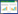中国科学院微生物研究所、中国微生物学会主办
文章信息
- 阿丽娅, 李子华, 吕金艳, 余柳松, 司徒卫, 薛凌云, 王辉, 陈国强
- A Liya, LI Zihua, LÜ Jinyan, YU Liusong, SITU Wei, XUE Lingyun, WANG Hui, CHEN Guoqiang
- 小分子,大作为:酮体D-β羟基丁酸在医疗领域的应用与展望
- Applications and perspectives of ketone body D-β-hydroxybutyrate in the medical fields
- 生物工程学报, 2022, 38(3): 976-989
- Chinese Journal of Biotechnology, 2022, 38(3): 976-989
- 10.13345/j.cjb.210343
-
文章历史
- Received: May 9, 2021
- Accepted: July 22, 2021
- Published: December 27, 2021
2. 清华大学 药学院, 北京 100084;
3. 中山大学附属第六医院结直肠外科 中山大学附属第六医院生物医学创新研究院 广东省生物医用材料转化与评估工程技术中心, 广东 广州 510655;
4. 清华大学 生命科学学院, 北京 100084;
5. 清华大学 化学工程系, 北京 100084
2. School of Pharmaceutical Sciences, Tsinghua University, Beijing 100084, China;
3. Guangdong Province Biomedical Material Conversion and Evaluation Engineering Technology Center, Institute of Biomedical Innovation, the Sixth Affiliated Hospital of Sun Yat-sen University, Department of Colorectal Surgery, the Sixth Affiliated Hospital of Sun Yat-sen University, Guangzhou 510655, Guangdong, China;
4. School of Life Sciences, Tsinghua University, Beijing 100084, China;
5. Department Chemical Engineering, Tsinghua University, Beijing 100084, China
在日常饮食中,给人体提供能量的物质主要为碳水化合物和脂肪两大营养元素,绝大部分人都会通过碳水化合物代谢产生的葡萄糖为身体提供能量。当碳水化合物不足时[1],身体才会更多地分解脂肪从而持续为身体供能。然而,近年来科学家们发现通过间歇性进食[2]、限时饮食[3]或生酮饮食[4]等方式能够使人体的代谢模式由葡萄糖代谢转化为脂肪代谢,这类饮食方式不仅能够长时间为身体供能,甚至可以改善人类健康。
1 生酮概述人体在长时间禁食、剧烈运动或特定饮食条件下会通过分解脂肪而产生酮体。脂肪酸在肝脏细胞中进行β氧化形成乙酰辅酶A (acetyl CoA),乙酰辅酶A在β-羟基-β-甲基戊二酰辅酶A合成酶(3-hydroxy-3-methylglutaryl CoA synthase, HMGCS2) 的催化下形成乙酰乙酸,乙酰乙酸通过β-羟基丁酸脱氢酶(β-hydroxybutyrate dehydrogenase, BDH1) 进一步形成3-羟基丁酸(D3HB)。肝脏细胞中产生的D3HB通过自由扩散的方式或细胞内膜上的单羧酸转运蛋白(monocarboxylate transporter, MCT) 进入血液。血液中的D3HB被肝外组织上的MCT再次转运到细胞质中,随后在细胞线粒体内BDH1的催化下逆向转化为乙酰乙酸。乙酰乙酸在酮脂酰辅酶A转移酶(succinyl-CoA: 3-ketoacid coenzyme A transferase, OXCT/SCOT) 催化下形成乙酰乙酰辅酶A,又经过乙酰辅酶A疏解酶形成乙酰辅酶A。随即,乙酰辅酶A进入三羧酸循环(tricarboxylic acid cycle, TCA) 被进一步代谢生成ATP (adenosine triphosphate) (图 1)[5]。
|
| 图 1 人体内源性D3HB的产生及代谢路径 Fig. 1 Endogenous production and metabolism of D3HB. D3HB在肝脏细胞中通过脂肪酸的分解而产生,随后进入血液并被运输到肝外组织,在细胞线粒体内经代谢产生ATP HMGCS2: 3-hydroxy-3-methylglutaryl CoA synthase 2; HMGCL: 3-hydroxy-3-methylglutaryl CoA lyase; BDH1: β-hydroxybutyrate dehydrogenase; OXCT/SCOT: succinyl-CoA: 3-ketoacid coenzyme A transferase; TCA: tricarboxylic acid. |
| |
人体内产生的酮体包括D3HB、乙酰乙酸和少量丙酮,其中D3HB在血液中的含量可占70%以上[6]。D3HB是一个具有高水溶性的小分子物质,能够快速穿过血脑屏障和毛细血管壁,抵达组织及器官并发挥功能。在正常饮食条件下,人体血液中的D3HB水平不超过0.5 mmol/L,但在长时间饥饿条件下,血液中的D3HB可升至4−6 mmol/L,甚至10 mmol/L[7-8]。
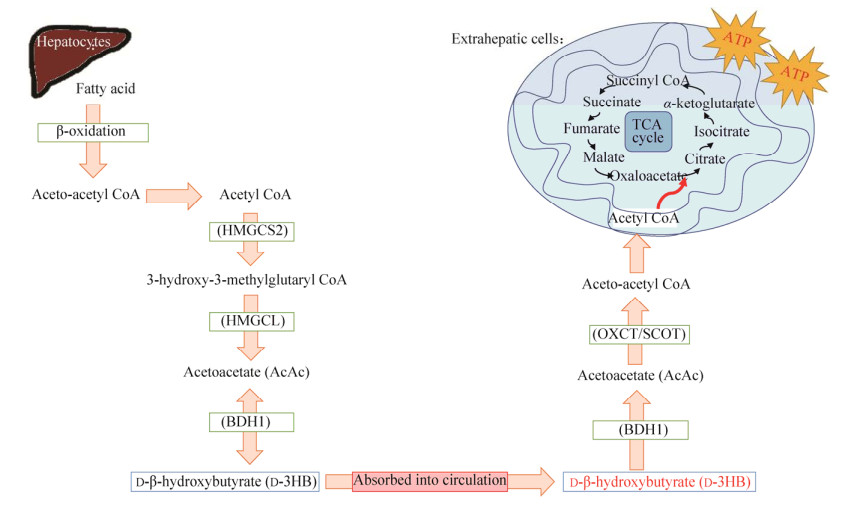
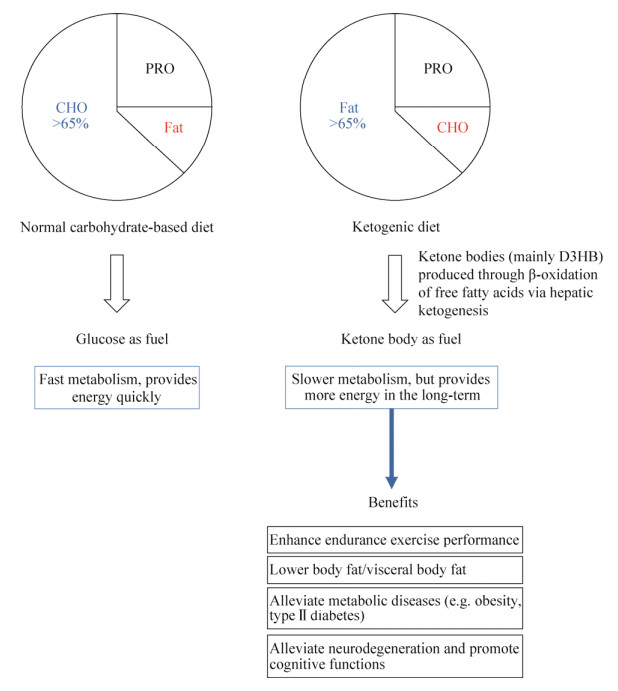
近年来,生酮饮食逐渐成为一种能够帮助人们提高血清中D3HB水平的特殊膳食结构,并且在运动员[9]、健身人群[10]、军队[11]等群体中备受欢迎。生酮饮食主要是以一种高脂肪、低碳水的饮食结构来诱导肝脏优先代谢脂肪,并通过产生大量的D3HB为机体供能的饮食方式(图 2)。研究显示,D3HB在人体肌肉组织[12]、心脏[13]、肾脏[14]、脑部神经元[15]与神经胶质细胞[16]中的吸收利用尤为活跃(图 3)。因此,许多科学家和医生们已经开始探索人体内源性D3HB可能会给不同代谢性与年龄相关性疾病带来的治疗作用[17-18],同时还可帮助运动员、军队等提高运动能力以实现更加高效的训练[19]。
|
| 图 2 碳水和生酮饮食在供能模式上的差异(葡萄糖vs D3HB供能)以及酮体代谢对机体的优点 Fig. 2 Carbohydrate-based & ketogenic diet in terms of energy metabolism and advantages of ketone metabolism. CHO: carbohydrate; PRO: protein. |
| |
|
| 图 3 D3HB在人体内的主要作用器官 Fig. 3 Major downstream target organs of D3HB. |
| |
事实上,酮体在很多年前已被用于复发性小儿癫痫疾病的治疗[20-21]。时至今日,人们发现D3HB在人体内的作用远远不仅限于此。在血清中D3HB含量被提高的状态下,人体会以酮体代谢供能为主并进入营养性酮症状态(nutritional ketosis, NK),即血清D3HB水平大于0.5 mmol/L[22]。研究发现,D3HB能够带来诸多好处,其中包括:提高运动耐力表现[23]、改善身体组成成分[24]、降低有害低密度胆固醇[25]、调节代谢相关的荷尔蒙[26]、控制食欲[27]和维持肠道健康[28]。在喂食生酮饮食的小鼠中发现其升高的D3HB水平对老年性肌肉萎缩起到了抑制作用[29]。
