biosci Bioeng, 2018, 126(4): 470-477. DOI:10.1016/j.jbiosc.2018.04.012
|
| [113] |
李强, 韩亚昆, 蒋帅, 等. 代谢工程改造大肠杆菌合成反式-4-羟基-L-脯氨酸. 食品科学, 2020, 41(2): 202-207. Li Q, Han YK, Jiang S. Metabolic engineering of Escherichia coli for production of trans-4-hydroxy-L-proline. Food Sci, 2020, 41(2): 202-207 (in Chinese). |
| [114] |
Zhang Y, Zhang Y, Shang X, et al. Reconstruction of tricarboxylic acid cycle in Corynebacterium glutamicum with a genome-scale metabolic network model for trans-4-hydroxyproline production. Biotechnol Bioeng, 2019, 116(1): 99-109. DOI:10.1002/bit.26818
|
| [115] |
Zhang HL, Zhang C, Pei CH, et al. Efficient production of trans-4-hydroxy-L-proline from glucose by metabolic engineering of recombinant Escherichia coli. Lett Appl Microbiol, 2018, 66(5): 400-408. DOI:10.1111/lam.12864
|
| [116] |
Wang XM, Han MN, Jiang JP, et al. Isolation of a Bacillus cereus strain HBL-AI and its application for production of trans-4-hydroxy-L-proline. Lett Appl Microbiol, 2021, 72(1): 53-59. DOI:10.1111/lam.13388
|
| [117] |
Long M, Xu M, Ma Z, et al. Significantly enhancing production of trans-4-hydroxy-L-proline by integrated system engineering in Escherichia coli. Sci Adv, 2020, 6(21): 1-10.
|
| [118] |
蔡萌萌, 刘子强, 户红通, 等. α-酮戊二酸对L-羟脯氨酸产量的影响. 中国酿造, 2018, 37(7): 47-50. Cai MM, Liu ZQ, Hu HT, et al. Effect of alpha-ketoglutarate on the yield of L-hydroxyproline. Chin Brew, 2018, 37(7): 47-50 (in Chinese). |
| [119] |
Zhao C, Cheng L, Xu Q, et al. Improvement of the production of L-tryptophan in Escherichia coli by application of a dissolved oxygen stage control strategy. Ann Microbiol, 2015, 66(2): 843-854.
|
| [120] |
Pablos TE, Sigala JC, Le Borgne S, et al. Aerobic expression of Vitreoscilla hemoglobin efficiently reduces overflow metabolism in Escherichia coli. Biotechnol J, 2014, 9(6): 791-799. DOI:10.1002/biot.201300388
|
| [121] |
Wei M, Webster DA, Stark BC. Genetic engineering of Serratia marcescens with bacterial hemoglobin gene: effects on growth, oxygen utilization, and cell size. Biotechnol Bioeng, 1998, 57(4): 477-483. DOI:10.1002/(SICI)1097-0290(19980220)57:4<477::AID-BIT12>3.0.CO;2-9
|
| [122] |
Zhao T, Li M, Zheng X, et al. Improved production of trans-4-hydroxy-L-proline by chromosomal integration of the Vitreoscilla hemoglobin gene into recombinant Escherichia coli with expression of proline-4-hydroxylase. J Biosci Bioeng, 2017, 123(1): 109-115. DOI:10.1016/j.jbiosc.2016.07.018
|
| [123] |
张胜利, 林凡, 刘合栋, 等. putA与vgb基因对反式-4-羟基-L-脯氨酸产量的影响. 食品与生物技术学报, 2016, 35(12): 1307-1316. Zhang SL, Lin F, Liu HD, et al. Effects of putA and vgb genes on trans-4-hydroxy-L-proline production. J Food Sci Biotechnol, 2016, 35(12): 1307-1316 (in Chinese). DOI:10.3969/j.issn.1673-1689.2016.12.012 |
| [124] |
Chen X, Yi J, Song W, et al. Chassis engineering of Escherichia coli for trans-4-hydroxy-L-proline production. Microb Biotechnol, 2021, 14(2): 392-402. DOI:10.1111/1751-7915.13573
|
| [125] |
Chen X, Yi J, Liu J, et al. Enzymatic production of trans-4-hydroxy-L-proline by proline 4‐hydroxylase. Microb Biotechnol, 2021, 14(2): 479-487. DOI:10.1111/1751-7915.13616
|
| [126] |
Liu X. Hydrolysing the soluble protein secreted by Escherichia coli in trans-4-hydroxy-L-proline fermentation increased dissolve oxygen to promote high-level trans-4-hydroxy-L-proline production. Bioengineered, 2019, 10(1): 52-58. DOI:10.1080/21655979.2019.1600966
|
| [127] |
蔡萌萌, 王健, 陈宁, 等. 溶氧对L-羟脯氨酸发酵的影响及其控制. 发酵科技通讯, 2018, 47(3): 166-169. Cai MM, Wang J, Chen N, et al. Effect of dissolved oxygen on the fermentation of L-hydroxyproline and its control. Bull Fermentation Sci Technol, 2018, 47(3): 166-169 (in Chinese). |
| [128] |
Luli GW, Strohl WR. Comparison of growth, acetate production, and acetate inhibition of Escherichia coli strains in batch and fed-batch fermentations. Appl Environ Microb, 1990, 56(4): 1004-1011. DOI:10.1128/aem.56.4.1004-1011.1990
|
| [129] |
Luo QP, Shen YL, Wei DZ, et al. Optimization of culture on the overproduction of TRAIL in high-cell-density culture by recombinant Escherichia coli. Appl Microbiol Biot, 2006, 71(2): 184-191. DOI:10.1007/s00253-005-0131-6
|
| [130] |
Wang JX, Zhang ZY, Liu HD, et al. Construction and optimization of trans-4-hydroxy-L-proline production recombinant E. coli strain taking the glycerol as carbon source. J Chem Technol Biotechnol, 2016, 91(9): 2389-2398. DOI:10.1002/jctb.5024
|
| [131] |
李浩. 改造谷氨酸棒杆菌及其利用秸秆酶解液发酵产羟脯氨酸[D]. 无锡: 江南大学, 2020. Li H. Engineering Corynebacterium glutamicum for producing trans-4-hydroxy-L-proline from corncob hydrolysates[D]. Wuxi: Jiangnan University, 2020 (in Chinese). |
| [132] |
Brandenburg F, Theodosiou E, Bertelmann C, et al. Trans-4-hydroxy-L-proline production by the cyanobacterium Synechocystis sp. PCC 6803. Metab Eng Commun, 2021, 12: 1-9.
|
 2022, Vol. 38
2022, Vol. 38