中国科学院微生物研究所、中国微生物学会主办
文章信息
- 刘经伟, 王健, 王琳
- LIU Jingwei, WANG Jian, WANG Lin
- 人工神经导管原材料选择与功能设计的研究进展
- Advances in the raw material selection and functional design of artificial nerve guidance conduits
- 生物工程学报, 2023, 39(10): 4057-4074
- Chinese Journal of Biotechnology, 2023, 39(10): 4057-4074
- 10.13345/j.cjb.221049
-
文章历史
- Received: December 29, 2022
- Accepted: April 11, 2023
- Published: April 13, 2023
2. 华中科技大学同济医学院附属协和医院检验科, 湖北 武汉 430022
2. Department of Clinical Laboratory, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430022, Hubei, China
周围神经损伤(peripheral nerve injury, PNI)是一种临床常见的疾病,多由交通事故、坠落伤、地震灾害、火器伤以及医源性损伤等引发。据统计约2.8%的外伤患者会累及周围神经,其中近4成患者出现神经器质性损伤[1],主要表现为运动与感觉功能部分或全部丧失、慢性疼痛和靶肌肉萎缩等[2]。目前PNI,尤其是神经离断伤会导致残疾,严重危害公共健康。
临床上短距离PNI的治疗多采用无张力神经吻合术,而长距离PNI治疗的金标准为自体神经移植。腓肠神经是最为常用的供体神经,手术方式为从腓肠神经中获取多束感觉神经移植到周围神经缺损处[3]。但是该疗法仍存在着诸多限制,如获取供体神经需要二次手术且会损害患者浅表感觉功能;自体神经的供给有限,无法有效治疗长段的周围神经离断伤[3-4]。因此PNI的治疗仍面临严峻挑战。
近年来随着组织工程学的发展,学者们提出开发人工神经导管(nerve guidance conduits, NGCs)用于替代自体神经移植。NGCs主要由天然或合成聚合物制备,旨在为神经再生提供引导与支持,并克服自体神经移植的限制[5]。20世纪70年代,第一代硅胶神经导管应运而生,随后各种材质、不同设计的NGCs迭代更新。第一代NGCs的设计宗旨是提供一种支撑结构,引导横断神经的再生并阻止结缔组织的浸入[6]。其中,典型的这类导管包括硅胶神经导管、聚四氟乙烯神经导管等[7-8],但它们在体内移植后均无法降解,会引发局部神经压迫以及纤维包膜的形成[9]。在此基础上,第二代神经导管提出使用可生物吸收、生物相容性好的原材料构建NGCs[10],这类导管包括聚乙交酯(polyglycolic acid, PGA)神经导管[11-12]、I型胶原神经导管[13]、己内酯神经导管[14-15]和聚乙烯醇(polyvinyl alcohol, PVA)神经导管[16]等。它们虽然能高效修复短距离的周围神经缺损,但是在治疗中、长距离PNI时神经再生与功能修复效果均不理想。针对上述问题,研究者提出在导管内搭载支持细胞、提供可控的治疗因子递送、给予物理电刺激、填充微-纳纤维导向材料或提供拓扑微结构等以研发第三代神经导管,目前该种导管的设计与制备仍为神经再生领域的研究热点。本文重点介绍第三代NGCs的研发以及其在PNI中的治疗现状,并将从原材料选择、结构设计、治疗因子搭载以及自供电元件集成等多个方面进行综述。
1 制备NGCs原材料 1.1 天然生物材料天然生物聚合物作为支架材料具有生物相容性好、支持细胞黏附与(部分)促细胞增殖等多种生物活性[17]。目前用于制备NGCs的天然生物材料包括多糖类生物材料如透明质酸、海藻酸盐和壳聚糖等与蛋白多肽类生物材料如胶原蛋白、丝素蛋白和纤维蛋白等(表 1)。对近年制备NGCs天然生物材料的优缺点进行归纳总结(表 2)。基于不同生物来源,天然生物材料有着各自独特的优势,如透明质酸的抗瘢痕组织再生、壳聚糖的抗菌活性与丝胶蛋白的促神经再生作用等。可惜天然生物材料都有相似的缺陷,即机械强度难以满足神经再生的需求。同时天然蛋白与多糖成分复杂,纯化工艺难以标准化,无法完全去除其免疫原性,因此天然生物材料的应用受到限制[44]。交联多种天然生物材料以互补彼此缺陷,如壳聚糖/海藻酸盐、壳聚糖/丝胶蛋白等,是目前克服上述困难的主要解决方案之一。
| Materials | Type | Animal model | Effects | Functional outcome | References |
| Chitosan/ Hyaluronic acid | Natural composite | Rats with sciatic nerve crush | Decrease of the formation of nerve scars and adhesions, improved neural regeneration and repair | N.A. | [18] |
| Collagen/ Hyaluronic acid/PCL | Natural- synthetic composite | N.A. | Improved attachment and proliferation of Schwann cells, and enhanced axon outgrowth | N.A. | [19] |
| Chitosan/ PLGA | Natural- synthetic composite | Rats with sciatic nerve transection | Improved migration and maturation of Schwann cells, and reduced inflammatory response | N.A. | [20] |
| Chitosan | Natural | Rats with sciatic nerve transection | Angiogenesis and improved regeneration of nerve and myelin | Sciatic nerve function index was improved | [21] |
| Alginate/ Chitosan | Natural composite | Rats with sciatic nerve crush | Improved cell viability of the transplanted extraolfactory mesenchymal stem cells | Sciatic function nerve index was improved and heat pain latency was shortened | [22] |
| Alginate/ Chitosan | Natural composite | Rats with sciatic nerve crush | Decreased levels in gastrocnemius atrophy and of nerve fiber demyelination | Sciatic function nerve index was improved and heat pain latency was shortened | [23] |
| Silk fibroin | Natural | N.A. | Improved migration and adhesion of Schwann cells | N.A. | [24] |
| Silk fibroin/ Magnesium filaments | Natural- synthetic composite | Rats with sciatic nerve transection | Improved adhesion and proliferation of Schwann cells, regeneration of nerve and myelin sheath | Sciatic nerve function index was improved | [25] |
| Sericin/ Chitosan | Natural composite | Rats with sciatic nerve crush | Improved regeneration of nerve and myelin sheath, reduced levels of inflammation and gastrocnemius atrophy | Better performances in mechanical withdrawal threshold test and thermal withdrawal latency test | [26] |
| Sericin/ CNT | Natural- synthetic composite | Rats with sciatic nerve transection | Myelin sheath regeneration, electrophysiological reconstruction and muscle reinnervation | Sciatic nerve function index was improved and thermal withdrawal latency was shortened | [27] |
| PLA/CNT | Synthetic composite | Rats with sciatic nerve transection | Myelin sheath and axon regeneration | Sciatic function nerve index was improved and heat pain latency was shortened | [28] |
| PLA | Synthetic | Rats with sciatic nerve transection | Myelin sheath and axon regeneration | Sciatic nerve function index was improved | [29] |
| PCL/PPy | Synthetic composite | N.A. | Improved cell proliferation and differentiation of human embryonic stem cells-derived neural crest stem cells | N.A. | [30] |
| PCL/ Gelatin | Synthetic composite | Rats with sciatic nerve transection | Neurotrophic factor expression and axon regeneration | Sciatic nerve function index was improved and hot plate latency time was shortened | [31] |