近期的研究中发现,D3HB还可以作为小信号分子抑制细胞内的组蛋白去乙酰基酶(histone deacetylase, HDAC) 并调控下游基因[30]、抑制细胞中的Nod-like receptor pyrin-domain containing 3 (NLRP3) 炎性小体引发抗炎和抗癌等作用[31-33]、激活G蛋白偶联受体109A (G-protein-coupled receptor, GPCR109A) 从而改善动脉粥样硬化[34]。最新研究显示,D3HB除了作为HDAC抑制剂调控基因表达外,还能够直接对蛋白起到修饰作用[35],其中最主要的修饰作用是在组蛋白赖氨酸位置(histone 3 lysine 9 or 14, H3K9 or H3K14) 上的β-羟基丁酰化(β-hydroxybutyrylation, Kbhb) 修饰作用[36]。Wu等[37]发现在H3K9的β-羟基丁酰化修饰后成功抑制了糖尿病小鼠的血管内壁损伤。
2.1 运动领域大量的研究已经证明人体在NK状态下能够更有效地减重和减脂,尤其以内脏脂肪的降低为主要表现形式[38]。同时,D3HB能够很好地被骨骼肌利用并提高骨骼肌线粒体的供能效率[12]。Vargas等[24]证明男性运动员在为期8周的生酮饮食中达到了很好的减重减脂的效果,并维持了良好的抗阻力运动能力。Chang等[23]发现在长时间有氧运动中,长跑运动员的最高脂肪氧化速率可达到1.5 g/min。总之,内源性D3HB水平的升高能够在运动中减少人体对糖原的利用,更多地消耗脂肪来维持运动表现;并且,在运动中储存的糖原与D3HB结合有利于运动后的肌肉恢复[39]。
2.2 肥胖症及代谢性疾病肥胖症是一种极为普遍且复杂的代谢病症,并容易引发其他继发性疾病。根据世界卫生组织的最新数据,全球在2016年有超过19亿成年人的体重属于超重范围(body mass index, BMI>25),其中有大约6.5亿人属于肥胖症(BMI>30)[40]。严重的患者往往需要去医院接受系统的治疗以避免危及健康。随着D3HB的研究进展越来越多,许多课题组陆续发现D3HB可以帮助肥胖人群达到更好的减重减脂效果,其作用机制一方面在于其能调节体内代谢相关的荷尔蒙(胃饥饿素、瘦素、胰淀素、肠抑胃肽),从而提升饱腹感、帮助控制食欲[26-27];另一方面D3HB在降低有害低密度胆固醇、增加高密度胆固醇上的功效[25, 41],使肥胖人群的血脂指标更加健康。
再者,研究发现D3HB可以激活肥胖小鼠体内的褐色脂肪组织(brown adipose tissue, BAT) 从而提高肥胖小鼠的基础代谢,促进能量消耗[42]。美国国立卫生研究院的Veech教授曾在喂食生酮饮食的小鼠中证明了小鼠BAT中的线粒体蛋白(mitochondrial uncoupling protein, UCP1) 表达明显增高,继而增加了BAT线粒体氧化磷酸化以及生热反应[43]。与之相似,人体内同样含有大量能够帮助人体调节能量代谢、维持体温及能量平衡的BAT[44]。因此,D3HB在BAT上的作用可能会为预防与治疗肥胖症带来新的可能。
2.3 肠道健康近年来,部分课题组发现D3HB能够辅助肠道细胞分化[45]、改善肠道菌群[46]、促进肠道干细胞自主更新从而维持肠道平衡[28]。Wang等[45]还发现当肝脏中促进D3HB生成的限速酶(HMGCS2) 表达增高时同样可以促肠道细胞分化,反之则起抑制作用。此外,另一课题组发现生酮小鼠肠道内的双歧杆菌数量减少并降低了肠道内辅助T细胞(helper T17 cells, Th17) 的数量,抑制促炎症因子的释放[46]。
3 提升内源性D3HB的困难尽管饮食干预能够使人体进入营养性酮症,其适应期却是一个十分漫长且不容易坚持的过程。有研究发现,过于严格的生酮饮食也可能导致不良副作用[47-50]。因此,为了避免严苛的饮食干预同时帮助人体快速提升血清D3HB水平,外源性D3HB补充提供了一种更加便捷的方式给身体提供能量、提高线粒体供能效率、保护体内不同的细胞及器官并预防代谢性与神经变性疾病。
4 外源D3HB的不同存在形式及应用研究外源性D3HB补充可分为酮盐(KS)、酮酯[(R)-hydroxybutyl-(R)-hydroxybutyrate (KME)、1, 3-butanediol diacetoacetate (BD-AcAc2) 或bis hexanoyl-(R)-1, 3-butanediol (BH-BD)]、D3HB前体(1, 3-丁二醇) 以及纯酸形式的D3HB。目前,绝大部分是由化学合成法制造的[51-52],其中也有少部分通过生物基制作D3HB的方法(lab-scale)[53]。酮酯和1, 3-丁二醇都属于D3HB的前体,需要在体内降解后释放D3HB[54]。
近年来全球已有大量的研究表明通过体外摄入D3HB盐或酯都能够引发不同的生理效应(表 1),例如:提高运动耐力[55]、帮助减肥[56]、促进运动后的肌肉修复[39, 57-58]、预防或治疗脑部相关疾病[59-60]、代谢疾病[61]、心血管疾病[34, 62-65]、慢性肾病[14, 66]、抑制肿瘤细胞滋生[67-68]甚至减缓细胞衰老[69-71]。
| No. | Functional area | Types of exogenous ketones used | References | |||
| Ketone salts | Ketone esters | 1, 3-butanediol | ||||
| 1 | Exercise performance |  Increased fat oxidation |
 Significant improvement in endurance |
 Minimal benefits |
[55, 72, 77, 84] | |
| 2 | Weight loss & anti-obesity treatment |  Appetite control, modulation of appetite-regulating hormones, activates BAT to help maintain energy balance & increase resting energy expenditure |
* | [41, 97-99] | ||
| 3 | Muscle recovery |  Glycogen-sparing effect during exercise Enhances post-exercise glucose uptake and increases muscle glycogen content |
* | [57-58, 100] | ||
| 4 | Prevent overtraining symptoms |
* |  Lowers GDF15 which is a key factor that increases when overtraining symptoms develop |
* | [101] |
|
| 5 | Neurodegenerative & psychiatric diseases (ALZ, PARK, PTSD) |
 Improves cognition and alleviates symptoms related to various neurodegenerative & psychiatric disorders |
* | [31, 78, 80, 102] | ||
| 6 | Metabolic deficiencies (MADD, FAO) |
 Observed gastrointestinal side effects |
 May better alleviate symptoms |
* | [61, 88-89] | |
| 7 | Cardiovascular health (Heart failure, stroke, atherosclerosis) |  Increases cardiac output in heart failure patients Alleviates atherosclerosis |
* | [64-65, 103] | ||
| 8 | Chronic kidney disease (DKD) |
* | * |  Inhibition of renal mTORC1 hyperactivation and minimizes renal tubular damage |
[66] | |
| 9 | Anti-aging |  Prevents cellular senescence |
* | * | [70-71, 82] | |
