中国科学院微生物研究所、中国微生物学会主办
文章信息
- 闫干干, 李东升, 戚海燕, 付正豪, 刘晓平, 张晶, 陈云雨
- YAN Gangan, LI Dongsheng, QI Haiyan, FU Zhenghao, LIU Xiaoping, ZHANG Jing, CHEN Yunyu
- 新冠病毒主蛋白酶小分子抑制剂荧光共振能量转移高通量筛选模型的优化与应用
- Discovery of SARS-CoV-2 main protease inhibitors using an optimized FRET-based high-throughput screening assay
- 生物工程学报, 2022, 38(6): 2236-2249
- Chinese Journal of Biotechnology, 2022, 38(6): 2236-2249
- 10.13345/j.cjb.210657
-
文章历史
- Received: August 28, 2021
- Accepted: November 26, 2021
- Published: December 15, 2021
2. 皖南医学院 安徽省皖南地区植物药活性筛选与再评价工程实验室, 安徽 芜湖 241002;
3. 中国医学科学院-北京协和医学院 医药生物技术研究所, 北京 100050
2. Anhui Provincial Engineering Laboratory for Screening and Reevaluation of Bioactive Compounds of Herbal Medicines in Southern Anhui, Wannan Medical College, Wuhu 241002, Anhui, China;
3. Institute of Medicinal Biotechnology, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100050, China
目前,由新冠病毒(severe acute respiratory syndrome coronavirus 2, SARS-CoV-2) 感染导致的新冠病毒肺炎(coronavirus disease, COVID-19) 席卷全球,已成为全球公共卫生关注的焦点[1]。截至2021年11月23日,COVID-19确诊病例达2.5亿人,死亡病例达515万人。虽然灭活疫苗、基因工程疫苗、5型腺病毒活载体疫苗和mRNA疫苗的上市有效地阻断了SARS-CoV-2的大流行并显著地降低了COVID-19重症发病率,但面对SARS-CoV-2高频基因突变导致的传染性升高和疫苗保护力下降等问题,尤其是德尔塔突变株的全球蔓延导致了COVID-19确诊病例数量再度激增,积极开发新型广谱抗冠状病毒药物刻不容缓[2-3]。
SARS-CoV-2是一种具有包膜的单股正链RNA病毒,具有高频的基因突变性,属于β-冠状病毒属。SARS-CoV-2主要通过刺突糖蛋白(spike glycoprotein) 的受体结合结构域(receptor-binding domain, RBD) 与宿主细胞表面的血管紧张素转化酶2 (angiotensin converting enzyme 2, ACE2) 结合,吸附到宿主呼吸道上皮细胞并注入病毒RNA到宿主细胞中[4]。病毒RNA利用宿主细胞的核糖体翻译成pp1a和pp1ab等2条多聚蛋白质体(polyprotein),并通过自剪切作用产生能够水解多聚蛋白质体为16个非结构蛋白质的主蛋白酶(main protease, Mpro) 和木瓜样蛋白酶(papain-like protease, PLpro)。其中,多聚蛋白质体在Mpro的水解作用下被切割成包括RNA依赖的RNA聚合酶(RNA-dependent RNA polymerase, RdRp) 等12个非结构蛋白质,调控病毒RNA的复制与转录,促进免疫逃逸[5]。Mpro对多聚蛋白质体的水解过程是病毒RNA复制与转录过程中不可或缺的关键步骤。
作为半胱氨酸蛋白酶的Mpro在致病性β-冠状病毒中的进化相对保守,不易突变。Mpro的活性形式为同源二聚体结构,其可塑性较小的活性中心主要由His41和Cys145组成,是调控病毒RNA复制与转录的关键蛋白酶。鉴于Mpro进化保守且人体缺乏其同源蛋白酶,这使Mpro成为广谱抗冠状病毒药物开发的理想靶标之一[6-8]。
自COVID-19疫情暴发以来,虽有大量的Mpro小分子抑制剂被相继发现与报道,但其中部分抑制剂已被证实是非特异性Mpro小分子抑制剂[9-10]。因此,建立灵敏可靠的Mpro小分子抑制剂高通量筛选模型具有重要意义。本研究在高活性Mpro制备的基础上,旨在利用荧光共振能量转移(fluorescence resonance energy transfer, FRET) 原理,通过一系列反应条件优化,建立灵敏、可靠的Mpro小分子抑制剂FRET高通量筛选模型,并筛选苗头化合物,为抗新型冠状病毒药物先导化合物的筛选与发现奠定基础。
1 材料与方法 1.1 材料 1.1.1 试剂大肠杆菌(Escherichia coli) Rosetta (DE3) 感受态细胞、质粒提取试剂盒、蛋白质标准分子量购自北京全式金生物技术有限公司;蛋白胨、琼脂粉、酵母粉购自Oxide公司;氨苄西林、异丙基-β-d-硫代半乳糖苷(isopropyl β-d-thiogalactoside, IPTG)、7-甲氧基香豆素-4-乙酸(7-methoxycoumarin-4-acetic acid, MCA)、二甲基亚砜(dimethyl sulfoxide, DMSO)、二硫苏糖醇(1, 4-dithiothreitol, DTT) 购自Aladdin公司;HisTrapTM层析柱购自Cytiva公司;全黑半底96孔板购自Corning公司;FRET底物(FRET substrate:MCA-AVLQSGFR-Lys(Dnp)-Lys-NH2,λex/λem:320/405 nm) 购自吉尔生化(上海) 有限公司;天然产物化合物库购自ChemDiv公司;GC-376、依布硒啉(ebselen)、黄芩素(baicalein)、白花丹素(plumbagin, PLB)、银杏酸(ginkgolic acid, GA) 购自TargetMol公司;其他试剂为国产分析纯试剂。
1.1.2 仪器超净工作台购自AIRTECH公司;细菌振荡培养箱购自上海知楚公司;超声波细胞粉碎仪购自SCIENTZ公司;蛋白质电泳仪购自Bio-Rad公司;高速冷冻离心机购自Eppendorf公司;AKTA Pure蛋白质纯化仪购自GE Healthcare公司;凝胶成像系统购自CLINX公司;多功能酶标仪购自BioTek公司。