| PLGA/ Collagen/ Hyaluronic acid | Natural- synthetic composite | Rats with sciatic nerve transection | Controlled release of neurotrophic factors | Better performances on electrical stimulation test | [32] |
| PLGA | Synthetic | Rats with sciatic nerve crush | The reduced release of proinflammatory factors | Sciatic nerve function index and BBB scores were improved, and withdrawal reflex latency was shortened | [33] |
| N.A.: Not applicable. | |||||
| Materials | Source | Advantage | Disadvantage | References |
| Hyaluronic acid | ECM | Hypo-immunogenicity Overcoming extra-neural scarring |
Very low mechanical strength Biodegradation |
[18-19, 34] |
| Chitosan | Carapace | Antibacterial activity Reducing neuroma formation and fibrosis |
Low mechanical strength High brittleness |
[20-21, 35-36] |
| Alginate | Brown algae | Remarkable chemical flexibility Physical crosslinking |
Low mechanical strength High degradation rate |
[22-23, 37] |
| Silk fibroin | Silk | Suitable mechanical strength Economically advantageous High resistance to fracture and compression |
Silk fibroin derived from solutions are generally weak and fragile. | [24-25, 38-41] |
| Sericin | Silk | Hypo-immunogenicity Suitable degradation rate Degradation products promoting nerve regeneration |
Low mechanical strength | [26-27, 42-43] |
透明质酸是一种天然线性多糖,由葡萄糖醛酸与N-乙酰氨基葡萄糖组成,属于细胞外基质中的糖胺聚糖成分[17-18]。在促进细胞信息交流中发挥重要作用。同时,透明质酸与血管形成、肿瘤发生和炎症反应等生理病理过程息息相关[18]。作为早期天然导管材料代表,透明质酸的优势在于可以有效减少神经溶解后的疤痕与神经再生后的粘连[34]。同时来源于细胞外基质,使其生物相容性与降解性优异,但无法满足神经导管对机械强度的需求。因此,透明质酸常与人工合成材料联合使用以达到更好的机械性能。Entekhabi等[19]利用了人工聚合物聚己内酯作为透明质酸神经导管的外壳,克服了纯透明质酸导管易形变的缺点,通过评价支架上的施万细胞增殖与轴突的生长情况,证明了其具有促神经再生的潜力。Li等[18]采用透明质酸/壳聚糖神经导管治疗周围神经卡压伤,发现透明质酸的引入有效抑制了瘢痕组织的形成与损伤处的组织黏附,有效促进神经再生。
1.1.2 壳聚糖壳聚糖是由甲壳素经过脱乙酰作用得到的碱性多糖。壳聚糖与糖胺聚糖的结构相似,故壳聚糖可以与细胞外基质中的分子相互作用。另外,壳聚糖具有多种生物学特性,且相较于透明质酸更易加工[35]。壳聚糖的物理性能比透明质酸更优秀,在为轴突再生提供物理支撑的同时,其降解产物壳寡糖可促进神经的再生[36]。但是壳聚糖在干燥状态下易碎,因此多与其他材料联合使用。Lu等[20]开发聚乳酸-羟基乙酸共聚物[poly(lactic-co-glycolic acid), PLGA)]与壳聚糖复合的神经导管,其降解产物改变局部的酸碱度从而调控神经再生中的炎症水平与再生微环境。Rao等[21]利用壳聚糖与细胞外基质类似的特点,将细胞因子模拟肽结合在壳聚糖导管上以模拟神经再生微环境,修复了大鼠15 mm的坐骨神经缺损。
1.1.3 海藻酸盐海藻酸盐主要来自于褐藻,与其他的天然生物材料相比,古洛糖酸醛与甘露糖酸醛的含量较高,赋予海藻酸盐生物材料更好的韧性。海藻酸盐可以通过钙离子进行物理交联,避免了戊二醛等共价交联剂引起的化学毒性。海藻酸盐作为支架材料的优势在于生物相容性好、可生物降解和螯合能力强,促细胞黏附。不同于壳聚糖的脆性,海藻酸盐凝胶有着更好的韧性与黏附性。因此海藻酸盐是一种极具潜力的组织修复与再生的支持基质或递送系统[37]。Salehi等[22]构建了一种海藻酸盐/壳聚糖支架,搭载嗅间充质干细胞用于治疗坐骨神经缺损。基于海藻酸盐的良好生物相容性与促细胞黏附的能力,该复合水凝胶显著延长了间充质干细胞在损伤部位的存活时间。此外,Rahmati等[23]利用海藻酸盐/壳聚糖支架搭载小檗碱治疗坐骨神经损伤,海藻酸盐提供了支架足够的机械强度与韧性,通过水凝胶的溶胀与降解实现小檗碱的缓释。
1.1.4 丝素蛋白蚕丝作为手术缝合线的原材料在医学领域被广泛应用。近10年来,蚕丝的核心蛋白——丝素蛋白作为第三代导管材料在生物医学工程领域受到关注。丝素蛋白具有良好生物相容性、低免疫原性、适宜的机械强度与韧性等优点[38-40]。丝素蛋白拥有促进神经再生的特性,其已被证明可支持神经轴突延伸与施万细胞增殖[41]。基于此,丝素蛋白也常作为组织工程神经导管的材料。Zhang等[24]开发了一种负载甲钴胺的丝素蛋白神经导管,丝素蛋白有效促进了施万细胞的黏附与迁移。Zhang等[25]构建了一种丝素蛋白/镁金属丝复合神经导管,丝素蛋白的包裹有效提高镁金属丝的生物相容性并且增强导管的机械强度,实现Mg2+缓释以促进神经再生。
1.1.5 丝胶蛋白丝胶蛋白作为一种水溶性黏蛋白,在天然蚕丝中维持其核壳结构的完整性。武汉协和医院王琳团队[42]发现温和的溴化锂提取法可以获取结构完整的丝胶蛋白,并证实丝胶水凝胶具有良好的生物相容性、可降解性与低免疫原性。丝胶水凝胶可以支持神经细胞的黏附和生长,并通过降解产物抑制细胞凋亡通路发挥神经保护作用[43];开发了一种壳聚糖/丝胶蛋白复合神经导管,通过丝胶蛋白的降解产物诱导神经营养因子与髓鞘生成相关蛋白表达升高,有效地促进神经的再生[26];构建了碳纳米管/丝胶蛋白神经导管,其中的丝胶蛋白提供了良好的神经保护与营养作用,促进神经轴突的延伸与髓鞘再生[27]。丝胶蛋白作为一种新兴的再生修复材料,在神经导管制备与研发方面极具潜力。
1.2 人工合成材料不同于天然生物材料的组分复杂,人工合成材料因为其可控的理化性能,在组织工程领域中拥有独特的优势[35]。但是,相较于天然生物材料,其生物相容性较差,需要进行表面修饰以改进生物亲和力。早在1970年,有学者尝试使用有机硅导管在猴子身上进行正中神经修复[45]。有机硅导管在短距离的周围神经修复中取得了效果[46],此后包括聚丙烯酸、聚乙烯等不可降解材料也被用于NGCs的制备[47]。但是,这些材料会引发慢性异物反应,同时阻碍导管内外物质的交换,这导致其临床应用受到限制。
为克服不可降解人工合成材料的上述缺点,研究者将目光转向了可降解人工合成材料的研发和应用上。可降解合成材料具有低免疫原性、降解产物可吸收等优点,并且可制备成疏松多孔结构,以支持轴突再生中代谢产物的交换[37]。常见的可降解合成材料包括聚乳酸(polylactic acid, PLA)、聚己内酯(polycaprolactone, PLC)、PLGA、聚丙交酯己内酯[poly(l-lactide- co-caprolactone), PLCL]及其它们的衍生物等(表 1)。不同共聚物单体组成与晶体结构不同,决定其物理性质的差异(表 3)。PLA的杨氏模量通常在2.7−4.1 GPa,拉伸强度在15−150 MPa,这使PLA导管拥有着更强的机械强度与抗拉伸性能,在体内不易形变[48-49]。PCL的杨氏模量与拉伸强度偏低,但是其高熔点温度与玻璃化转变温度分别为224 ℃与36 ℃,因此PCL易于加工并适配于3D打印技术[50, 54]。而PLGA作为PLA与PGA的共聚物,其物理性能受到两者配比调控,在保证杨氏模量与拉伸强度可控的同时,可以实现快速降解[54]。
| Materials | Tensile modulus (GPa) | Tensile strength (MPa) | Elongation at break (%) | Degradation time (months) | Degradation products | References |
| PLA | 2.70–4.10 | 15–150 | 3–10 | > 24 | Lactic acid | [48-49] |
| PCL | 0.20–0.35 | 20–34 | 300–1 000 | > 24 | Acetic acid | [50-51] |
| PLGA (80% PDLA) | 3.30–3.50 | 40–55 | 2–10 | > 1.5 | Lactic acid and glycolic acid | [52-53] |
PLA是一种生物降解材料,可使用淀粉为原料进行合成。PLA的杨氏模量与拉伸强度显著高于多数合成导管材料,这意味着PLA神经导管在复杂体内环境中可以有效维持形貌并支持神经再生。其降解产物为乳酸,可迅速被机体代谢,但是其较差的细胞黏附性与极高的疏水性限制了PLA的应用[28, 55]。Zheng等[29]构建了一种有序PLA纤维神经导管,利用去细胞化的基质水凝胶改善导管的疏水性,这种神经导管为神经髓鞘的再生与功能恢复提供了拓扑信号。Jahromi等[28]将表面改性碳纳米管掺入PLA神经导管,以改善导管的机械性能,同时在导管中填充含有施万细胞与姜黄素的纤维蛋白水凝胶。这种复合支架可获得与自体神经移植相媲美的修复效果。
1.2.2 聚己内酯PCL是一种脂肪族聚酯,降解时间要远长于PLA等工合成材料,完全降解时间长达24个月。所以对于PCL是否属于可降解材料仍存在争议[49, 56]。但是,PCL具有低熔点与易于加工定型等特点。伴随着3D打印技术的发展,PCL重新成为神经导管研发的热门材料[50]。Vijayavenkataraman等[30]利用3D打印技术制备PCL/聚吡咯复合导电神经导管,PCL的使用使得三维多孔结构得以实现。Qian等[31]利用PCL在3D打印技术上的优势构建了一种多孔复层的神经导管,将多巴胺、氨基酸、石墨烯与PCL按照设计逐层铸造喷涂,利用复杂的导管结构实现了细胞黏附、导电性与机械强度等优势的有机统一。