 : very effective; : very effective;  : not very effective; *: haven’t been explored. BAT: brown adipose tissue; ALZ: Alzheimer’s disease; PARK: Parkinson’s disease; PTSD: post-traumatic stress disorder; MADD: multi-acyl CoA dehydrogenase deficiency; FAO: fatty acid oxidation disorders; ATH: atherosclerosis; DKD: diabetic kidney disease; mTORC1: mechanistic target of rapamycin complex 1. : not very effective; *: haven’t been explored. BAT: brown adipose tissue; ALZ: Alzheimer’s disease; PARK: Parkinson’s disease; PTSD: post-traumatic stress disorder; MADD: multi-acyl CoA dehydrogenase deficiency; FAO: fatty acid oxidation disorders; ATH: atherosclerosis; DKD: diabetic kidney disease; mTORC1: mechanistic target of rapamycin complex 1. |
||||||
酮盐是目前全球市面上最普遍的酮体补充剂,主要以D3HB钠、钙、钾或镁盐的形式存在。研究表明,酮盐的摄入可以将血酮升至大约1–1.5 mmol/L并提高脂肪酸氧化的效率,但是并未对改善高强度运动表现起到明显的效果[72]。Kackley等[73]在近期发现酮盐与其他作用物质的复配产品对提升高强度运动起到了一定的作用。从成分角度分析,大部分酮盐都是混旋手性而l-3HB并非人体内自然产生的物质,而以d/l形式存在的酮盐其中l-手性的量若高于d-手性,则容易导致原本促发酮症所需的时间被延长[74]。
4.2 酮酯(KE)酯类酮体补充剂和盐类最大的区别在于酮酯的吸收率和提升血酮的能力比酮盐更快[54, 75]。英国牛津大学的Clarke教授在2014年发明了全球第一款纯酮酯——KME,并已经取得了美国食品药品监督局的GRAS证明[76]。该团队也做了非常多的研究证明酮酯在提高运动能力[77]、改善认知[78-80]、治疗或抑制神经性损伤[81]以及抗衰老[82]方面的相关作用。Cox等[55]发现专业运动员饮用KME 0.5 h后的D3HB水平可以提升至3−5 mmol/L,并且有效地增强了骑行选手的耐力表现。
4.3 1, 3-丁二醇(BD)除了已知的酮盐和酮酯外,1, 3-丁二醇也是一种常见的酮体前体,并同样在肝脏中降解形成D3HB。Kesl等[83]对比了不同外源性酮体补充剂在快速提高血液中D3HB水平上的效果。试验中所有外源酮体种类都达到了类似的生酮效果,然而1, 3-丁二醇相比酮酯的效果较为欠佳。再者,Scott等[84]通过有氧运动试验发现饮用了复配1, 3-丁二醇-碳水饮料的跑者血液中的D3HB水平相比碳水对照组也提高了至少一倍;同时在运动中产生的乳酸有所减少且运动后的葡萄糖水平高于对照组。虽然在最后的5 km计时冲刺中并没有和对照组显现出很大差异,但通过乳酸和葡萄糖水平的变化可以认为饮用了1, 3-丁二醇的跑者在运动过程中更依赖于脂肪代谢所产生的酮体供能。
4.4 最新一代酮体补充剂:酮酸(D3HB)化学合成的酮盐与酮酯不管是从工艺角度还是生酮效果(主要针对盐类)都存在一定的劣势以及副作用,因此,纯酸形式的D3HB可能会开拓出更加新颖且具有疗效的临床治疗方法。Wu等[85]在5XFAD转基因阿尔茨海默小鼠体内注入1.5 mmol/kg D3HB后发现,小鼠海马体内的beta淀粉样蛋白沉淀和小神经胶质细胞活性有明显降低,减少了Aβ毒性,并提高了小鼠的认知与记忆功能。Kraeuter等[86]在药物促发(MK-801-induced) 的精神分裂症小鼠中发现短时间或长期注入高剂量的D3HB (10−20 mmol/kg) 成功缓解了实验小鼠的精神分裂症状,其中包括抑制过度自主活动、提高社交能力、改善受损的惊跳反射弱刺激抑制(pre-pulse inhibition) 和恢复感觉运动门控(sensorimotor gating) 功能。
5 未来与展望生酮饮食目前在国内正受到科学家与医生们的广泛关注,也有越来越多的课题组发现D3HB或可被用于各种不同疾病的治疗,甚至包括一些极罕见的遗传性病症或严重的代谢疾病[61, 87-88]。市面上现有的类似D3HB产品主要为含有钠、钙、钾、镁的酮盐,或是经过化学合成法制造的酮酯。酮盐的制造工艺相对简单、价格便宜,但容易导致肠胃副作用[89],同时,D3HB钠盐易于导致人体摄入过多的钠,并引发高血压[90]。相对而言,酮酯在人体内的安全性和耐受性远远大于酮盐[91-93],但其需要经过肝脏代谢后才能降解成酸被吸收。然而,纯酸形式的D3HB目前在市场上的供应极少,大部分为化学合成,而现有唯一的纯D3HB酮酸也仅作为标样销售因此价格十分昂贵。
陈国强等[94]在2002年发明了一种利用重组大肠杆菌发酵制作D3HB的方法;而在2021年,麦得发公司首次开发出了一种全新的通过生物合成法大量生产纯酸形式的D3HB的制作工艺[95],并已实现百公斤级规模化生产。由于纯酸形式的D3HB自身的新颖性,其在人体内的药代动力学数据、剂量-效果关系(dose-response)、人体对外源性D3HB的耐受性是目前仍需要进一步研究和探索的主要方向。
然而值得关注的是,近年来已经有不少课题组利用纯酸形式的D3HB在动物模型中开展了针对不同疾病的探究(表 2)。其中,Fischer等[96]甚至运用了阳离子交换树脂或草酸沉淀法将D3HB钙盐中的钙离子成分剔除从而得到d/l-3HB酮酸,并给一名12岁的MADD (multi Acyl-CoA dehydrogenase deficiency) 患者进行过为期5 d的治疗。虽然d/l-3HB未能将血酮提升至理想的治疗水平,其中的因素可能来源于酮盐的混旋结构(l-3HB在血液中停留时间长但其作用和代谢还有待研究),因此为D3HB的未来应用发展提供了更多可能。总而言之,D3HB作为一种人体可以通过自然代谢所产生的小分子物质或许有望给运动及医学领域带来更大的突破与应用;而随着外源性酮体补充剂的普及与发展,可能会为改善人类健康提供一个比饮食干预更加有效且便捷的平台。
| No. | Functional area | Experimental model | Dose/ concentration |
Main findings | Reference |
| 1 | Alzheimer’s disease | 5XFAD mice | 156.0 mg/(kg·d) (osmotic pump) | ↑ Cognitive function ↓ amyloid-β (Aβ) deposition and neuroinflammation ↑ Mitochondrial respiratory function ↓ Aβ toxicity and reactive oxygen species |
[85] |
| 2 | Alzheimer’s disease & atherosclerosis | ApoE–/– mice | 156.0 mg/(kg·d) (s.c.) | Anti-inflammatory effects of D3HB alters pathology of both conditions | [104] |
| 3 | Schizophrenia | MK-801-induced SCZ mice | 208.0 mg/(kg·d) 1.0 g/(kg·d) 2.1 g/(kg·d) |
Both acute injection of highest dose and chronic injection of lower dose for 3 weeks inhibited the MK-801-induced SCZ symptoms | [86] |