1.2 方法 1.2.1 SARS-CoV-2 Mpro原核表达、分离纯化与比活力的测定将含有重组质粒pET-21a-Mpro的E. coli DH5α甘油菌以平板划线法复苏后,提取重组质粒。再将重组质粒以冷CaCl2化学转化法转化至E. coli Rosetta (DE3) 感受态细胞中,以氨苄西林抗性法筛选重组子。按照参考文献[11]所述的方法进行Mpro原核表达、分离纯化及比活力的测定。
1.2.2 Mpro小分子抑制剂FRET高通量筛选模型的优化1) Mpro水解反应的缓冲液优化
将Mpro以4-吗啉乙磺酸(4-morpholineethanesulfonic acid, MES) 缓冲液(10 mmol/L MES, 50 mmol/L NaCl, 1 mmol/L EDTA, pH 6.0) 稀释至0.25 μmol/L,加入FRET底物使其终浓度为10 μmol/L。将上述反应液以50 μL/孔加入到全黑半底96孔板中,设置增益值为58,检测温度为室温(25 ℃),激发光为320 nm,发射光为405 nm,检测间隔为1 s,检测总时间为2 min,以多功能酶标仪检测相对荧光强度(relative fluorescence unit, RFU) 值。根据30 s内酶促反应曲线的斜率计算其反应初速度(V=ΔRFU/s)。设定反应缓冲液为羟乙基哌嗪乙磺酸(4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, HEPES) 缓冲液(10 mmol/L HEPES, 50 mmol/L NaCl, 1 mmol/L EDTA, pH 7.0) 与Tris缓冲液(10 mmol/L Tris, 50 mmol/L NaCl, 1 mmol/L EDTA, pH 8.0),同法检测酶促反应的初速度,确定Mpro水解反应的最佳缓冲液。
2) Mpro最佳反应浓度的确定
以HEPES缓冲液将Mpro稀释至1.5、1.25、1、0.75、0.5、0.25、0.125、0.062 μmol/L,分别在各组稀释液中加入FRET底物使其终浓度为5 μmol/L,再以50 μL/孔加入到全黑半底96孔板中,以多功能酶标仪检测RFU值。以GraphPad Prism 8.0拟合Mpro水解反应曲线,计算Mpro的半数有效浓度(half concentration of maximal effect, EC50) 值。
3) Mpro水解反应的最佳反应温度的优化
将FRET底物以含0.4 μmol/L Mpro的HEPES缓冲液稀释至5 μmol/L,再以50 μL/孔加入到全黑半底96孔板中,设置反应温度为4 ℃,以多功能酶标仪检测RFU值。设置反应温度为25、30 ℃,同法检测RFU值。通过计算各组酶促反应的初速度,确定Mpro水解反应的最佳反应温度。
4) DMSO最大耐受浓度的测定
设定上述反应体系中DMSO终浓度分别为0、2%、5%和10%,按照上述方法检测各组酶促反应的RFU值并计算其酶促反应的初速度,确定Mpro水解反应中DMSO最大耐受浓度。
5) Mpro水解反应的最佳反应时间的确定
将FRET底物以含0.4 μmol/L Mpro的HEPES缓冲液稀释至5 μmol/L,再以50 μL/孔加入到全黑半底96孔板中,设置检测温度为25 ℃,分别在酶促反应的第0、1、2、3、4、5、6 min,以多功能酶标仪检测RFU值。通过计算各组酶促反应的ΔRFU值,确定Mpro水解反应的最佳反应时间。
1.2.3 DTT对FRET筛选模型可靠性的影响将20 mmol/L依布硒啉以含0.8 μmol/L Mpro的HEPES缓冲液稀释至2 μmol/L,以2倍倍比稀释法,共稀释8个浓度梯度,以25 μL/孔加入到全黑半底96孔板中,室温孵育30 min。再分别以25 μL/孔加入10 μmol/L FRET底物到各组梯度溶液中,以多功能酶标仪检测RFU值,分别计算各组酶促反应的初速度。设定DMSO组为阴性对照组。依布硒啉的抑制率计算公式如下:
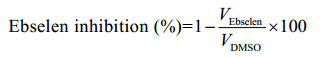
|
(Eq. 1) |
式中VEbselen代表含既定依布硒啉浓度下的酶促反应的初速度,VDMSO代表阴性对照组的酶促反应的初速度。以GraphPad Prism 8.0拟合依布硒啉的抑制曲线,计算半数抑制浓度(half maximal inhibitory concentration, IC50) 值。同法检测在HEPES缓冲液中含1 mmol/L DTT条件下,依布硒啉(起始浓度100 μmol/L) 在FRET筛选模型中的IC50值。
设定黄芩素的起始终浓度为100 μmol/L,以2倍倍比稀释法,共稀释8个浓度梯度。按照上述操作方法,分别计算在不含或含有1 mmol/L DTT条件下,黄芩素在FRET筛选模型中的IC50值。考虑到DTT对FRET筛选模型的可靠性有重要影响,本研究所述的苗头化合物高通量筛选实验中,所使用的HEPES缓冲液均含1 mmol/L DTT。
1.2.4 FRET筛选模型的特异性评价设定GC-376起始终浓度为1 μmol/L,以2倍倍比稀释法,共稀释8个浓度梯度,按照依布硒啉抑制活性测定中所述的方法,计算GC-376在FRET筛选模型中的IC50值。
在含0.8 μmol/L Mpro的HEPES缓冲液中分别加入GC-376 (2 μmol/L) 和2% DMSO,以25 μL/孔加入到全黑半底96孔板中,室温孵育30 min。其中A板#1–#50孔反应体系中含GC-376,设定为阳性对照组;B板#1–#50孔反应体系中含DMSO,设定为阴性对照组。再以25 μL/孔加入10 μmol/L FRET底物到上述反应孔中,室温继续孵育5 min,以多功能酶标仪检测RFU值。按照参考文献[12]所述的方法计算FRET筛选模型的Z′因子值。同时计算本筛选模型的信号窗值(signal window, SW)、信号本底比值(signal to background, S/B) 与信噪比值(signal to noise, S/N)。其中,Z´因子值计算方法如下所述:

|
(Eq. 2) |
式中阴性对照组RFU值的标准差为SDDMSO,阳性对照组RFU值的标准差为SDGC-376,阴性对照组RFU值的平均值为μDMSO,阳性对照组RFU值的平均值为μGC-376。
1.2.5 天然产物化合物库的高通量筛选将含0.8 μmol/L Mpro的HEPES缓冲液以24 μL/孔加入到全黑半底96孔板中,再以1 μL/孔加入天然产物化合物库(1 mg/mL),室温孵育30 min。以25 μL/孔加入10 μmol/L FRET底物,室温继续孵育5 min,再以多功能酶标仪检测RFU值。设定GC-376孔为阳性对照组,DMSO孔为阴性对照组。在初次筛选过程中,确定淬灭率(quenching ratio, Qr) 大于50%的初筛化合物为候选苗头化合物(hit),再进行第二轮复筛。