1.2.3 聚乳酸-羟基乙酸共聚物PLGA是由PLA与聚乙醇酸以不同比例共聚而成,这使得其物理性能可控。虽然PLGA的降解速率随着其中PLA含量升高而放缓,但其降解速率远高于PLA与PCL材料[53]。由于神经营养因子的半衰期短,而且在时间维度上不同神经营养因子有各自的分泌峰,所以PLGA这种可控降解对于实现神经营养因子多相释放有重要价值。Lackington等[32]开发一种PLGA微粒,使其中包裹的NGF与GDNF在28 d内依次缓释到神经再生微环境中,契合神经再生的时空过程。同样,PLGA也可作为一种优良的药物缓释平台,Haidar等[33]将α-硫辛酸与阿托伐他汀负载纳米颗粒加载在PLGA神经导管上以实现药物的局部长期缓释。
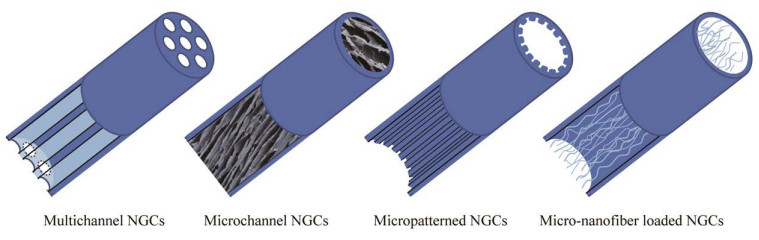
2 NGCs的物理结构与设计随着电纺与生物3D打印技术的成熟与发展,拓扑结构的概念被引入神经导管的设计之中(表 4)。神经元拥有独特的极化特性,这使神经轴突再生方向有重要意义。在体内外实验中,拓扑形貌信号对神经突的引导作用均被证实,并有效促进了神经再生[65]。图 1展现了4种主流的NGCs拓扑形貌设计。
| Materials | Type | Animal model | Effects | Functional outcome | References |
| Collagen/ Hyaluronic acid/PCL | Natural- synthetic composite | N.A. | Improved cell attachment and proliferation of Schwann cells, and enhanced axon outgrowth | N.A. | [57] |
| Chitosan/ Hyaluronic acid | Natural composite | Rats with sciatic nerve crush | Reduction of extraneural scaring and adhesion, and improved nerve regeneration | N.A. | [58] |
| Chitosan/PLGA | Natural- synthetic composite | Rats with sciatic nerve transection | Neurotrophic factor release and nerve regeneration | N.A. | [59] |
| Silk fibroin/ Collagen | Natural- synthetic composite | Rats with sciatic nerve transection | Axonal regeneration that was induced by the NGF gradient and longitudinal oriented microstructure | Static sciatic function index was improved | [60] |
| PLGA | Synthetic | N.A. | This system delivering FK506 that also guides enhanced axon growth induced by released FK506 and the microgroove microstructures | N.A. | [61] |
| PLCL | Synthetic | Rats with sciatic nerve severe traction injury | The groove and ridge morphologic surfaces of the NGCs | N.A. | [62] |
| Collagen or Laminin | Natural | Rats with facial nerve transection | The ordered growth of axons | Whisker movements index was improved | [63] |
| Spider fibroin/Silk fibroin/Hyaluronic acid | Natural composite | Rats with sciatic nerve transection | Regeneration of nerve and myelination | Better performances on footprint analysis and von frey test | [64] |
| N.A.: Not applicable. | |||||
|
| 图 1 人工神经导管的结构设计示意图 Fig. 1 Structure design of NGCs. |
| |
单通道作为神经导管最基础的设计之一,凭借其制备方式简单、技术门槛低的优势,仍是神经导管设计中的主流。王琳团队[42]研发的单通道丝胶神经导管成功修复5 mm的周围神经缺损。但是周围神经在生理结构上是由多条神经束共同组成,单通道结构未考虑仿生理念,因此模拟神经束膜将神经导管分隔成多通道结构的NGCs受到越来越多的关注[66]。
多通道导管的制造工艺复杂,目前多以注塑、电纺以及3D打印等技术来实现[57]。Yao等[58]研究不同通道数的多通道胶原导管与商业化单通道导管的治疗效果差异,发现多通道NGCs可以促进神经再支配,达到更好的术后运动功能恢复。Wang等[57]将一种形状记忆性聚合物聚丙交酯与共三甲基碳酸酯制备成一种四通道的神经导管,形状记忆性使其在生物体内自形成四通道结构,同时多通道的设计促进了神经轴突的再生。
2.2 微通道结构相较于宏观尺度上的多通道,微观尺度上的微通道结构与神经轴突的真实尺寸更加匹配,可给予新生的轴突更加仿生的指引信号[67]。微通道结构导管多采用冻干技术,利用冰晶生长方向与尺寸来控制微通道的方向与内径大小,形成单方向的多孔结构[68]。Manoukian等[59]通过液氮速冻制备具有微通道结构的壳聚糖神经导管,搭载4-氨基吡啶实现神经电生理的恢复,而有序微通道结构提供了轴突延伸的物理导向信号。Huang等[60]制备了一种具有神经营养因子梯度分布的有序微通道结构神经导管,实现化学与物理线索协同促进神经再生的效果。
2.3 微槽结构微槽结构是在材料平面上印刻出一系列平行规整的纵向沟槽,通过导管表面的形貌设计来影响细胞的生理活动,进而促进神经轴突的再生。微槽结构的构建主要采用刻蚀与冲压技术[61-62]。Davis等[61]利用光刻蚀技术在搭载有他克莫司的PLGA薄膜上刻蚀了不同宽带比的微槽,发现微槽的形貌不仅会影响神经再生的效率还会影响导管搭载药物的释放曲线。Yu等[62]利用冲压技术在搭载了人工多肽与NGF的多孔PCL膜上印刻纵向分布的微槽,这些微槽在神经再生过程中可有效引导施万细胞迁移,促进大鼠坐骨神经的再生。
2.4 微-纳纤维结构微-纳纤维的设计理念是为神经再生提供支持与引导信号,微丝与纳米纤维在直径上的差异与排布密度会直接影响神经修复效果[69]。早在1946年Weiss等[70]就展开了对于微丝的探究。Li等[71]通过在PLGA导管中填充基于自组装肽的有序纳米纤维,证实这种有序纤维结构有利于施万细胞的增殖与迁移。Cao等[63]将神经营养因子交联在层粘连蛋白与胶原蛋白上,并构建微丝填充的NGCs,该导管有效促进了大鼠面神经再生与功能恢复。Huang等[64]利用透明质酸包裹的蛛丝作为微丝填充到丝素神经导管中,发现这种天然的蛛丝纤维具有促神经轴突与施万细胞附着的作用,同时也证实过多或过少地填充纤维都会影响到微-纳纤维导管的治疗效果。这表明为神经再生提供物理支持与再生空间之间存在着平衡,需要进一步研究与探索。
3 支持细胞搭载 3.1 施万细胞施万细胞是周围神经修复的主要支持细胞,可形成神经髓鞘并分泌多种神经营养因子。将自体施万细胞搭载于NGCs中,通过在神经损伤处补充施万细胞来提高神经再生效果。但是,自体施万细胞存在提取与纯化困难、细胞扩增时间长以及难以标准化等缺陷,而同种异体施万细胞存在免疫原性及疫源性等问题,这限制了它们的临床应用[72]。Wang等[73]利用Büngner带中成纤维细胞与施万细胞的协同作用,将二者共同搭载于NGCs中,成纤维细胞增强施万细胞的迁移和分泌行为,促进神经再生与功能修复。
施万细胞的基因改造也是近年研究热点。PNI发生后,施万细胞上调多种神经再生相关细胞因子的表达。通过病毒转染等方式进行施万细胞基因改造,使其过表达神经再生细胞因子以促进神经的再生。Wu等[74]通过慢病毒转染使施万细胞过表达血管内皮生长因子,随后负载于NGCs中,促进周围血管再生与神经修复。
3.2 干细胞干细胞发挥促神经再生的机制与施万细胞相似,它们递送到损伤部位后可分化为施万细胞样细胞并分泌神经营养因子。胚胎干细胞、诱导多能干细胞、间充质干细胞(mesenchymal stem cells, MSCs)和神经干细胞等多种干细胞已被应用到NGCs的设计中[4]。其中MSCs因易于获取而成为被广泛搭载的干细胞[75]。而在不同来源的间充质干细胞中,又以骨髓间充质干细胞(bone marrow mesenchymal stem cells, BMSCs)与脂肪来源间充质干细胞(adipose- derived stem cells, ADSCs)研究得最为广泛。
虽然未分化BMSCs的疗效存在着争议,但是施万细胞样分化BMSCs (d-BMSCs)的治疗效果已被证实[76]。d-BMSCs被认为与轴突再生蛋白以及神经营养因子高表达密切相关[77-78]。Wang等[79]通过体外实验发现了缺氧处理有利于BMSCs向d-BMSCs分化并释放神经营养因子,负载缺氧处理BMSCs的NGCs取得更好的促神经再生效果。同时Zhou等[80]对BMSCs与ADSCs的应用进行比较,发现二者在疗效上没有显著的差异,且ADSCs有着更加安全易行的获取方式。
4 治疗因子递送神经营养因子作为一种高生物活性的蛋白多肽,调控多种神经再生相关的生理活动,但是其存在价格高昂以及生物半衰期短等局限性[81]。这注定神经营养因子的递送需要精准且缓释的递送系统,因此神经导管便成为了一种理想的载体。王琳团队[26]在壳聚糖/丝胶导管上搭载了神经生长因子,通过导管的缓慢降解实现了神经生长因子的缓释,调控了施万细胞的基因表达并促进了神经再生。
在神经再生过程中,神经营养因子之间存在着时间与空间上的动态变化。设计搭载神经营养因子的导管需要兼顾时间和空间维度的药物控释。Hong等[82]开发一种由PCL与PLGA不同构成比的3层复合神经导管。他们将神经营养素-3、脑源性神经营养因子以及血小板源生长因子搭载在不同的层面上,利用不同层面的降解速率差异实现了这3种神经营养因子的时空控释,并证实了这3种神经营养因子在神经再生的不同阶段发挥不同作用。