| 4 | Glioma treatment | Rat C6 glioma cells (in vitro) | 1.0 mmol/L 10.0 mmol/L 25.0 mmol/L |
Cells incubated with mid- or high doses of D3HB demonstrated inhibition of NLRP3 inflammasome via activating GPR109A, which in turn inhibits the migration of C6 glioma cells, activation of caspase-1, and IL-1β release | [68] |
| 5 | Prevents diabetic vascular injuries | Diabetic rats | 160.0 mg/kg 200.0 mg/kg 240.0 mg/kg |
D3HB promotes β-hydroxybutyrylation on lysine residues of histone 3 (H3K9bhb) and results in increased gene expression of vascular endothelial growth factor (VEGF) | [37] |
| 6 | Anti-depression | Mice | 100.0−300.0 mg/kg (i.p.) |
Alleviates depressive behaviors of mice after chronic administration for 11 days; lower doses combined with fluoxetine exerts better anti-depressive effect and alleviate symptoms | [105] |
| 7 | Prevention of liver ischemic reperfusion injury (IRI) | Mice | 1.0 g/kg (i.p.) | Following 12 h fast or intraperitoneal injection of D3HB, mice had reduced inflammation and enhanced FOXO expression in which contributed to protection of the liver from IRI | [106] |
| 8 | Multi-acyl CoA dehydrogenase deficiency (MADD) | Patient case-study (age 12) |
d/l-3HB 0.8−2.0 g/kg |
d/l-3HB acid may be used as an adjunct to sodium-d/l-3HB salt as a potential novel way of treating MADD | [96] |
| 5XFAD: transgenic mice model of Alzheimer’s disease; GPR109A: G-protein-coupled receptor 109A; NLRP3: nod-like receptor pyrin-domain containing 3 inflammasome; IL-1β: interleukin-1β; HDAC: histone deacetylase; IRI: ischemic reperfusion injury; FOXO: transcription factor; i.p.: intraperitoneal; s.c.: subcutaneous. | |||||
| [1] |
Sherwood LM, Parris EE, Cahill GF Jr. Starvation in man. N Engl J Med, 1970, 282(12): 668-675. DOI:10.1056/NEJM197003192821209
|
| [2] |
De Cabo R, Mattson MP. Effects of intermittent fasting on health, aging, and disease. N Engl J Med, 2019, 381(26): 2541-2551. DOI:10.1056/NEJMra1905136
|
| [3] |
Wilkinson MJ, Manoogian ENC, Zadourian A, et al. Ten-hour time-restricted eating reduces weight, blood pressure, and atherogenic lipids in patients with metabolic syndrome. Cell Metab, 2020, 31(1): 92-104.e5. DOI:10.1016/j.cmet.2019.11.004
|
| [4] |
Harvey KL, Holcomb LE, Kolwicz SC Jr. Ketogenic diets and exercise performance. Nutrients, 2019, 11(10): 2296. DOI:10.3390/nu11102296
|
| [5] |
Newman JC, Verdin E. Ketone bodies as signaling metabolites. Trends Endocrinol Metab, 2014, 25(1): 42-52. DOI:10.1016/j.tem.2013.09.002
|
| [6] |
Balasse EO, Féry F. Ketone body production and disposal: effects of fasting, diabetes, and exercise. Diabetes Metab Rev, 1989, 5(3): 247-270. DOI:10.1002/dmr.5610050304
|
| [7] |
Rich AJ. Ketone bodies as substrates. Proc Nutr Soc, 1990, 49(3): 361-373. DOI:10.1079/PNS19900042
|
| [8] |
Veech RL, Chance B, Kashiwaya Y, et al. Ketone bodies, potential therapeutic uses. IUBMB Life Int Union Biochem Mol Biol: Life, 2001, 51(4): 241-247. DOI:10.1080/152165401753311780
|
| [9] |
Bailey CP, Hennessy E. A review of the ketogenic diet for endurance Athletes: performance enhancer or placebo effect. J Int Soc Sports Nutr, 2020, 17(1): 33. DOI:10.1186/s12970-020-00362-9
|
| [10] |
McSwiney FT, Wardrop B, Hyde PN, et al. Keto-adaptation enhances exercise performance and body composition responses to training in endurance athletes. Metabolism, 2018, 81: 25-34. DOI:10.1016/j.metabol.2017.10.010
|
| [11] |
LaFountain RA, Miller VJ, Barnhart EC, et al. Extended ketogenic diet and physical training intervention in military personnel. Mil Med, 2019, 184: e538-e547. DOI:10.1093/milmed/usz046
|
| [12] |