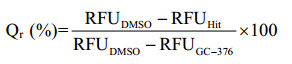
|
(Eq. 3) |
设定白花丹素与银杏酸的起始终浓度为100 μmol/L,以2倍倍比稀释法,共稀释8个浓度梯度。按照依布硒啉抑制活性测定中所述的方法,根据酶促反应的初速度,分别计算在不含或含有1 mmol/L DTT条件下,白花丹素与银杏酸在FRET筛选模型中的IC50值,确定其抑制活性。
将20 mmol/L白花丹素以0.8 μmol/L Mpro稀释至0、12、24、36 μmol/L,以25 μL/孔加入至全黑半底96孔板中,室温孵育30 min。在白花丹素单一抑制浓度条件下,再以25 μL/孔加入10、20、30、40 μmol/L FRET底物,测定白花丹素在不同浓度的FRET底物中酶促反应的初速度。利用Lineweaver-Burk双倒数作图法,计算白花丹素的抑制常数(inhibitory constant, Ki) 值。
将50 mmol/L银杏酸以0.8 μmol/L Mpro稀释至0、3.2、6.4、9.6 μmol/L。同法检测银杏酸的Ki值。
2 结果与分析 2.1 SARS-CoV-2 Mpro原核表达、分离纯化与比活力的测定将以氨苄西林抗性法筛选的重组子扩增培养,经IPTG诱导后,工程菌在相对分子量34 kDa位置有明显的目的蛋白质表达条带,其与Mpro预期分子量基本一致,并呈可溶表达。由于在Mpro的羧基端融合有多聚组氨酸标签,故此菌体裂解上清液经25%饱和硫酸铵沉淀法制备粗体液后,采用HisTrapTM亲和层析柱成功地纯化了重组Mpro。SDS-PAGE结果显示,纯化的Mpro在相对分子量34 kDa位置呈单一条带,纯度大于90% (图 1A)。纯化的Mpro经透析和定量后,浓度达2 mg/mL。比活力测定实验结果表明,纯化的Mpro具有良好的水解活性,比活力不低于40 000 U/mg (图 1B)。利用MCA标准曲线和米氏方程,计算Mpro的酶促反应动力学参数,其中米氏常数(Michaelis constant, Km) 值为19.71 μmol/L,最大反应速度(maximal velocity, Vmax) 值为149.7 ΔRFU/s,催化常数(catalytic number, kcat) 值为0.137/s,专一性常数(kcat/Km) 值为6 990 L/(mol·s) (图 1C–1D)。为了保持FRET筛选模型较低的本底值和较高的灵敏度,选用5 μmol/L FRET底物作为其最佳工作浓度。
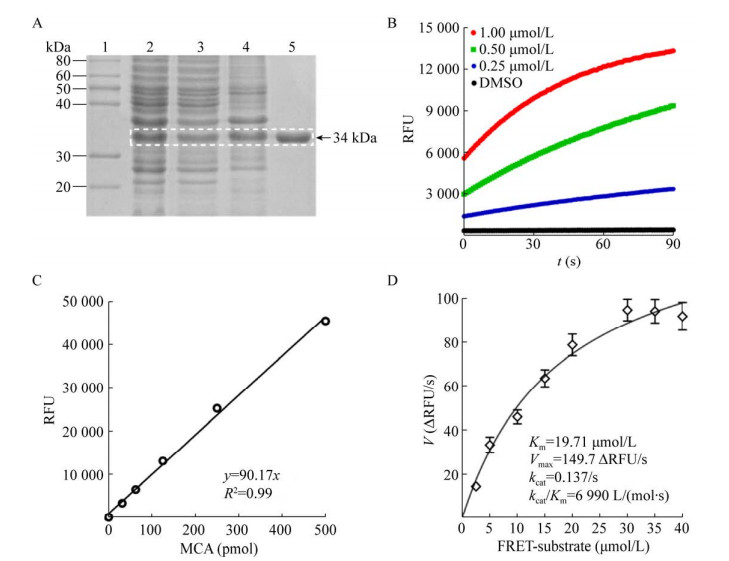
|
| 图 1 SARS-CoV-2 Mpro原核表达、分离纯化与比活力测定 Fig. 1 Preparation of highly active recombinant SARS-CoV-2 Mpro in E. coli cells. (A) Expression and purification of Mpro. The quality assessment of purified Mpro was analyzed by coomassie brilliant blue (CBB)-stained SDS-PAGE assay, and the target protein was highlighted by a white dashed box. 1: protein marker; 2: total cell extracts after IPTG induction; 3: supernatant; 4: crude extracts in 25% ammonium sulfate; 5: purified Mpro band (34 kDa). (B) Proteolytic reaction curve of purified Mpro in a FRET assay. The specific activity of Mpro was measured by a FRET assay as described in the published protocol[11]. The sample of 10 μmol/L FRET substrate was gently mixed with the indicated concentrations of purified Mpro, and the RFU value was continuously measured every second by a microplate reader (BioTek) at 320/405 nm. DMSO was used as a negative control. (C) MCA standard curve. This equation was used to convert RFU value to the amount of the cleaved FRET substrate (pmol) in a FRET assay. MCA: 7-methoxycoumarin-4-acetic acid. (D) Determination of the enzymatic parameters of purified Mpro. According to the initial velocity (V), the Km, Vmax and kcat values were calculated using a Michaelis-Menten equation. |
| |
将Mpro水解反应置于pH 6.0、7.0、8.0的缓冲液中,检测不同pH条件下Mpro酶促反应的初速度。酶促反应实验结果表明,在pH 7.0缓冲液中,Mpro的酶促反应初速度较快,此时Mpro水解活性最佳(图 2A)。因此,选用HEPES (pH 7.0) 缓冲液作为Mpro水解反应的最佳反应缓冲液。
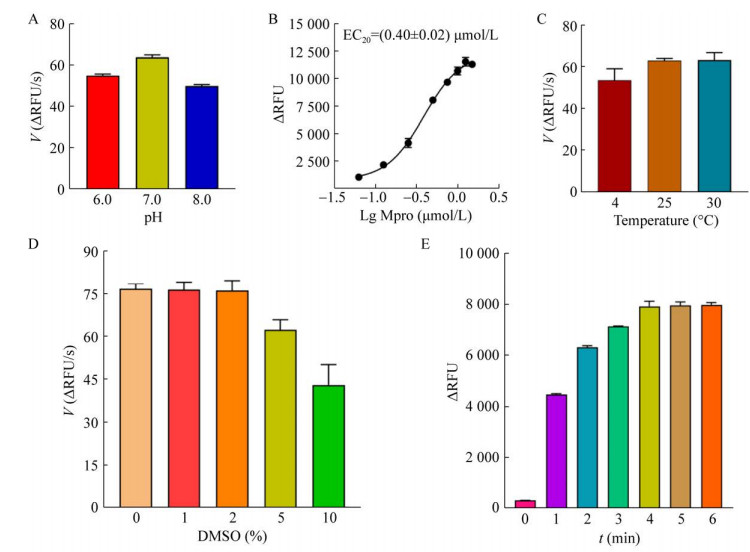
|