除去神经营养因子,也有少量的临床药物被证明有益于神经的再生,如阿托伐他汀、他克莫司与氯倍他索等[33, 59, 83]。但是这些药物常是其他疾病的临床用药,对于PNI治疗的效果尚不明确。氯倍他索先前被证明可以促进少突胶质细胞的增生,进而影响中枢神经的髓鞘再生。王琳团队[83]首次将其搭载于丝胶导管上,证明了氯倍他索在促外周神经再生中的作用。
5 外泌体递送迄今为止,已有研究证实外泌体在细胞信息交流中发挥关键作用。在神经再生相关细胞中,MSCs、施万细胞与巨噬细胞都可以通过分泌外泌体影响相关细胞增殖与迁移行为、损伤处血管再生以及免疫炎症反应等,达到调控神经再生的目的[84]。
搭载外泌体的NGCs实现疗效的关键是实现外泌体与支持细胞的长时间交互,这要求导管在拥有孔隙的同时也具有缓释能力,因此天然生物材料是制备这类导管的主要原材料。Hsu等[85]开发一种海绵状的海藻酸盐导管以缓释MSCs的外泌体,该外泌体作用于再生微环境的多种成分,抑制炎症因子和疼痛相关分子的表达,并显著促进髓鞘再生。在坐骨神经损伤模型的治疗中,Rao等[86]开发的甲壳素NGCs缓释MSCs外泌体显著促进施万细胞增殖与背根神经节的生长。
6 电刺激在NGCs中的运用近年几项随机临床试验已证实围手术期的低频电刺激可促进轴突生长与神经功能恢复,目前也有临床Ⅰ期的积极试验结果[87]。但是目前临床电刺激均需在术中外接电极并且难以在术后维持刺激,这限制其治疗效果。为了实现体内长程电刺激,多种自供电元件集成的NGCs被陆续研发(表 5)。
| Power supply | Animal model | Electrical stimulation parameter | Design of self-powered components | References |
| Electric conduction | Rats with sciatic nerve transection | N.A. | CNTs were dispersed into sericin solution to construct hollow tubular catheter with genipin as a cross-linker | [27] |
| Electric conduction | Rats with sciatic nerve transection | N.A. | The reduced graphene oxide (RGO) was coated onto ApF/PLCL nanofibrous scaffolds | [88] |
| Electromagnetic coupling | Rats with sciatic nerve transection or sciatic nerve crush | 2.5 V voltage, 20 Hz frequency, immediate stimulation for 1 h after operation | The wireless receiving coil was connected with the silicone nerve catheter through Pt/Ir microwire | [89] |
| Electromagnetic coupling | Rats with sciatic nerve transection | Over minimum threshold voltage, 20 Hz frequency, daily stimulation after operation | The wireless receiving coil and wire was constructed by biodegradable metals such as magnesium | [90] |
| Primary battery | Rats with sciatic nerve transection | 0.984 V on day 1, 0.450 V on day 2, and 0.068 V on day 3 | The electroactive NGCs consisted of thin-film magnesium and iron-manganese alloy electrodes on a polymer-based biodegradable scaffold | [91] |
| Primary battery | Rats with sciatic nerve transection | 350 mV maximum voltage, maintaining the electrical stimulation of 100 mV voltage for 6 h | The multiblock NGCs realized self-powered electrical stimulation by the consumption of glucose and oxygen | [92] |
| Mechanical energy | Sciatic nerve of rat | Extracorporeal ultrasound stimulation, 50 μA to 400 μA of current amplitude | As cuff-type nerve attachment, the system for wireless neural stimulation elicited highly repeatable compound action potentials in the sciatic nerve | [93] |
| Mechanical energy | Rats with sciatic nerve transection | Thoracic friction stimulation, 2.7 V, 0.12 µA at the active state and 2.2 V, 0.1 µA at the calm state | A self-powered system developed by integrating a novel tribo/piezoelectric hybrid nanogenerator and a multifunctional nanoporous NGCs | [94] |
| N.A.: Not applicable. | ||||
电信号传递是神经纤维的基本功能之一,因此导电神经导管具有仿生概念。在过往10年间,学界已经证明了包括聚吡咯、聚噻吩、碳纳米管与石墨烯在内的诸多导电材料可以有效促进横断神经纤维之间的神经冲动传递并促进神经再生[27, 88]。Wang等[88]选用氧化石墨烯掺入PLCL导管以赋予其导电性,利用大鼠体内生物电加速了神经修复过程。王琳团队[27]选择了性质类似的碳纳米管来增强神经导管的导电性,通过术后结合单次电刺激显著改善了髓鞘再生与神经再支配。
6.2 体外无线供电NGCsMacewan等[89]将硅胶导管与无线电接收线圈相连接,构建了一个基于电磁耦合原理的体外无线供电NGCs。将无线电接收器植入小鼠的皮下可以有效地提高能量转化效率,而基础导管结构则提供电极平台支持并引导神经生长的作用。同样的策略Koo等[90]也有采用,进一步使用PLGA、氧化硅与镁等可降解材料制备了柔性无线电接收器,克服了植入无线电线圈易形变损耗、诱导排异反应与不可降解等缺陷,使得整个体系生物相容性更加优异。
6.3 化学原电池供电NGCs除去无线供电的方式,Wang等[91]将原电池与NGCs相结合以实现自供电刺激。镁电极与铁锰合金电极位于两端并用多孔膜相夹,最终形成一种双层导管结构。这样的复合结构可以在植入导管后,以原电池供电原理持续给予神经微弱的电刺激促进神经的再生。Sun等[92]采用了燃料电池的原理设计了原电池供电NGCs,由Pt纳米粒子与碳纳米管构成的电极对可以有效氧化体液中的葡萄糖。葡萄糖转化为葡萄糖酸内酯并在导管内形成电流,同样实现导管的自供电与对神经的电刺激。
6.4 机械能供电NGCs机械能供电NGCs的构建主要基于压电材料,这类材料在发生机械形变时出现电荷分离进而形成局部电场,给予其超声、振动刺激或者生理压力变化即可实现对组织的电刺激[95]。常见压电材料包括压电聚合物聚偏二氟乙烯(polyvinylidene difluoride, PVDF)、压电陶瓷钛酸钡(BaTiO3)和氮化硼(BN)等,其中压电陶瓷纳米材料的应用最为广泛[96]。
Piech等[93]利用压电陶瓷、集成电路与电容等构建了神经袖带,压电陶瓷将体外超声转换成神经电刺激。压电陶瓷实现了高达7 V的电压,但不得不使用惰性材料封装以提高生物相容性。而压电聚合物的生物相容性优于压电陶瓷,更适于NGCs的开发。Jin等[94]制备了一种基于PVDF的摩擦发电机,通过患者手腕脉搏跳动实现90 mV的电压,达到电刺激神经再生阈值。将发电机置于大鼠胸部皮下并连接NGCs,成功促进大鼠坐骨神经再生的同时未引发显著的排斥反应。
7 讨论与展望近几十年,随着导管设计理念的革新以及工程技术的进步,各式各样的NGCs被研发与报道。在原材料的选择方面,人们最初选用惰性生物材料(如硅胶)来制备第一代NGCs,但是在动物实验中发现其无法降解,需要2次手术取出。随后,人工合成可降解聚合物和天然生物材料被用于新型NGCs的制备中。人工合成聚合物具有组分单一和理化性能可控等优势,而天然生物材料对支持细胞的亲和性更好,因此合成聚合物/天然复合生物材料可以继承二者优势,并越来越多地用于第三代NGCs的制备中。在NGCs的结构设计上,各种方案层出不穷,如导管内表面构建有序拓扑结构、导管内填充微-纳纤维、设计多通道及微通道的导管类型等。现有的结果显示,相较于传统的单通道神经导管,结构优化的NGCs具有更好的神经再生效果。但是,大部分NGCs的结构设计方案具有排他性,无法兼容其他设计方案。因此,在选择NGCs的结构设计方案时,需要统筹兼顾,不可盲目地做加法。在设计治疗因子搭载的NGCs时,要特别关注治疗因子的活性保持和可控释放,这对神经再生效果至关重要。而在导管内搭载施万细胞或者干细胞时,NGCs的研发者需要考虑如下2个问题:(1) 如何提高植入细胞的活率并长期保持其生物活性;(2) 如何降低细胞增殖失控而引发的肿瘤发生风险。在传统NGCs的研发中,原材料选择、结构设计以及治疗因子搭载是需要重点考虑的问题,研究者需要在统筹兼顾这些设计的基础上,提出自己的设计理念。
自供电元件集成NGCs为当前神经导管的一个研究热点。研究表明,物理电刺激可诱导施万细胞分泌神经营养因子,并通过促进Ca离子内流上调轴突表面神经营养因子受体的表达,二者共同作用提升再生轴突延伸速率并促进神经功能修复[97-98]。基于以上论点,多种类型的自供电元件被集成到NGCs中,包括磁耦合自供电,压电材料自发电和原电池自发电等。动物实验数据表明,自供电元件集成的NGCs可提供物理电刺激,促进神经功能恢复。但是,已报道的自供电元件均需要连接必要的配件,如金属导线、电容器何刺激电极等,这会引起人们对其降解性和生物安全性的担忧。此外,过强的电刺激会造成机体组织的损伤而过低的电刺激生物学效果不明显,如何保证自供电元件输出电刺激参数稳定并能维持在合适范围是一个需要重点关注的问题。尽管如此,自供电元件集成NGCs提供了一种可行的物理刺激方法促进神经再生,此外其提供的电信号可为传感微器件供能,为神经再生检测元件的集成打下基础。