Parker B, Walton C, Carr S, et al. β-hydroxybutyrate elicits favorable mitochondrial changes in skeletal muscle. Int J Mol Sci, 2018, 19(8): 2247. DOI:10.3390/ijms19082247
|
| [13] |
Cuenoud B, Hartweg M, Godin JP, et al. Metabolism of exogenous d-beta-hydroxybutyrate, an energy substrate avidly consumed by the heart and kidney. Front Nutr, 2020, 7: 13. DOI:10.3389/fnut.2020.00013
|
| [14] |
Hattori, Y. Beneficial effects on kidney during treatment with sodium-glucose cotransporter 2 inhibitors: proposed role of ketone utilization. Heart Fail Rev, 2021, 26: 947-952. DOI:10.1007/s10741-020-10065-7
|
| [15] |
Kashiwaya Y, Takeshima T, Mori N, et al. D-beta -hydroxybutyrate protects neurons in models of Alzheimer's and Parkinson's disease. PNAS, 2000, 97(10): 5440-5444. DOI:10.1073/pnas.97.10.5440
|
| [16] |
Xiao XQ, Zhao Y, Chen GQ. The effect of 3-hydroxybutyrate and its derivatives on the growth of glial cells. Biomaterials, 2007, 28(25): 3608-3616. DOI:10.1016/j.biomaterials.2007.04.046
|
| [17] |
Kumar S, Behl T, Sachdeva M, et al. Implicating the effect of ketogenic diet as a preventive measure to obesity and diabetes mellitus. Life Sci, 2021, 264: 118661. DOI:10.1016/j.lfs.2020.118661
|
| [18] |
Lee THY, Yau SY. From obesity to hippocampal neurodegeneration: pathogenesis and non-pharmacological interventions. Int J Mol Sci, 2021, 22(1): 201.
|
| [19] |
Sherrier M, Li H. The impact of keto-adaptation on exercise performance and the role of metabolic-regulating cytokines. Am J Clin Nutr, 2019, 110(3): 562-573. DOI:10.1093/ajcn/nqz145
|
| [20] |
Hallböök T, Köhler S, Rosén I, et al. Effects of ketogenic diet on epileptiform activity in children with therapy resistant epilepsy. Epilepsy Res, 2007, 77: 134-140. DOI:10.1016/j.eplepsyres.2007.09.008
|
| [21] |
Wells J, Swaminathan A, Paseka J, et al. Efficacy and safety of a ketogenic diet in children and adolescents with refractory epilepsy—a review. Nutrients, 2020, 12: 1809. DOI:10.3390/nu12061809
|
| [22] |
Poff AM, Koutnik AP, Egan B. Nutritional ketosis with ketogenic diets or exogenous ketones: features, convergence, and divergence. Curr Sports Med Rep, 2020, 19(7): 251-259. DOI:10.1249/JSR.0000000000000732
|
| [23] |
Chang CK, Borer K, Lin PJ. Low-carbohydrate- high-fat diet: can it help exercise performance. J Hum Kinetics, 2017, 56: 81-92. DOI:10.1515/hukin-2017-0025
|
| [24] |
Vargas S, Romance R, Petro JL, et al. Efficacy of ketogenic diet on body composition during resistance training in trained men: a randomized controlled trial. J Int Soc Sports Nutr, 2018, 15: 31. DOI:10.1186/s12970-018-0236-9
|
| [25] |
Choi HR, Kim J, Lim H, et al. Two-week exclusive supplementation of modified ketogenic nutrition drink reserves lean body mass and improves blood lipid profile in obese adults: a randomized clinical trial. Nutrients, 2018, 10: 1895. DOI:10.3390/nu10121895
|
| [26] |
Sumithran P, Prendergast LA, Delbridge E, et al. Ketosis and appetite-mediating nutrients and hormones after weight loss. Eur J Clin Nutr, 2013, 67(7): 759-764. DOI:10.1038/ejcn.2013.90
|
| [27] |
Mohorko N, Černelič-Bizjak M, Poklar-Vatovec T, et al. Weight loss, improved physical performance, cognitive function, eating behavior, and metabolic profile in a 12-week ketogenic diet in obese adults. Nutr Res, 2019, 62: 64-77. DOI:10.1016/j.nutres.2018.11.007
|
| [28] |
Cheng CW, Biton M, Haber AL, et al. Ketone body signaling mediates intestinal stem cell homeostasis and adaptation to diet. Cell, 2019, 178(5): 1115-1131. DOI:10.1016/j.cell.2019.07.048
|
| [29] |
Wallace MA, Aguirre NW, Marcotte GR, et al. The ketogenic diet preserves skeletal muscle with aging in mice. Aging Cell, 2021, 20(4): e13322.