| 图 2 FRET筛选模型的优化 Fig. 2 Optimization for the setup of a FRET screening assay. (A) Reaction buffer optimization. The proteolytic reaction was initiated at different pH buffers and the initial velocity (V) was separately compared to determine an optimal reaction buffer. (B) Proteolytic reaction curve of Mpro. The sample of 5 μmol/L FRET substrate was incubated with the 2-fold dilutions of Mpro. After the measurement of RFU value, the proteolytic reaction curve was plotted to calculate an EC50 value using GraphPad Prism 8.0. (C) Incubation temperature optimization. As described above, the initial velocities (V) at the indicated incubation temperatures were calculated. (D) DMSO tolerance assay. The proteolytic reaction was performed in the presence of the indicated DMSO concentrations, and the initial velocities (V) were compared. (E) Incubation length optimization. The reaction mixture containing 5 μmol/L FRET substrate and 0.4 μmol/L Mpro was incubated at room temperature (RT) from 0 to 6 min, and the RFU value was separately recorded by a microplate reader (BioTek). The maximal RFU value indicated an endpoint of Mpro proteolytic reaction. |
| |
将5 μmol/L FRET底物与倍比稀释的Mpro孵育,进行Mpro水解反应。拟合的Mpro水解反应曲线表明,在上述水解反应中,Mpro的EC50值为0.4 μmol/L (图 2B)。为了保持FRET筛选模型的较高灵敏度,选用0.4 μmol/L作为Mpro的最佳工作浓度。
将5 μmol/L FRET底物与0.4 μmol/L Mpro分别在4 ℃、25 ℃、30 ℃条件下孵育,进行Mpro水解反应。酶促反应实验结果表明,Mpro水解反应在上述条件下,其酶促反应的初速度无显著差异(图 2C),说明建立的FRET筛选模型具有良好的温度耐受性与稳定性,环境温度的波动对FRET筛选模型的稳定性没有显著的影响。为了方便FRET筛选模型的便利使用,选择室温作为Mpro水解反应的最佳反应温度。
将5 μmol/L FRET底物与0.4 μmol/L Mpro分别在不同DMSO条件下孵育,进行Mpro水解反应。酶促反应实验结果表明,当Mpro水解反应中DMSO浓度低于2%时,其对酶促反应的初速度无显著影响(图 2D)。为了保证FRET筛选模型的稳定性与可靠性,DMSO工作浓度不应高于2%。
将5 μmol/L FRET底物与0.4 μmol/L Mpro分别在不同时间点孵育,进行Mpro水解反应。酶促反应实验结果表明,从4 min开始,酶促反应的ΔRFU值基本达到最大,说明Mpro水解反应彻底完成(图 2E)。为了保证FRET筛选模型的高灵敏度与高效性,确定FRET底物与Mpro的最佳孵育时间为5 min。
综上所述,通过上述系统的优化实验,确定了FRET筛选模型的最佳工作条件为:在HEPES缓冲液中,5 μmol/L FRET底物与0.4 μmol/L Mpro室温孵育5 min,再进行RFU值检测。
2.3 DTT对FRET筛选模型可靠性的影响依布硒啉与黄芩素是已报道的Mpro小分子抑制剂[6, 13-15]。上述优化的FRET筛选模型中的抑制活性实验表明,当HEPES缓冲液中不含DTT时,依布硒啉与黄芩素具有良好的抑制活性,其IC50值分别为(0.21±0.03) μmol/L、(2.18±0.2) μmol/L (图 3A, 3C),与文献报道基本一致[6, 13-15]。当HEPES缓冲液中含有1 mmol/L DTT时,依布硒啉与黄芩素对Mpro的抑制活性极大地减弱,IC50值均大于50 μmol/L (图 3B–3D)。这表明依布硒啉与黄芩素是非特异性的Mpro小分子抑制剂,DTT是影响FRET筛选模型可靠性的重要因素。因此,为了保证FRET筛选模型具有良好的可靠性,在苗头化合物高通量筛选与活性鉴定的实验中,均应在含有DTT的HEPES缓冲液中进行。
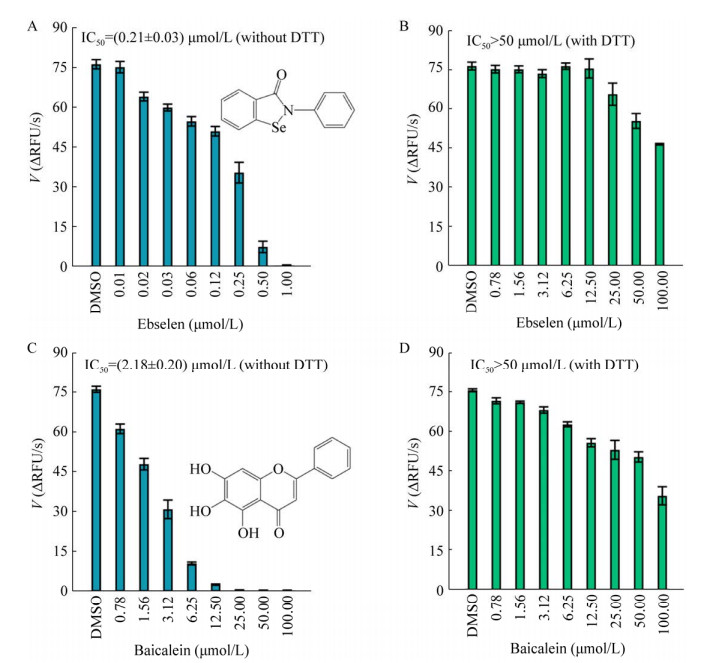
|
| 图 3 DTT对FRET筛选模型可靠性的影响 Fig. 3 Effect of DTT addition on the reliability of a FRET screening assay. (A, B) The inhibitory activity of ebselen in the absence or presence of 1 mmol/L DTT in HEPES buffer. Tested ebselen was diluted by 0.8 μmol/L Mpro solution to generate 8 dilutions and added to a 96-well microplate (25 μL/well). The mixture was further incubated for 30 min at RT. After addition of 25 μL solution of 10 μmol/L FRET dilution, the RFU value was monitored by a microplate reader (BioTek). The initial velocity (V) was generated according to the slop of the enzymatic reaction progress. The IC50 value was calculated using the plotted inhibitory curve. (C, D) The inhibitory activity of baicalein in the absence or presence of 1 mmol/L DTT in HEPES buffer. As mentioned above, the inhibitory activity of baicalein against Mpro was carried out using a FRET screening assay. All experiments were performed in triplicate. |