未来,随着材料科学的发展和工程技术的革新,NGCs的研发必定会迎来暴发式增长,越来越多的NGCs会从动物实验走向临床转化。在这个过程中,周围神经损伤与再生的基础研究至关重要,这需要多背景、多领域专家通力合作,希望早日研发出促进长距离离断神经高效再生修复的NGCs,造福广大患者。
| [1] |
NOBLE J, MUNRO CA, PRASAD VSSV, MIDHA R. Analysis of upper and lower extremity peripheral nerve injuries in a population of patients with multiple injuries. The Journal of Trauma: Injury, Infection, and Critical Care, 1998, 45(1): 116-122. DOI:10.1097/00005373-199807000-00025
|
| [2] |
WANG ML, RIVLIN M, GRAHAM JG, BEREDJIKLIAN PK. Peripheral nerve injury, scarring, and recovery. Connective Tissue Research, 2019, 60(1): 3-9. DOI:10.1080/03008207.2018.1489381
|
| [3] |
HALLGREN A, BJÖRKMAN A, CHEMNITZ A, DAHLIN LB. Subjective outcome related to donor site morbidity after sural nerve graft harvesting: a survey in 41 patients. BMC Surgery, 2013, 13(1): 1-7. DOI:10.1186/1471-2482-13-1
|
| [4] |
BOECKER A, DAESCHLER SC, KNESER U, HARHAUS L. Relevance and recent developments of chitosan in peripheral nerve surgery. Frontiers in Cellular Neuroscience, 2019, 13: 104. DOI:10.3389/fncel.2019.00104
|
| [5] |
VIJAYAVENKATARAMAN S. Nerve guide conduits for peripheral nerve injury repair: a review on design, materials and fabrication methods. Acta Biomaterialia, 2020, 106: 54-69. DOI:10.1016/j.actbio.2020.02.003
|
| [6] |
QUIGLEY AF, BULLUSS KJ, KYRATZIS IB, GILMORE K, MYSORE T, SCHIRMER KU, KENNEDY EL, O'SHEA M, TRUONG YB, EDWARDS SL, PEETERS G, HERWIG P, RAZAL JM, CAMPBELL TE, LOWES KN, HIGGINS MJ, MOULTON SE, MURPHY MA, COOK MJ, CLARK GM, et al. Engineering a multimodal nerve conduit for repair of injured peripheral nerve. Journal of Neural Engineering, 2013, 10(1): 016008. DOI:10.1088/1741-2560/10/1/016008
|
| [7] |
STANEC S, STANEC Z. Reconstruction of upper-extremity peripheral-nerve injuries with ePTFE conduits. Journal of Reconstructive Microsurgery, 1998, 14(4): 227-232. DOI:10.1055/s-2007-1000173
|
| [8] |
LUNDBORG G, GELBERMAN RH, LONGO FM, POWELL HC, VARON S. In vivo regeneration of cut nerves encased in silicone tubes. Journal of Neuropathology and Experimental Neurology, 1982, 41(4): 412-422. DOI:10.1097/00005072-198207000-00004
|
| [9] |
KONOFAOS P, VER HALEN J. Nerve repair by means of tubulization: past, present, future. Journal of Reconstructive Microsurgery, 2013, 29(3): 149-164. DOI:10.1055/s-0032-1333316
|
| [10] |
GAUDIN R, KNIPFER C, HENNINGSEN A, SMEETS R, HEILAND M, HADLOCK T. Approaches to peripheral nerve repair: generations of biomaterial conduits yielding to replacing autologous nerve grafts in craniomaxillofacial surgery. BioMed Research International, 2016, 2016, 1-18.
|
| [11] |
WEBER RA, BREIDENBACH WC, BROWN RE, JABALEY ME, MASS DP. A randomized prospective study of polyglycolic acid conduits for digital nerve reconstruction in humans. Plastic and Reconstructive Surgery, 2000, 106(5): 1036-1045. DOI:10.1097/00006534-200010000-00013
|
| [12] |
CRAWLEY WA, DELLON LA. Inferior alveolar nerve reconstruction with a polyglycolic acid bioabsorbable nerve conduit. Plastic and Reconstructive Surgery, 1992, 90(2): 300-302. DOI:10.1097/00006534-199290020-00022
|
| [13] |
WANGENSTEEN K, KALLIAINEN L. Collagen tube conduits in peripheral nerve repair: a retrospective analysis. Hand, 2010, 5: 273-277. DOI:10.1007/s11552-009-9245-0
|
| [14] |
JANSEN K, MEEKMF, van der WERFF JFA, van WACHEM PB, van LUYN MJA. Long-term regeneration of the rat sciatic nerve through a biodegradable poly(dl-lactide-epsilon-caprolactone) nerve guide: tissue reactions with focus on collagen Ⅲ/Ⅳ reformation. Journal of Biomedical Materials Research, 2004, 69A(2): 334-341. DOI:10.1002/jbm.a.30004
|
| [15] |
DUDA S, DREYER L, BEHRENS P, WIENECKE S, CHAKRADEO T, GLASMACHER B, HAASTERT-TALINI K. Outer electrospun polycaprolactone shell induces massive foreign body reaction and impairs axonal regeneration through 3D multichannel chitosan nerve guides. BioMed Research International, 2014, 2014, 1-16.
|
| [16] |
DEUMENS R, BOZKURT A, MEEK MF, MARCUS MAE, JOOSTEN EAJ, WEIS J, BROOK GA. Repairing injured peripheral nerves: bridging the gap. Progress in Neurobiology, 2010, 92(3): 245-276. DOI:10.1016/j.pneurobio.2010.10.002
|
| [17] |
POONGODI R, CHEN YL, YANG TH, HUANG YH, YANG KD, LIN HC, CHENG JK. Bio-scaffolds as cell or exosome carriers for nerve injury repair. International Journal of Molecular Sciences, 2021, 22(24): 13347. DOI:10.3390/ijms222413347
|
| [18] |
LI RX, LIU HW, HUANG HT, BI WT, YAN RZ, TAN XY, WEN WS, WANG C, SONG WL, ZHANG YH, ZHANG F, HU M. Chitosan conduit combined with hyaluronic acid prevent sciatic nerve scar in a rat model of peripheral nerve crush injury. Molecular Medicine Reports, 2018, 17(3): 4360-4368.