|
| [30] |
Dąbek A, Wojtala M, Pirola L, et al. Modulation of cellular biochemistry, epigenetics and metabolomics by ketone bodies. implications of the ketogenic diet in the physiology of the organism and pathological states. Nutrients, 2020, 12(3): 788. DOI:10.3390/nu12030788
|
| [31] |
Yamanashi T, Iwata M, Shibushita M, et al. Beta-hydroxybutyrate, an endogenous NLRP3 inflammasome inhibitor, attenuates anxiety-related behavior in a rodent post-traumatic stress disorder model. Sci Rep, 2020, 10: 21629. DOI:10.1038/s41598-020-78410-2
|
| [32] |
Goldberg EL, Asher JL, Molony RD, et al. Β-hydroxybutyrate deactivates neutrophil NLRP3 inflammasome to relieve gout flares. Cell Rep, 2017, 18(9): 2077-2087. DOI:10.1016/j.celrep.2017.02.004
|
| [33] |
Tengesdal IW, Menon DR, Osborne DG, et al. Targeting tumor-derived NLRP3 reduces melanoma progression by limiting MDSCs expansion. PNAS, 2021, 118(10): e2000915118. DOI:10.1073/pnas.2000915118
|
| [34] |
Zhang SJ, Li ZH, Zhang YD, et al. Ketone body 3-hydroxybutyrate ameliorates atherosclerosis via receptor Gpr109a-mediated calcium influx. Adv Sci, 2021, 2003, 410.
|
| [35] |
Xie Z, Zhang D, Chung D, et al. Metabolic regulation of gene expression by histone lysine β-hydroxybutyrylation. Mol Cell, 2016, 62(2): 194-206. DOI:10.1016/j.molcel.2016.03.036
|
| [36] |
Koronowski KB, Greco CM, Huang H, et al. Ketogenesis impact on liver metabolism revealed by proteomics of lysine β-hydroxybutyrylation. BioRxiv. DOI:10.1101/2021.01.21.427645
|
| [37] |
Wu XL, Miao DZ, Liu ZJ, et al. β-hydroxybutyrate antagonizes aortic endothelial injury by promoting generation of VEGF in diabetic rats. Tissue Cell, 2020, 64: 101345. DOI:10.1016/j.tice.2020.101345
|
| [38] |
Kang J, Ratamess NA, Faigenbaum AD, et al. Ergogenic properties of ketogenic diets in normal-weight individuals: a systematic review. J Am Coll Nutr, 2020, 39(7): 665-675. DOI:10.1080/07315724.2020.1725686
|
| [39] |
Mansor LS, Woo GH. Ketones for post-exercise recovery: potential applications and mechanisms. Front Physiol, 2021, 11: e613648. DOI:10.3389/fphys.2020.613648
|
| [40] |
World Health Organization, WHO: obesity and Overweight. [2021-03-01]. https://www.who.int/news-room/fact-sheets/detail/o.
|
| [41] |
Caminhotto RDO, Komino ACM, de Fatima Silva F, et al. Oral β-hydroxybutyrate increases ketonemia, decreases visceral adipocyte volume and improves serum lipid profile in Wistar rats. Nutr Metab, 2017, 14: 31. DOI:10.1186/s12986-017-0184-4
|
| [42] |
Srivastava S, Kashiwaya Y, King MT, et al. Mitochondrial biogenesis and increased uncoupling protein 1 in brown adipose tissue of mice fed a ketone ester diet. Faseb J, 2012, 26(6): 2351-2362. DOI:10.1096/fj.11-200410
|
| [43] |
Srivastava S, Baxa U, Niu G, et al. A ketogenic diet increases brown adipose tissue mitochondrial proteins and UCP1 levels in mice. IUBMB Life, 2013, 65(1): 58-66. DOI:10.1002/iub.1102
|
| [44] |
Enerbäck S. Brown adipose tissue in humans. Int J Obes, 2010, 34: S43-S46. DOI:10.1038/ijo.2010.183
|
| [45] |
Wang Q, Zhou Y, Rychahou P, et al. Ketogenesis contributes to intestinal cell differentiation. Cell Death Differ, 2017, 24(3): 458-468. DOI:10.1038/cdd.2016.142
|
| [46] |
Ang QY, Alexander M, Newman JC, et al. Ketogenic diets alter the gut microbiome resulting in decreased intestinal Th17 cells. Cell, 2020, 181(6): 1263-1275. DOI:10.1016/j.cell.2020.04.027
|
| [47] |
Ding JY, Xu XL, Wu XH, et al. Bone loss and biomechanical reduction of appendicular and axial bones under ketogenic diet in rats. Exp Ther Med, 2019, 17: 2503-2510.
|
| [48] |
Malik N, Tonstad S, Paalani M, et al. Are long-term FAD diets restricting micronutrient intake? A randomized controlled trial. Food Sci Nutr, 2020, 8: 6047-6060. DOI:10.1002/fsn3.1895
|
| [49] |
Sjödin A, Hellström F, Sehlstedt E, et al. Effects of a ketogenic diet on muscle fatigue in healthy, young, normal-weight women: a randomized controlled feeding trial. Nutrients, 2020, 12: 955. DOI:10.3390/nu12040955
|
| [50] |
Iacovides S, Goble D, Paterson B, et al. Three consecutive weeks of nutritional ketosis has no effect on cognitive function, sleep, and mood compared with a high-carbohydrate, low-fat diet in healthy individuals: a randomized, crossover, controlled trial. Am J Clin Nutr, 2019, 110(2): 349-357. DOI:10.1093/ajcn/nqz073
|
| [51] |
Haas T, Hecker A, Potter M, et al. A method of synthesizing 3-hydroxybutyric acid: Germany, WO2017/016902, 2017-02-02.
|
| [52] |
Zaccone F, Venturi V, Giovannini PP, et al. An alternative enzymatic route to the ergogenic ketone body ester (R)-3-hydroxybutyl (R)-3-hydroxybutyrate, Catalysts, 2021, 11 (1) : 1–8.
|
| [53] |
Martin CH, Prather KLJ. Microbial production of 3-hydroxyacids from glucose and glycolate: USA, US8361760B2, 2013-01-29.
|
| [54] |
Stubbs BJ, Cox PJ, Evans RD, et al. On the metabolism of exogenous ketones in humans. Front Physiol, 2017, 8: e848. DOI:10.3389/fphys.2017.00848
|
| [55] |
Cox PJ, Kirk T, Ashmore T, et al. Nutritional ketosis alters fuel preference and thereby endurance performance in athletes. Cell Metab, 2016, 24(2): 256-268. DOI:10.1016/j.cmet.2016.07.010
|
| [56] |
Walton CM, Jacobsen SM, Dallon BW, et al. Ketones elicit distinct alterations in adipose mitochondrial bioenergetics. Int J Mol Sci, 2020, 21: 6255. DOI:10.3390/ijms21176255
|
| [57] |
Vandoorne T, De Smet S, Ramaekers M, et al. Intake of a ketone ester drink during recovery from exercise promotes mTORC1 signaling but not glycogen resynthesis in human muscle. Front Physiol, 2017, 8: e310. DOI:10.3389/fphys.2017.00310
|
| [58] |
Holdsworth DA, Cox PJ, Kirk T, et al. A ketone ester drink increases postexercise muscle glycogen synthesis in humans. Med Sci Sports Exerc, 2017, 49(9): 1789-1795. DOI:10.1249/MSS.0000000000001292
|
| [59] |
Kovács Z, D'Agostino DP, Diamond D, et al. Therapeutic potential of exogenous ketone supplement induced ketosis in the treatment of psychiatric disorders: review of current literature. Front Psychiatry, 2019, 10: e363.