| |
GC-376是公认的、具有良好抑制活性的Mpro小分子抑制剂[16-18]。GC-376的抑制活性实验结果表明,GC376对Mpro酶活性具有良好的抑制作用,在所建立的FRET筛选模型中的IC50值为0.12 μmol/L,与文献报道基本一致[17-18] (图 4A–4B),说明建立的FRET筛选模型具有良好的特异性与灵敏性。
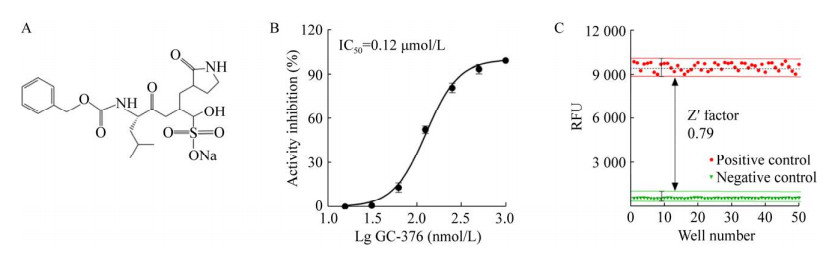
|
| 图 4 FRET筛选模型的特异性评价 Fig. 4 Quality assessment of an optimized FRET screening assay. (A) The chemical structure of GC-376. (B) The inhibitory activity of GC-376 against Mpro was determined using an optimized FRET screening assay, and all the tests in the presence of 1 mmol/L DTT were performed in triplicate. (C) Determination of Z′ factor of a FRET screening assay. GC-376 and DMSO were used as positive and negative controls, respectively. A high signal window was highlighted by a straight line. The Z′ factor of 0.79 indicated that this screening assay is reliable, robust and amenable for large-scale screening. |
| |
设定GC376与DMSO作为FRET筛选模型的阳性对照组与阴性对照组。通过系统的计算,本筛选模型的各项筛选指标均满足高通量筛选的基本要求(表 1)。其中Z′因子值为0.79,满足其大于0.5的基本要求(图 4C)。说明已成功建立了适用于Mpro小分子抑制剂高通量筛选的FRET筛选模型。
| Indicators | Requirements of HTS | Results of HTS |
| SW | Not determined | 9 368.00 |
| S/N | > 10 | 11.88 |
| S/B | > 3 | 18.38 |
| Z′ factor | > 0.5 | 0.79 |
根据上述优化的实验结果,使用0.4 μmol/L Mpro与5 μmol/L FRET底物成功建立了Mpro小分子抑制剂高通量筛选模型(图 5A)。利用已建立的FRET筛选模型对本室天然产物化合物库进行了高通量筛选,以淬灭率大于50%作为候选苗头化合物的判断标准,初筛共获得24个候选阳性化合物(图 5B)。
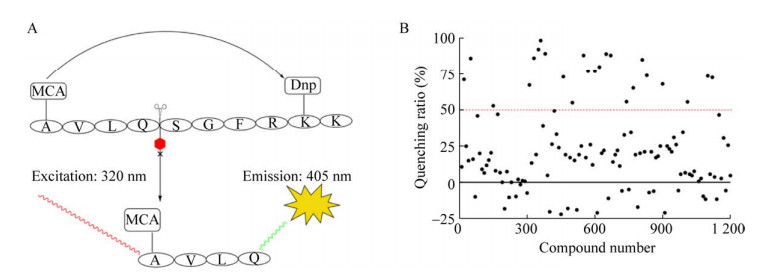
|
| 图 5 天然产物化合物库的高通量筛选 Fig. 5 High-throughput screening of a natural product library using an optimized FRET screening assay. (A) Sketch map of the FRET screening assay construction. The quenched peptide was used as a FRET substrate, and the Mpro cleaved site was represented by a scissor. After cleavage by active Mpro, the cleaved MCA-AVLQ fragment was released, resulting in a high RFU value. Hence, the bioactive compound exhibited a low RFU value in this FRET screening assay. Dnp: 2, 4-dinitropheno. (B) The general layout in the primary screening cycle. In brief, the mixture containing Mpro (0.8 μmol/L) and natural product (1 mg/mL) was incubated for 30 min at RT in a 96-well microplate (25 μL/well). After addition of 25 μL FRET substrate (10 μmol/L), the mixture was continued to incubate for 5 min. Subsequently, the RFU value was measured using a microplate reader (BioTek). The red dashed line represented a quenching ratio (Qr) value of 50%, and all candidate hits (Qr > 50%) were further validated in second FRET assay. |
| |
在第2轮复筛中,发现白花丹素与银杏酸对Mpro酶活性的抑制作用具有明显的剂量依赖性,且DTT对其抑制活性无显著影响,说明白花丹素与银杏酸是新型特异性Mpro小分子抑制剂(图 6A–6B, 6E–6F)。其中,白花丹素的IC50值为(19.15±0.94) μmol/L,且为非竞争型抑制剂,其Ki值为11.89 μmol/L (图 6B–6D)。银杏酸的IC50值为(4.89±0.3) μmol/L,且为竞争型抑制剂,其Ki值为3.52 μmol/L (图 6F–6H)。
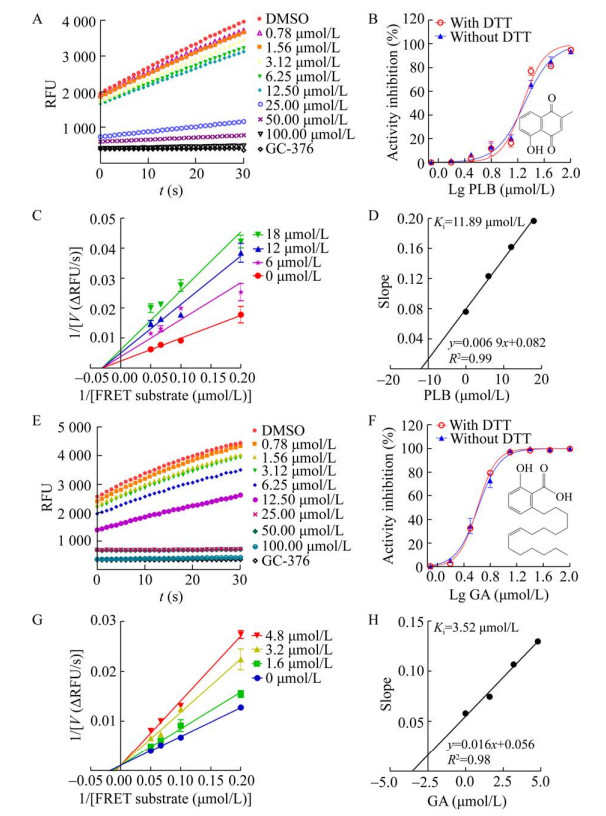
|