|
| [19] |
ENTEKHABI E, HAGHBIN NAZARPAK M, SHAFIEIAN M, MOHAMMADI H, FIROUZI M, HASSANNEJAD Z. Fabrication and in vitro evaluation of 3D composite scaffold based on collagen/hyaluronic acid sponge and electrospun polycaprolactone nanofibers for peripheral nerve regeneration. Journal of Biomedical Materials Research Part A, 2021, 109(3): 300-312. DOI:10.1002/jbm.a.37023
|
| [20] |
LU PJ, WANG G, QIAN TM, CAI XD, ZHANG P, LI MY, SHEN YY, XUE CB, WANG HK. The balanced microenvironment regulated by the degradants of appropriate PLGA scaffolds and chitosan conduit promotes peripheral nerve regeneration. Materials Today Bio, 2021, 12: 100158. DOI:10.1016/j.mtbio.2021.100158
|
| [21] |
RAO F, WANG YH, ZHANG DY, LU CF, CAO Z, SUI JJ, WU MJ, ZHANG YW, PI W, WANG B, KOU YH, WANG XM, ZHANG PX, JIANG BG. Aligned chitosan nanofiber hydrogel grafted with peptides mimicking bioactive brain-derived neurotrophic factor and vascular endothelial growth factor repair long-distance sciatic nerve defects in rats. Theranostics, 2020, 10(4): 1590-1603. DOI:10.7150/thno.36272
|
| [22] |
SALEHI M, BAGHER Z, KAMRAVA SK, EHTERAMI A, ALIZADEH R, FARHADI M, FALAH M, KOMEILI A. Alginate/chitosan hydrogel containing olfactory ectomesenchymal stem cells for sciatic nerve tissue engineering. Journal of Cellular Physiology, 2019, 234(9): 15357-15368. DOI:10.1002/jcp.28183
|
| [23] |
RAHMATI M, EHTERAMI A, SABERANI R, ABBASZADEH-GOUDARZI G, REZAEI KOLARIJANI N, KHASTAR H, GARMABI B, SALEHI M. Improving sciatic nerve regeneration by using alginate/chitosan hydrogel containing berberine. Drug Delivery and Translational Research, 2021, 11(5): 1983-1993. DOI:10.1007/s13346-020-00860-y
|
| [24] |
ZHANG LZ, XU L, LI GC, YANG YM. Fabrication of high-strength mecobalamin loaded aligned silk fibroin scaffolds for guiding neuronal orientation. Colloids and Surfaces B: Biointerfaces, 2019, 173: 689-697. DOI:10.1016/j.colsurfb.2018.10.053
|
| [25] |
ZHANG SJ, WANG J, ZHENG ZZ, YAN J, ZHANG L, LI Y, ZHANG JH, LI G, WANG XQ, KAPLAN D. Porous nerve guidance conduits reinforced with braided composite structures of silk/magnesium filaments for peripheral nerve repair. Acta Biomaterialia, 2021, 134: 116-130. DOI:10.1016/j.actbio.2021.07.028
|
| [26] |
ZHANG L, YANG W, TAO KX, SONG Y, XIE HJ, WANG J, LI XL, SHUAI XM, GAO JB, CHANG PP, WANG GB, WANG Z, WANG L. Sustained local release of NGF from a chitosan-sericin composite scaffold for treating chronic nerve compression. ACS Applied Materials & Interfaces, 2017, 9(4): 3432-3444.
|
| [27] |
LI XL, YANG W, XIE HJ, WANG J, ZHANG L, WANG Z, WANG L. CNT/sericin conductive nerve guidance conduit promotes functional recovery of transected peripheral nerve injury in a rat model. ACS Applied Materials & Interfaces, 2020, 12(33): 36860-36872.
|
| [28] |
JAHROMI HK, FARZIN A, HASANZADEH E, BAROUGH SE, MAHMOODI N, NAJAFABADI MRH, FARAHANI MS, MANSOORI K, SHIRIAN S, AI J. Enhanced sciatic nerve regeneration by poly-l-lactic acid/multi-wall carbon nanotube neural guidance conduit containing Schwann cells and curcumin encapsulated chitosan nanoparticles in rat. Materials Science and Engineering: C, 2020, 109: 110564. DOI:10.1016/j.msec.2019.110564
|
| [29] |
ZHENG CS, YANG ZH, CHEN SH, ZHANG F, RAO ZL, ZHAO CL, QUAN DP, BAI Y, SHEN J. Nanofibrous nerve guidance conduits decorated with decellularized matrix hydrogel facilitate peripheral nerve injury repair. Theranostics, 2021, 11(6): 2917-2931. DOI:10.7150/thno.50825
|
| [30] |
VIJAYAVENKATARAMAN S, KANNAN S, CAO T, FUH JYH, SRIRAM G, LU WF. 3D-printed PCL/PPy conductive scaffolds as three-dimensional porous nerve guide conduits (NGCs) for peripheral nerve injury repair. Frontiers in Bioengineering and Biotechnology, 2019, 7: 266. DOI:10.3389/fbioe.2019.00266
|
| [31] |
QIAN Y, ZHAO XT, HAN QX, CHEN W, LI H, YUAN WE. An integrated multi-layer 3D-fabrication of PDA/RGD coated graphene loaded PCL nanoscaffold for peripheral nerve restoration. Nature Communications, 2018, 9(1): 323. DOI:10.1038/s41467-017-02598-7
|
| [32] |
LACKINGTON WA, KOČÍ Z, ALEKSEEVA T, HIBBITTS AJ, KNEAFSEY SL, CHEN G, O'BRIEN FJ. Controlling the dose-dependent, synergistic and temporal effects of NGF and GDNF by encapsulation in PLGA microparticles for use in nerve guidance conduits for the repair of large peripheral nerve defects. Journal of Controlled Release, 2019, 304: 51-64. DOI:10.1016/j.jconrel.2019.05.001
|
| [33] |
HAIDAR MK, TIMUR SS, KAZANCI A, TURKOGLU OF, GÜRSOY RN, NEMUTLU E, SARGON MF, BODUR E, GÖK M, ULUBAYRAM K, ÖNER L, EROĞLU H. Composite nanofibers incorporating alpha lipoic acid and atorvastatin provide neuroprotection after peripheral nerve injury in rats. European Journal of Pharmaceutics and Biopharmaceutics, 2020, 153: 1-13. DOI:10.1016/j.ejpb.2020.05.032
|
| [34] |
HEMSHEKHAR M, THUSHARA RM, CHANDRANAYAKA S, SHERMAN LS, KEMPARAJU K, GIRISH KS. Emerging roles of hyaluronic acid bioscaffolds in tissue engineering and regenerative medicine. International Journal of Biological Macromolecules, 2016, 86: 917-928. DOI:10.1016/j.ijbiomac.2016.02.032
|
| [35] |
BENOWITZ LI, POPOVICH PG. Inflammation and axon regeneration. Current Opinion in Neurology, 2011, 24(6): 577-583. DOI:10.1097/WCO.0b013e32834c208d
|
| [36] |
ZHAO YH, WANG YJ, GONG JH, YANG L, NIU CM, NI XJ, WANG YL, PENG S, GU XS, SUN C, YANG YM. Chitosan degradation products facilitate peripheral nerve regeneration by improving macrophage-constructed microenvironments. Biomaterials, 2017, 134: 64-77. DOI:10.1016/j.biomaterials.2017.02.026
|
| [37] |
SUN JC, TAN HP. Alginate-based biomaterials for regenerative medicine applications. Materials, 2013, 6(4): 1285-1309. DOI:10.3390/ma6041285
|
| [38] |
YI BC, ZHANG HL, YU ZP, YUAN HH, WANG XL, ZHANG YZ. Fabrication of high performance silk fibroin fibers via stable jet electrospinning for potential use in anisotropic tissue regeneration. Journal of Materials Chemistry B, 2018, 6(23): 3934-3945. DOI:10.1039/C8TB00535D
|
| [39] |
CHEN ZY, ZHANG Q, LI HM, WEI Q, ZHAO X, CHEN FL. Elastin-like polypeptide modified silk fibroin porous scaffold promotes osteochondral repair. Bioactive Materials, 2021, 6(3): 589-601. DOI:10.1016/j.bioactmat.2020.09.003
|
| [40] |
NGUYEN TP, NGUYEN QV, NGUYEN VH, LE TH, HUYNH VQN, VO DV N, TRINH QT, KIM SY, van LE Q. Silk fibroin-based biomaterials for biomedical applications: a review. Polymers, 2019, 11(12): 1933. DOI:10.3390/polym11121933
|
| [41] |
MAGAZ A, FARONI A, GOUGH JE, REID AJ, LI X, BLAKER JJ. Bioactive silk-based nerve guidance conduits for augmenting peripheral nerve repair. Advanced Healthcare Materials, 2018, 7(23): 1800308. DOI:10.1002/adhm.201800308
|
| [42] |
XIE HJ, YANG W, CHEN JH, ZHANG JX, LU XC, ZHAO XB, HUANG K, LI HL, CHANG PP, WANG Z, WANG L. A silk sericin/silicone nerve guidance conduit promotes regeneration of a transected sciatic nerve. Advanced Healthcare Materials, 2015, 4(15): 2195-2205. DOI:10.1002/adhm.201500355
|
| [43] |
WANG Z, WANG J, JIN Y, LUO Z, YANG W, XIE HJ, HUANG K, WANG L. A neuroprotective sericin hydrogel as an effective neuronal cell carrier for the repair of ischemic stroke. ACS Applied Materials & Interfaces, 2015, 7(44): 24629-24640.