|
| [60] |
Jensen NJ, Wodschow HZ, Nilsson M, et al. Effects of ketone bodies on brain metabolism and function in neurodegenerative diseases. Int J Mol Sci, 2020, 21: 8767. DOI:10.3390/ijms21228767
|
| [61] |
Fischer T, Och U, Marquardt T. Long-term ketone body therapy of severe multiple acyl-CoA dehydrogenase deficiency: a case report. Nutrition, 2019, 60: 122-128. DOI:10.1016/j.nut.2018.10.014
|
| [62] |
Yurista SR, Chong CR, Badimon JJ, et al. Therapeutic potential of ketone bodies for patients with cardiovascular disease: JACC state-of-the-art review. J Am Coll Cardiol, 2021, 77: 1660-1669.
|
| [63] |
Brown SM, Larsen NK, Thankam FG, et al. Fetal cardiomyocyte phenotype, ketone body metabolism, and mitochondrial dysfunction in the pathology of atrial fibrillation. Mol Cell Biochem, 2021, 476(2): 1165-1178. DOI:10.1007/s11010-020-03980-8
|
| [64] |
Yurista SR, Matsuura TR, Silljé HHW, et al. Ketone ester treatment improves cardiac function and reduces pathologic remodeling in preclinical models of heart failure. Circ Heart Fail, 2021, 14(1): 112-124.
|
| [65] |
Nielsen R, Møller N, Gormsen LC, et al. Cardiovascular effects of treatment with the ketone body 3-hydroxybutyrate in chronic heart failure patients. Circulation, 2019, 139(18): 2129-2141. DOI:10.1161/CIRCULATIONAHA.118.036459
|
| [66] |
Tomita I, Kume S, Sugahara S, et al. SGLT2 inhibition mediates protection from diabetic kidney disease by promoting ketone body-induced mTORC1 inhibition. Cell Metab, 2020, 32(3): 404-419. DOI:10.1016/j.cmet.2020.06.020
|
| [67] |
Winter SF, Loebel F, Dietrich J. Role of ketogenic metabolic therapy in malignant glioma: a systematic review. Crit Rev Oncol Hematol, 2017, 112: 41-58. DOI:10.1016/j.critrevonc.2017.02.016
|
| [68] |
Shang S, Wang L, Zhang Y, et al. The beta-hydroxybutyrate suppresses the migration of glioma cells by inhibition of NLRP3 inflammasome. Cell Mol Neurobiol, 2018, 38(8): 1479-1489. DOI:10.1007/s10571-018-0617-2
|
| [69] |
Han YM, Bedarida T, Ding Y, et al. β-hydroxybutyrate prevents vascular senescence through hnRNP A1-mediated upregulation of Oct4. Mol Cell, 2018, 71(6): 1064-1078. DOI:10.1016/j.molcel.2018.07.036
|
| [70] |
Park JS, Kim YJ. Anti-aging effect of the ketone metabolite β-hydroxybutyrate in Drosophila intestinal stem cells. Int J Mol Sci, 2020, 21(10): 3497. DOI:10.3390/ijms21103497
|
| [71] |
Habieb ME, Mohamed MA, El Gamal DM, et al. Anti-aging effect of DL-β-hydroxybutyrate against hepatic cellular senescence induced by d-galactose or γ-irradiation via autophagic flux stimulation in male rats. Arch Gerontol Geriatr, 2021, 92: 104288. DOI:10.1016/j.archger.2020.104288
|
| [72] |
O'Malley T, Myette-Cote E, Durrer C, et al. Nutritional ketone salts increase fat oxidation but impair high-intensity exercise performance in healthy adult males. Appl Physiol Nutr Metab, 2017, 42(10): 1031-1035. DOI:10.1139/apnm-2016-0641
|
| [73] |
Kackley ML, Short JA, Hyde PN, et al. A pre-workout supplement of ketone salts, caffeine, and amino acids improves high-intensity exercise performance in keto-naïve and keto-adapted individuals. J Am Coll Nutr, 2020, 39(4): 290-300. DOI:10.1080/07315724.2020.1752846
|
| [74] |
Millet G. Non-racemic beta-hydroxybutyrate compounds and compositions enriched with the S - enantiomer and methods of use: USA, US10245243B1, 2019-04-02.
|
| [75] |
Stubbs BJ, Blade T, Mills S, et al. In vitro stability and in vivo pharmacokinetics of the novel ketogenic ester, bis hexanoyl (R)-1, 3-butanediol. Food Chem Toxicol, 2021, 147: 111859. DOI:10.1016/j.fct.2020.111859
|
| [76] |
US FDA GRAS Notices GRN No. 515 d-beta-hydroxybutyrate ester. [2021-03-01]. GRAS Notices (fda. gov).
|
| [77] |
Murray AJ, Knight NS, Cole MA, et al. Novel ketone diet enhances physical and cognitive performance. Faseb J, 2016, 30(12): 4021-4032. DOI:10.1096/fj.201600773R
|
| [78] |
Newport MT, VanItallie TB, Kashiwaya Y, et al. A new way to produce hyperketonemia: use of ketone ester in a case of Alzheimer's disease. Alzheimers Dement, 2015, 11(1): 99-103. DOI:10.1016/j.jalz.2014.01.006
|
| [79] |
Kashiwaya Y, Bergman C, Bergman C, et al. A ketone ester diet exhibits anxiolytic and cognition-sparing properties, and lessens amyloid and tau pathologies in a mouse model of Alzheimer's disease. Neurobiol Aging, 2013, 34(6): 1530-1539. DOI:10.1016/j.neurobiolaging.2012.11.023
|
| [80] |
Pawlosky RJ, Kemper MF, Kashiwaya Y, et al. Effects of a dietary ketone ester on hippocampal glycolytic and tricarboxylic acid cycle intermediates and amino acids in a 3xTgAD mouse model of Alzheimer's disease. J Neurochem, 2017, 141(2): 195-207. DOI:10.1111/jnc.13958
|
| [81] |
Almeida-Suhett C, Namboodiri AM, Clarke K, et al. The ketone ester, 3-hydroxybutyl-3-hydroxybutyrate, attenuates neurobehavioral deficits and improves neuropathology following controlled cortical impact in male rats[EB/OL]. (2020-12-09) [2021-03-10]. https://www.tandfonline.com/doi/full/10.1080/1028415X.2020.1853414.