| 图 6 白花丹素与银杏酸在FRET筛选模型中的抑制活性与抑制机制 Fig. 6 Inhibitory activities and inhibition mechanisms of plumbagin (PLB) and ginkgolic acid (GA). (A) The change of RFU value in the presence of PLB. The slope of the colored lines represented the initial velocities (V) at the indicated concentrations of PLB. (B) The inhibitory curve of PLB in the absence or presence of DTT using a FRET assay. The chemical structure of PLB was presented. (C) The Lineweaver-Burk double-reciprocal plots of PLB using a FRET assay. (D) The second plots for a Ki value of PLB. (E) The change of RFU value in the presence of GA at the indicated concentrations. (F) The inhibitory curve of GA in the absence or presence of DTT using a FRET assay. The chemical structure of GA was showed. All the inhibitory curves were plotted using GraphPad Prism 8.0, and then the IC50 values were calculated. All mentioned FRET assays were carried out in triplicate. (G) The Lineweaver-Burk double-reciprocal plots of GA using a FRET assay. (H) The second plots for a Ki value of GA. |
| |
自SARS-CoV-2感染导致的COVID-19疫情暴发以来,始终具有较高的传染性。SARS-CoV-2德尔塔突变株使其传染性和致病性进一步增强,降低了疫苗接种的保护力[3]。因此,积极开发广谱抗冠状病毒药物具有重要意义。进化保守的Mpro在调控新冠病毒RNA复制中具有重要的作用且人体缺乏其同源蛋白酶。因此,Mpro被认为是广谱抗冠状病毒药物开发的理想靶标之一[6-8]。
制备高活性的Mpro是药物高通量筛选模型建立的重要基础。本研究利用大肠杆菌原核表达技术,基于羧基端融合多聚组氨酸标签的构建策略,成功地进行了重组Mpro在大肠杆菌中的原核表达与分离纯化。纯化的Mpro比活力不低于40 000 U/mg,且kcat/Km值为6 990 L/(mol·s),不低于已报道的酶活力[11],说明制备的Mpro具有良好的水解活性,这为Mpro小分子抑制剂高通量筛选模型的建立奠定了坚实基础。
目前,已报道了多种筛选方法用于Mpro小分子抑制剂的筛选与发现,主要包括虚拟筛选法、FRET筛选法、细胞模型筛选法和表型筛选法等[19]。其中,经典的FRET筛选法主要基于电偶极相互作用时,能量从起始被激发的供体分子(donor) 转移到受体分子(acceptor) 不产生任何光子的原理,具有操作简便、灵敏度高、分析快速等优点,从而使FRET技术在药物筛选、生物分析与食品科学等领域被广泛应用[19-20]。基于FRET技术原理,以荧光基团MCA与淬灭基团Dnp标记来源于Mpro天然剪切序列的多肽作为Mpro水解底物。当Mpro水解FERT底物产生MCA-AVLQ片段时,由于底物失去FRET效应会产生较高的RFU值。但活性化合物可以抑制Mpro对FRET底物的水解作用,产生较低的RFU值[19]。虽然已有报道利用FRET筛选法成功地发现了大量的Mpro抑制剂,但非特异性抑制剂的高频出现,迫切需求建立更可靠、更稳定的FRET筛选法应用于Mpro小分子抑制剂的快速筛选与发现[9-10]。本研究在高活性Mpro成功制备的基础上,通过一系列反应条件优化,成功地建立了灵敏、可靠的FRET筛选模型。通过依布硒啉与黄芩素在FRET筛选模型中的抑制实验,证实了DTT是影响FRET筛选模型可靠性的重要因素。
Mpro作为半胱氨酸蛋白酶,Cys145是重要的活性中心。DTT作为一种小分子还原剂,可使含有游离巯基的Mpro更加稳定。某些化合物(如依布硒啉、黄芩素等) 可能通过非特异性的烷基化或氧化作用影响Cys145巯基使Mpro失活,表现出非特异性的表观抑制作用[10]。但加入DTT可以更好地保护Cys145巯基,防止上述非特异性表观抑制作用的发生,提高了FRET筛选模型的可靠性。本研究证实,DTT的存在可以消除筛选化合物潜在的非特异性影响,进一步提高FRET筛选模型的可靠性与稳定性。GC-376作为共价结合的Mpro小分子抑制剂,其在FRET筛选模型中的IC50值与文献报道基本一致[19-20],且本筛选模型的Z′值为0.79,再次证实了所建立的FRET筛选模型具有良好的可靠性、灵敏性和稳定性。本研究建立的FRET筛选模型较好地模拟了Mpro的水解反应,但由于标记底物多肽的荧光分子易于淬灭,所以需要新鲜配制并使用。另外,由于某些天然产物分子可能具有荧光特性,能够对FRET筛选模型的RFU值检测造成较大的干扰,所以需要及时利用化合物库的理化信息,有效地排除此类化合物的干扰以提高筛选效率。考虑到Mpro的酶动力学反应易受环境、温度等因素影响,为了更好地保持FRET筛选模型的稳定性与重复性,需要尽量保证FRET反应条件的一致性。
利用所建立的FRET筛选模型,通过对本室天然产物化合物库进行高通量筛选,发现了具有良好抑制活性的白花丹素与银杏酸是一类新型特异性Mpro小分子抑制剂。白花丹素是传统中药白花丹的主要药效成分,其具有广泛的药理活性,包括抗病毒、抗肿瘤、抗炎症等[21-22]。本实验室曾报道白花丹素是一类靶向β-catenin/ TCF4相互作用的新型Wnt抑制剂,具有良好的抗肿瘤活性[23]。但白花丹素也对Mpro酶活性展现出了中等强度的非竞争性抑制作用。白花丹素作为Mpro抑制剂的新型苗头化合物,需要深入进行化学改造与结构优化,以提高其成药性与靶向性。相较白花丹素的中等抑制活性,来源于银杏叶提取物的银杏酸却展现出了较强的竞争性抑制活性。已有研究证实,银杏酸对埃博拉病毒、流感病毒、EB病毒、基孔肯亚病毒、马亚罗病毒、寨卡病毒等展现出了良好的广谱抗病毒活性[24-25]。近期研究证实,银杏酸也是靶向新冠病毒Mpro的小分子抑制剂,具有较好的抗新冠病毒活性,其EC50值为8.3 μmol/L[26-27]。本研究再次证实了银杏酸是新冠病毒Mpro的竞争型小分子抑制剂,可能是一类具有广阔开发前景的新型苗头化合物。