|
| [44] |
FORNASARI BE, CARTA G, GAMBAROTTA G, RAIMONDO S. Natural-based biomaterials for peripheral nerve injury repair. Frontiers in Bioengineering and Biotechnology, 2020, 8: 554257. DOI:10.3389/fbioe.2020.554257
|
| [45] |
CAMPBELL JB. Peripheral nerve repair. Neurosurgery, 1970, 17(supplement 1): 77-98. DOI:10.1093/neurosurgery/17.CN_suppl_1.77
|
| [46] |
ABBASIPOUR-DALIVAND S, MOHAMMADI R, MOHAMMADI V. Effects of local administration of platelet rich plasma on functional recovery after bridging sciatic nerve defect using silicone rubber chamber; an experimental study. Bulletin of Emergency and Trauma, 2015, 3(1): 1-7.
|
| [47] |
SUNDERLAND S. A classification of peripheral nerve injuries producing loss of function. Brain, 1951, 74(4): 491-516. DOI:10.1093/brain/74.4.491
|
| [48] |
MIDDLETON JC, TIPTON AJ. Synthetic biodegradable polymers as orthopedic devices. Biomaterials, 2000, 21(23): 2335-2346. DOI:10.1016/S0142-9612(00)00101-0
|
| [49] |
CHANG SH, LEE HJ, PARK S, KIM Y, JEONG B. Fast degradable polycaprolactone for drug delivery. Biomacromolecules, 2018, 19(6): 2302-2307. DOI:10.1021/acs.biomac.8b00266
|
| [50] |
LI C, LIU IKK, TSAO CY, CHAN V. Neuronal differentiation of human placenta-derived multi-potent stem cells enhanced by cell body oscillation on gelatin hydrogel. Journal of Bioactive and Compatible Polymers, 2014, 29(6): 529-544. DOI:10.1177/0883911514553903
|
| [51] |
VROMAN I, TIGHZERT L. Biodegradable polymers. Materials, 2009, 2: 307-344. DOI:10.3390/ma2020307
|
| [52] |
VELDE K, KIEKENS P. Biopolymers: overview of several properties and consequences on their applications. Polymer Testing, 2002, 21(4): 433-442. DOI:10.1016/S0142-9418(01)00107-6
|
| [53] |
MAKADIA HK, SIEGEL SJ. Poly lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers, 2011, 3(3): 1377-1397. DOI:10.3390/polym3031377
|
| [54] |
DUFFY P, MCMAHON S, WANG X, KEAVENEY S, O'CEARBHAILL ED, QUINTANA I, RODRÍGUEZ FJ, WANG WX. Synthetic bioresorbable poly-α-hydroxyesters as peripheral nerve guidance conduits; a review of material properties, design strategies and their efficacy to date. Biomaterials Science, 2019, 7(12): 4912-4943. DOI:10.1039/C9BM00246D
|
| [55] |
LASPRILLA AJR, MARTINEZ GAR, LUNELLI BH, JARDINI AL, MACIEL FILHO R. Poly-lactic acid synthesis for application in biomedical devices—a review. Biotechnology Advances, 2012, 30(1): 321-328. DOI:10.1016/j.biotechadv.2011.06.019
|
| [56] |
WOODRUFF MA, HUTMACHER DW. The return of a forgotten polymer—polycaprolactone in the 21st century. Progress in Polymer Science, 2010, 35(10): 1217-1256. DOI:10.1016/j.progpolymsci.2010.04.002
|
| [57] |
WANG J, XIONG H, ZHU TH, LIU Y, PAN HB, FAN CY, ZHAO XL, LU WW. Bioinspired multichannel nerve guidance conduit based on shape memory nanofibers for potential application in peripheral nerve repair. ACS Nano, 2020, 14(10): 12579-12595. DOI:10.1021/acsnano.0c03570
|
| [58] |
YAO L, RUITER GC, WANG H, KNIGHT AM, SPINNER RJ, YASZEMSKI MJ, WINDEBANK AJ, PANDIT A. Controlling dispersion of axonal regeneration using a multichannel collagen nerve conduit. Biomaterials, 2010, 31(22): 5789-5797. DOI:10.1016/j.biomaterials.2010.03.081
|
| [59] |
MANOUKIAN OS, ARUL MR, RUDRAIAH S, KALAJZIC I, KUMBAR SG. Aligned microchannel polymer-nanotube composites for peripheral nerve regeneration: small molecule drug delivery. Journal of Controlled Release, 2019, 296: 54-67. DOI:10.1016/j.jconrel.2019.01.013
|
| [60] |
HUANG LL, GAO JB, WANG HR, XIA B, YANG YJ, XU F, ZHENG XF, HUANG JH, LUO ZJ. Fabrication of 3D scaffolds displaying biochemical gradients along longitudinally oriented microchannels for neural tissue engineering. ACS Applied Materials & Interfaces, 2020, 12(43): 48380-48394.
|
| [61] |
DAVIS B, WOJTALEWICZ S, LABROO P, SHEA J, SANT H, GALE B, AGARWAL J. Controlled release of FK506 from micropatterned PLGA films: potential for application in peripheral nerve repair. Neural Regeneration Research, 2018, 13(7): 1247-1252. DOI:10.4103/1673-5374.235063
|
| [62] |
YU X, ZHANG DT, LIU C, LIU ZD, LI YJ, ZHAO QZ, GAO CY, WANG Y. Micropatterned poly(d, l-lactide-co-caprolactone) conduits with KHI-peptide and NGF promote peripheral nerve repair after severe traction injury. Frontiers in Bioengineering and Biotechnology, 2021, 9: 744230. DOI:10.3389/fbioe.2021.744230
|
| [63] |
CAO JN, XIAO ZF, JIN W, CHEN B, MENG DQ, DING WY, HAN SF, HOU XS, ZHU TS, YUAN BY, WANG J, LIANG WB, DAI JW. Induction of rat facial nerve regeneration by functional collagen scaffolds. Biomaterials, 2013, 34(4): 1302-1310. DOI:10.1016/j.biomaterials.2012.10.031
|
| [64] |
HUANG W, BEGUM R, BARBER T, IBBA V, TEE NCH, HUSSAIN M, ARASTOO M, YANG Q, ROBSON LG, LESAGE S, GHEYSENS T, SKAER NJV, KNIGHT DP, PRIESTLEY JV. Regenerative potential of silk conduits in repair of peripheral nerve injury in adult rats. Biomaterials, 2012, 33(1): 59-71. DOI:10.1016/j.biomaterials.2011.09.030
|
| [65] |
YANG CY, HUANG WY, CHEN LH, LIANG NW, WANG HC, LU JJ, WANG XM, WANG TW. Neural tissue engineering: the influence of scaffold surface topography and extracellular matrix microenvironment. Journal of Materials Chemistry B, 2021, 9(3): 567-584. DOI:10.1039/D0TB01605E
|
| [66] |
MA Y, GAO HC, WANG H, CAO XD. Engineering topography: effects on nerve cell behaviors and applications in peripheral nerve repair. Journal of Materials Chemistry B, 2021, 9(32): 6310-6325. DOI:10.1039/D1TB00782C
|
| [67] |
YU TH, WEN LL, HE J, XU YX, LI T, WANG WZ, MA YZ, AHMAD MA, TIAN XH, FAN J, WANG XH, HAGIWARA H, AO Q. Fabrication and evaluation of an optimized acellular nerve allograft with multiple axial channels. Acta Biomaterialia, 2020, 115: 235-249. DOI:10.1016/j.actbio.2020.07.059
|
| [68] |
OLIVEIRA AL, SUN L, KIM HJ, HU X, RICE W, KLUGE J, REIS RL, KAPLAN DL. Aligned silk-based 3-D architectures for contact guidance in tissue engineering. Acta Biomaterialia, 2012, 8(4): 1530-1542. DOI:10.1016/j.actbio.2011.12.015
|
| [69] |
LIZARRAGA-VALDERRAMA LR, TAYLOR CS, CLAEYSSENS F, HAYCOCK JW, KNOWLES JC, ROY I. Unidirectional neuronal cell growth and differentiation on aligned polyhydroxyalkanoate blend microfibres with varying diameters. Journal of Tissue Engineering and Regenerative Medicine, 2019, 13(9): 1581-1594. DOI:10.1002/term.2911
|
| [70] |
WEISS P, TAYLOR AC. Guides for nerve regeneration across gaps. Journal of Neurosurgery, 1946, 3(5): 375-389. DOI:10.3171/jns.1946.3.5.0375
|
| [71] |
LI A, HOKUGO A, YALOM A, BERNS EJ, STEPHANOPOULOS N, MCCLENDON MT, SEGOVIA LA, SPIGELMAN I, STUPP SI, JARRAHY R. A bioengineered peripheral nerve construct using aligned peptide amphiphile nanofibers. Biomaterials, 2014, 35(31): 8780-8790. DOI:10.1016/j.biomaterials.2014.06.049
|
| [72] |
SARKER MD, NAGHIEH S, MCINNES AD, SCHREYER DJ, CHEN XB. Regeneration of peripheral nerves by nerve guidance conduits: influence of design, biopolymers, cells, growth factors, and physical stimuli. Progress in Neurobiology, 2018, 171: 125-150. DOI:10.1016/j.pneurobio.2018.07.002
|
| [73] |
WANG Y, LI D, WANG GY, CHEN LL, CHEN J, LIU ZY, ZHANG ZF, SHEN H, JIN YQ, SHEN ZL. The effect of co-transplantation of nerve fibroblasts and Schwann cells on peripheral nerve repair. International Journal of Biological Sciences, 2017, 13(12): 1507-1519. DOI:10.7150/ijbs.21976
|
| [74] |
WU P, TONG Z, LUO LH, ZHAO YN, CHEN FX, LI YP, HUSELSTEIN C, YE QF, YE QS, CHEN Y. Comprehensive strategy of conduit guidance combined with VEGF producing Schwann cells accelerates peripheral nerve repair. Bioactive Materials, 2021, 6(10): 3515-3527. DOI:10.1016/j.bioactmat.2021.03.020
|
| [75] |
MATHOT F, SHIN AY, van WIJNEN AJ. Targeted stimulation of MSCs in peripheral nerve repair. Gene, 2019, 710: 17-23. DOI:10.1016/j.gene.2019.02.078
|
| [76] |
LI Y, KAMEI Y, KAMBE M, EBISAWA K, OISHI M, TAKANARI K. Peripheral nerve regeneration using different germ layer-derived adult stem cells in the past decade. Behavioural Neurology, 2021, 2021, 1-15.