|
| [82] |
Veech RL, Bradshaw PC, Clarke K, et al. Ketone bodies mimic the life span extending properties of caloric restriction. IUBMB Life, 2017, 69(5): 305-314. DOI:10.1002/iub.1627
|
| [83] |
Kesl SL, Poff AM, Ward NP, et al. Effects of exogenous ketone supplementation on blood ketone, glucose, triglyceride, and lipoprotein levels in Sprague-Dawley rats. Nutr Metab, 2016, 13: 9. DOI:10.1186/s12986-016-0069-y
|
| [84] |
Scott BE, Laursen PB, James LJ, et al. The effect of 1, 3-butanediol and carbohydrate supplementation on running performance. J Sci Med Sport, 2019, 22(6): 702-706. DOI:10.1016/j.jsams.2018.11.027
|
| [85] |
Wu Y, Gong Y, Luan Y, et al. BHBA treatment improves cognitive function by targeting pleiotropic mechanisms in transgenic mouse model of Alzheimer's disease. Faseb J, 2020, 34(1): 1412-1429. DOI:10.1096/fj.201901984R
|
| [86] |
Kraeuter AK, Mashavave T, Suvarna A, et al. Effects of beta-hydroxybutyrate administration on MK-801-induced schizophrenia-like behaviour in mice. Psychopharmacology, 2020, 237(5): 1397-1405. DOI:10.1007/s00213-020-05467-2
|
| [87] |
Carriazo S, Perez-Gomez MV, Cordido A, et al. Dietary care for ADPKD patients: current status and future directions. Nutrients, 2019, 11: 1576. DOI:10.3390/nu11071576
|
| [88] |
Bleeker JC, Visser G, Clarke K, et al. Nutritional ketosis improves exercise metabolism in patients with very long-chain acyl-CoA dehydrogenase deficiency. J Inherit Metab Dis, 2020, 43(4): 787-799. DOI:10.1002/jimd.12217
|
| [89] |
Fischer T, Och U, Klawon I, et al. Effect of a sodium and calcium DL-β-hydroxybutyrate salt in healthy adults. J Nutr Metab, 2018, 2018, 9812806.
|
| [90] |
Strazzullo P, D'Elia L, Kandala NB, et al. Salt intake, stroke, and cardiovascular disease: meta-analysis of prospective studies. BMJ, 2009, 339: b4567. DOI:10.1136/bmj.b4567
|
| [91] |
Clarke K, Tchabanenko K, Pawlosky R, et al. Kinetics, safety and tolerability of (R)-3-hydroxybutyl (R)-3-hydroxybutyrate in healthy adult subjects. Regul Toxicol Pharmacol, 2012, 63(3): 401-408. DOI:10.1016/j.yrtph.2012.04.008
|
| [92] |
Clarke K, Tchabanenko K, Pawlosky R, et al. Oral 28-day and developmental toxicity studies of (R)-3-hydroxybutyl (R)-3-hydroxybutyrate. Regul Toxicol Pharmacol, 2012, 63(2): 196-208. DOI:10.1016/j.yrtph.2012.04.001
|
| [93] |
Soto-Mota A, Vansant H, Evans RD, et al. Safety and tolerability of sustained exogenous ketosis using ketone monoester drinks for 28 days in healthy adults. Regul Toxicol Pharmacol, 2019, 109: 104506. DOI:10.1016/j.yrtph.2019.104506
|
| [94] |
Chen GQ, Xi J, Wu Q, et al. A biological method of producing (R)-β-hydroxybutyric acid using recombinant E. coli: China, CN1357629A, 2002-07-10.
|
| [95] |
Lyu J, Wei S, Yu L, et al. A novel method for producing (R)-3-hydroxybutyric acid: China, CN112176003A, 2021-01-05.
|
| [96] |
Fischer T, Elpers C, Och U, et al. Ketone body therapy with D/L-β-hydroxybutyric acid solution in severe MADD. Mol Genet Metab Rep, 2019, 20: 100491. DOI:10.1016/j.ymgmr.2019.100491
|
| [97] |
Stubbs BJ, Cox PJ, Evans RD, et al. A ketone ester drink lowers human ghrelin and appetite. Obesity, 2018, 26(2): 269-273. DOI:10.1002/oby.22051
|
| [98] |
Deemer SE, Davis RAH, Roberts BM, et al. Exogenous dietary ketone ester decreases body weight and adiposity in mice housed at thermoneutrality. Obesity, 2020, 28(8): 1447-1455. DOI:10.1002/oby.22855
|
| [99] |
Davis RAH, Deemer SE, Bergeron JM, et al. Dietary R, S-1, 3-butanediol diacetoacetate reduces body weight and adiposity in obese mice fed a high-fat diet. Faseb J, 2019, 33(2): 2409-2421. DOI:10.1096/fj.201800821RR
|
| [100] |
Takahashi Y, Terada S, Banjo M, et al. Effects of β-hydroxybutyrate treatment on glycogen repletion and its related signaling cascades in epitrochlearis muscle during 120 min of postexercise recovery. Appl Physiol Nutr Metab, 2019, 44(12): 1311-1319. DOI:10.1139/apnm-2018-0860
|
| [101] |
Poffé C, Ramaekers M, Van Thienen R, et al. Ketone ester supplementation blunts overreaching symptoms during endurance training overload. J Physiol, 2019, 597(12): 3009-3027. DOI:10.1113/JP277831
|
| [102] |
Ari C, Kovács Z, Juhasz G, et al. Exogenous ketone supplements reduce anxiety-related behavior in sprague-dawley and wistar albino glaxo/Rijswijk rats. Front Mol Neurosci, 2016, 9: e137.
|
| [103] |
Monzo L, Sedlacek K, Hromanikova K, et al. Myocardial ketone body utilization in patients with heart failure: the impact of oral ketone ester. Metabolism, 2021, 115: e154452. DOI:10.1016/j.metabol.2020.154452
|
| [104] |
Krishnan M, Hwang JS, Kim M, et al. β-hydroxybutyrate impedes the progression of Alzheimer's disease and atherosclerosis in ApoE-deficient mice. Nutrients, 2020, 12: 471. DOI:10.3390/nu12020471
|
| [105] |
Pan SY, Hu PL, You QS, et al. Evaluation of the antidepressive property of β-hydroxybutyrate in mice. Behav Pharmacol, 2020, 31(4): 322-332. DOI:10.1097/FBP.0000000000000535
|
| [106] |
Miyauchi T, Uchida Y, Kadono K, et al. Up-regulation of FOXO1 and reduced inflammation by β-hydroxybutyric acid are essential diet restriction benefits against liver injury. PNAS, 2019, 116(27): 13533-13542. DOI:10.1073/pnas.1820282116
|
 2022, Vol. 38
2022, Vol. 38