综上所述,本研究通过系统的优化方案,成功地建立了适用于新冠病毒Mpro小分子抑制剂快速筛选的FRET高通量筛选模型,证实了白花丹素与银杏酸是一类新型苗头化合物,为抗新型冠状病毒药物先导化合物的筛选与发现奠定了基础。
| [1] |
Zheng J. SARS-CoV-2: an emerging coronavirus that causes a global threat. Int J Biol Sci, 2020, 16(10): 1678-1685. DOI:10.7150/ijbs.45053
|
| [2] |
Korber B, Fischer WM, Gnanakaran S, et al. Tracking changes in SARS-CoV-2 spike: evidence that D614G increases infectivity of the COVID-19 virus. Cell, 2020, 182(4): 812-827.e19. DOI:10.1016/j.cell.2020.06.043
|
| [3] |
Lopez Bernal J, Andrews N, Gower C, et al. Effectiveness of covid-19 vaccines against the B.1.617.2 (delta) variant. N Engl J Med, 2021, 385(7): 585-594. DOI:10.1056/NEJMoa2108891
|
| [4] |
Shang J, Wan YS, Luo CM, et al. Cell entry mechanisms of SARS-CoV-2. PNAS, 2020, 117(21): 11727-11734. DOI:10.1073/pnas.2003138117
|
| [5] |
Anirudhan V, Lee H, Cheng H, et al. Targeting SARS-CoV-2 viral proteases as a therapeutic strategy to treat COVID-19. J Med Virol, 2021, 93(5): 2722-2734. DOI:10.1002/jmv.26814
|
| [6] |
Jin Z, Du X, Xu Y, et al. Structure of Mpro from SARS-CoV-2 and discovery of its inhibitors. Nature, 2020, 582(7811): 289-293. DOI:10.1038/s41586-020-2223-y
|
| [7] |
Jin Z, Wang H, Duan Y, et al. The main protease and RNA-dependent RNA polymerase are two prime targets for SARS-CoV-2. Biochem Biophys Res Commun, 2021, 538: 63-71. DOI:10.1016/j.bbrc.2020.10.091
|
| [8] |
Zhu W, Shyr Z, Lo DC, et al. Viral proteases as targets for coronavirus disease 2019 drug development. J Pharmacol Exp Ther, 2021, 378(2): 166-172. DOI:10.1124/jpet.121.000688
|
| [9] |
Ma CL, Wang J. Dipyridamole, chloroquine, montelukast sodium, candesartan, oxytetracycline, and atazanavir are not SARS-CoV-2 main protease inhibitors. PNAS, 2021, 118(8): e2024420118. DOI:10.1073/pnas.2024420118
|
| [10] |
Ma CL, Hu YM, Townsend JA, et al. Ebselen, disulfiram, carmofur, PX-12, tideglusib, and shikonin are nonspecific promiscuous SARS-CoV-2 main protease inhibitors. ACS Pharmacol Transl Sci, 2020, 3(6): 1265-1277. DOI:10.1021/acsptsci.0c00130
|
| [11] |
陈云雨, 付正豪, 闫干干, 等. 基于密码子优化策略的新型冠状病毒主蛋白酶在大肠杆菌中的表达条件优化与活性鉴定. 生物工程学报, 2021, 37(4): 1334-1345. Chen YY, Fu ZH, Yan GG, et al. Optimization of expression conditions and determination the proteolytic activity of codon-optimized SARS-CoV-2 main protease in Escherichia coli. Chin J Biotech, 2021, 37(4): 1334-1345 (in Chinese). |
| [12] |
Chen Y, Fu Z, Li D, et al. Optimizations of a novel fluorescence polarization-based high-throughput screening assay for β-catenin/LEF1 interaction inhibitors. Anal Biochem, 2021, 612: 113966. DOI:10.1016/j.ab.2020.113966
|
| [13] |
Amporndanai K, Meng X, Shang W, et al. Inhibition mechanism of SARS-CoV-2 main protease by ebselen and its derivatives. Nat Commun, 2021, 12(1): 3061. DOI:10.1038/s41467-021-23313-7