|
| [77] |
MA YB, GE SH, ZHANG JH, ZHOU D, LI L, WANG XF, SU JH. Mesenchymal stem cell-derived extracellular vesicles promote nerve regeneration after sciatic nerve crush injury in rats. International journal of clinical and experimental pathology, 2017, 10(9): 10032-10039.
|
| [78] |
MAHAY D, TERENGHI G, SHAWCROSS SG. Schwann cell mediated trophic effects by differentiated mesenchymal stem cells. Experimental Cell Research, 2008, 314(14): 2692-2701. DOI:10.1016/j.yexcr.2008.05.013
|
| [79] |
WANG JP, LIAO YT, WU SH, CHIANG ER, HSU SH, TSENG TC, HUNG SC. Mesenchymal stem cells from a hypoxic culture improve nerve regeneration. Journal of Tissue Engineering and Regenerative Medicine, 2020, 14(12): 1804-1814. DOI:10.1002/term.3136
|
| [80] |
ZHOU LN, WANG JC, ZILUNDU PLM, WANG YQ, GUO WP, ZHANG SX, LUO H, ZHOU JH, DENG RD, CHEN DF. A comparison of the use of adipose-derived and bone marrow-derived stem cells for peripheral nerve regeneration in vitro and in vivo. Stem Cell Research & Therapy, 2020, 11(1): 153.
|
| [81] |
PFISTER LA, PAPALOÏZOS M, MERKLE HP, GANDER B. Nerve conduits and growth factor delivery in peripheral nerve repair. Journal of the Peripheral Nervous System, 2007, 12(2): 65-82. DOI:10.1111/j.1529-8027.2007.00125.x
|
| [82] |
HONG MH, HONG HJ, PANG H, LEE HJ, YI S, KOH WG. Controlled release of growth factors from multilayered fibrous scaffold for functional recoveries in crushed sciatic nerve. ACS Biomaterials Science & Engineering, 2018, 4(2): 576-586.
|
| [83] |
ZHANG L, YANG W, XIE HJ, WANG H, WANG J, SU QF, LI XL, SONG Y, WANG GB, WANG L, WANG Z. Sericin nerve guidance conduit delivering therapeutically repurposed clobetasol for functional and structural regeneration of transected peripheral nerves. ACS Biomaterials Science & Engineering, 2019, 5(3): 1426-1439.
|
| [84] |
QING LM, CHEN HW, TANG JY, JIA XF. Exosomes and their microRNA cargo: new players in peripheral nerve regeneration. Neurorehabilitation and Neural Repair, 2018, 32(9): 765-776. DOI:10.1177/1545968318798955
|
| [85] |
HSU JM, SHIUE SJ, YANG KD, SHIUE HS, HUNG YW, PANNURU P, POONGODI R, LIN HY, CHENG JK. Locally applied stem cell exosome-scaffold attenuates nerve injury-induced pain in rats. Journal of Pain Research, 2020, 13: 3257-3268. DOI:10.2147/JPR.S286771
|
| [86] |
RAO F, ZHANG DY, FANG T, LU CF, WANG B, DING X, WEI S, ZHANG YR, PI W, XU HL, WANG YH, JIANG BG, ZHANG PX. Exosomes from human gingiva-derived mesenchymal stem cells combined with biodegradable chitin conduits promote rat sciatic nerve regeneration. Stem Cells International, 2019, 2019, 1-12.
|
| [87] |
ZUO KJ, GORDON T, CHAN KM, BORSCHEL GH. Electrical stimulation to enhance peripheral nerve regeneration: update in molecular investigations and clinical translation. Experimental Neurology, 2020, 332: 113397. DOI:10.1016/j.expneurol.2020.113397
|
| [88] |
WANG J, CHENG Y, CHEN L, ZHU TH, YE KQ, JIA C, WANG HJ, ZHU MF, FAN CY, MO XM. In vitro and in vivo studies of electroactive reduced graphene oxide-modified nanofiber scaffolds for peripheral nerve regeneration. Acta Biomaterialia, 2019, 84: 98-113. DOI:10.1016/j.actbio.2018.11.032
|
| [89] |
MACEWAN MR, GAMBLE P, STEPHEN M, RAY WZ. Therapeutic electrical stimulation of injured peripheral nerve tissue using implantable thin-film wireless nerve stimulators. Journal of Neurosurgery, 2019, 130(2): 486-495. DOI:10.3171/2017.8.JNS163020
|
| [90] |
KOO J, MACEWAN MR, KANG SK, WON SM, STEPHEN M, GAMBLE P, XIE ZQ, YAN Y, CHEN YY, SHIN J, BIRENBAUM N, CHUNG S, KIM SB, KHALIFEH J, HARBURG DV, BEAN K, PASKETT M, KIM J, ZOHNY ZS, LEE SM, et al. Wireless bioresorbable electronic system enables sustained nonpharmacological neuroregenerative therapy. Nature Medicine, 2018, 24(12): 1830-1836. DOI:10.1038/s41591-018-0196-2
|
| [91] |
WANG L, LU CF, YANG SH, SUN PC, WANG Y, GUAN YJ, LIU S, CHENG DL, MENG HY, WANG Q, HE JG, HOU HQ, LI H, LU W, ZHAO YX, WANG J, ZHU YQ, LI YX, LUO D, LI T, et al. A fully biodegradable and self-electrified device for neuroregenerative medicine. Science Advances, 2020, 6(50): eabc6686. DOI:10.1126/sciadv.abc6686
|
| [92] |
SUN Y, QUAN Q, MENG HY, ZHENG YD, PENG J, HU YX, FENG ZX, SANG X, QIAO K, HE W, CHI XQ, ZHAO L. Enhanced neurite outgrowth on a multiblock conductive nerve scaffold with self-powered electrical stimulation. Advanced Healthcare Materials, 2019, 8(10): 1900127. DOI:10.1002/adhm.201900127
|
| [93] |
PIECH DK, JOHNSON BC, SHEN K, GHANBARI MM, LI KY, NEELY RM, KAY JE, CARMENA JM, MAHARBIZ MM, MULLER R. A wireless millimetre-scale implantable neural stimulator with ultrasonically powered bidirectional communication. Nature Biomedical Engineering, 2020, 4(2): 207-222.
|
| [94] |
JIN F, LI T, YUAN T, DU LJ, LAI CT, WU Q, ZHAO Y, SUN FY, GU L, WANG T, FENG ZQ. Physiologically self-regulated, fully implantable, battery-free system for peripheral nerve restoration. Advanced Materials, 2021, 33(48): 2104175. DOI:10.1002/adma.202104175
|
| [95] |
FERSON ND, UHL AM, ANDREW JS. Piezoelectric and magnetoelectric scaffolds for tissue regeneration and biomedicine: a review. IEEE Transactions on Ultrasonics Ferroelectrics and Frequency Control, 2021, 68(2): 229-241.
|
| [96] |
KAPAT K, SHUBHRA QTH, ZHOU M, LEEUWENBURGH S. Piezoelectric nano-biomaterials for biomedicine and tissue regeneration. Advanced Functional Materials, 2020, 30(44): 1909045.
|
| [97] |
CHU XL, SONG XZ, LI Q, LI YR, HE F, GU XS, MING D. Basic mechanisms of peripheral nerve injury and treatment via electrical stimulation. Neural Regeneration Research, 2022, 17(10): 2185-2193.
|
| [98] |
KAWAMURA K, KANO Y. Electrical stimulation induces neurite outgrowth in PC12m3 cells via the p38 mitogen-activated protein kinase pathway. Neuroscience Letters, 2019, 698: 81-84.
|
 2023, Vol. 39
2023, Vol. 39