|
| [14] |
Su HX, Yao S, Zhao WF, et al. Anti-SARS-CoV-2 activities in vitro of Shuanghuanglian preparations and bioactive ingredients. Acta Pharmacol Sin, 2020, 41(9): 1167-1177. DOI:10.1038/s41401-020-0483-6
|
| [15] |
Liu H, Ye F, Sun Q, et al. Scutellaria baicalensis extract and baicalein inhibit replication of SARS-CoV-2 and its 3C-like protease in vitro. J Enzyme Inhib Med Chem, 2021, 36(1): 497-503. DOI:10.1080/14756366.2021.1873977
|
| [16] |
Hung HC, Ke YY, Huang SY, et al. Discovery of M protease inhibitors encoded by SARS-CoV-2. Antimicrob Agents Chemother, 2020, 64(9): e00872-20.
|
| [17] |
Vuong W, Khan MB, Fischer C, et al. Feline coronavirus drug inhibits the main protease of SARS-CoV-2 and blocks virus replication. Nat Commun, 2020, 11(1): 4282. DOI:10.1038/s41467-020-18096-2
|
| [18] |
Fu L, Ye F, Feng Y, et al. Both boceprevir and GC376 efficaciously inhibit SARS-CoV-2 by targeting its main protease. Nat Commun, 2020, 11(1): 4417. DOI:10.1038/s41467-020-18233-x
|
| [19] |
戚海燕, 闫干干, 付正豪, 等. 新型冠状病毒主蛋白酶抑制剂的筛选方法研究进展. 生命的化学, 2021, 41(2): 207-214. Qi HY, Yan GG, Fu ZH, et al. Miniaturized high-throughput screening assays for the discovery of SARS-CoV-2 main protease inhibitors. Chem Life, 2021, 41(2): 207-214 (in Chinese). |
| [20] |
Imani M, Mohajeri N, Rastegar M, et al. Recent advances in FRET-based biosensors for biomedical applications. Anal Biochem, 2021, 630: 114323. DOI:10.1016/j.ab.2021.114323
|
| [21] |
Panichayupakaranant P, Ahmad MI. Plumbagin and its role in chronic diseases. Adv Exp Med Biol, 2016, 929: 229-246.
|
| [22] |
Roy A. Plumbagin: a potential anti-cancer compound. Mini Rev Med Chem, 2021, 21(6): 731-737. DOI:10.2174/1389557520666201116144421
|
| [23] |
付正豪, 闫干干, 朱小红, 等. 靶向β-catenin/TCF4相互作用小分子抑制剂酶联免疫吸附法高通量筛选模型的优化与应用. 生物工程学报, 2021, 37(8): 2878-2889. Fu ZH, Yan GG, Zhu XH, et al. Optimizations of an ELISA-like high-throughput screening assay for the discovery of β-catenin/TCF4 interaction antagonists. Chin J Biotech, 2021, 37(8): 2878-2889 (in Chinese). |
| [24] |
Borenstein R, Hanson BA, Markosyan RM, et al. Ginkgolic acid inhibits fusion of enveloped viruses. Sci Rep, 2020, 10(1): 4746. DOI:10.1038/s41598-020-61700-0
|
| [25] |
Campos D, Navarro S, Llamas-González YY, et al. Broad antiviral activity of ginkgolic acid against chikungunya, mayaro, una, and zika viruses. Viruses, 2020, 12(4): 449. DOI:10.3390/v12040449
|
| [26] |
Chen ZN, Cui QH, Cooper L, et al. Ginkgolic acid and anacardic acid are specific covalent inhibitors of SARS-CoV-2 cysteine proteases. Cell Biosci, 2021, 11(1): 45. DOI:10.1186/s13578-021-00564-x
|
| [27] |
Xiong Y, Zhu GH, Wang HN, et al. Discovery of naturally occurring inhibitors against SARS-CoV-2 3CLpro from Ginkgo biloba leaves via large-scale screening. Fitoterapia, 2021, 152: 104909. DOI:10.1016/j.fitote.2021.104909
|
 2022, Vol. 38
2022, Vol. 38