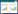中国科学院微生物研究所、中国微生物学会主办
文章信息
- 潘剑锋, 尚方正, 马荣, 戎友俊, 张燕军
- PAN Jianfeng, SHANG Fangzheng, MA Rong, RONG Youjun, ZHANG Yanjun
- 周期蛋白和周期蛋白依赖性激酶及相关激酶抑制剂在细胞周期进程中的调控机制研究进展
- Advances of the regulatory mechanism of cyclin, cyclin- dependent kinases and related kinase inhibitors in cell cycle progression
- 生物工程学报, 2023, 39(4): 1525-1547
- Chinese Journal of Biotechnology, 2023, 39(4): 1525-1547
- 10.13345/j.cjb.220478
-
文章历史
- Received: June 16, 2022
- Accepted: November 14, 2022
- Published: December 8, 2022
2. 农业农村部肉羊遗传育种重点实验室, 内蒙古 呼和浩特 010018;
3. 内蒙古自治区动物遗传育种与繁殖重点实验室, 内蒙古 呼和浩特 010018;
4. 内蒙古自治区山羊遗传育种工程技术研究中心, 内蒙古 呼和浩特 010018
2. Key Laboratory of Meat Sheep Genetics and Breeding, Ministry of Agriculture and Rural Affairs of the people's Republic of China, Hohhot 010018, Inner Mongolia, China;
3. Key Laboratory of Animal Genetics, Breeding and Reproduction in Inner Mongolia Autonomous Region, Hohhot 010018, Inner Mongolia, China;
4. Goat Genetics and Breeding in Inner Mongolia Autonomous Region Engineering Technology Research Center, Hohhot 010018, Inner Mongolia, China
细胞周期蛋白依赖性激酶(cyclin dependent kinase, CDK)是丝氨酸/苏氨酸蛋白激酶家族成员,通过介导不同底物磷酸化,参与细胞周期调控和转录调节[1]。大多数CDK具有CDK激酶结构域、周期蛋白结合位点、磷酸化修饰位点和T-loop (称为激活环)基序等[2-3]。当CDK与其特定的周期蛋白非共价结合时CDK的T-loop被置换,从而暴露底物ATP结合位点并重新排列活性位点的关键残基,激活CDK活性[4-5]。迄今为止,已有数十种CDK (CDK1/2/4/6/7/9等)和周期蛋白(cyclinA/B/D/ E/F/G/H等)被确认,并在细胞周期调控中发挥重要作用,例如cyclinD-CDK4/6启动细胞周期进程,cyclinE-CDK2调控S期进入,cyclinA-CDK2调控S期DNA复制,cyclinA/ B-CDK1触发有丝分裂等[6-7]。
由于大多数CDK异常表达与癌症进展有关,因此研发CDK抑制剂作为抗癌靶向药物引起了学界极大关注[8]。近20年来,至少有数十种靶向CDK的药物在临床试验中进行了研究,然而却仅有极少数被批准用于临床治疗[9-10]。在研究早期,发现的CDK抑制剂多为泛CDK抑制剂与多CDK抑制剂,其虽可有效抑制多种CDK或其他激酶,但表现出来的明显副作用,阻碍了这两类抑制剂进入市场与开展临床治疗。因此,为获得更安全、有效、副作用小的CDK抑制剂用于临床治疗,研究者对选择性CDK抑制剂展开了研究。2015年第一种选择性CDK4/6抑制剂帕博西尼(palbociclib)被美国食品药品监督管理局(Food and Drug Administration, FDA)批准用于治疗乳腺癌[11]。之后,3种选择性CDK4/6抑制剂瑞博西尼(ribociclib)、阿贝西利(abemaciclib)、曲拉西利(trilaciclib)也相继上市并用于临床治疗[12]。此外,当前被鉴定的选择性CDK抑制剂,主要靶向CDK1、CDK2、CDK4/6、CDK7等[13-14]。在临床研究中CDK抑制剂不仅可以被用于治疗各类癌症,还可以被用于治疗各类非癌症疾病,例如炎症性疾病、中枢神经系统疾病和感染性疾病等[15]。这表明CDK在许多癌症与非癌症疾病的病理过程中发挥着重要作用。
本文介绍了CDK激活或灭活过程的关键事件、特定时期及位置的周期蛋白-CDK的研究进展、相关CDK抑制剂在癌症和非癌症疾病中的应用情况及研发进展。最后,简单阐述了细胞周期研究领域面临的问题和存在的挑战,旨在为细胞周期研究领域的发展提供研究思路。
1 CDK活性激活的关键事件CDK是驱动真核细胞周期的丝氨酸/苏氨酸特异性蛋白激酶,在其特定时期激活或灭活可使细胞周期进程有序进行[16-17]。CDK激活需要满足如下条件:(1) 需要与周期蛋白结合;(2) CDK激活位点的磷酸化和抑制位点的去磷酸化;(3) 不与CDK抑制剂(cyclin-dependent kinase inhibitors, CKI)结合等。有趣的是只有当周期蛋白与CDK结合形成周期蛋白-CDK复合物时,CDK上的激活位点磷酸化和抑制位点去磷酸化,才会激活CDK的活性,而活化的CDK则会在CKI的作用下被抑制,因此CDK的激活需要满足这3个关键事件。
1.1 与周期蛋白结合形成复合物周期蛋白(cyclin)是一类在细胞周期调控过程中表达量呈周期性变化的蛋白质,周期蛋白最初是由Tom Evans在1983年研究海胆细胞周期时发现,主要通过激活CDK活性和其他细胞周期相关的酶,进而控制细胞周期进程的蛋白质家族[18-19]。在脊椎动物中,周期蛋白包括A、B、D、E、F、G和H等类型,可在特定时期与特定CDK结合构成周期蛋白-CDK复合物,例如cyclinD-CDK4/6、cyclinE-CDK2、cyclinA-CDK2、cyclinH-CDK7、cyclinA-CDK1和cyclinB-CDK1等[3, 20]。每一组周期蛋白-CDK可通过触发下一组周期蛋白-CDK以及其他相关细胞周期蛋白的表达,调控各个阶段细胞周期的有序进行[7]。另外,周期蛋白拥有如下基本结构与特征,包括氨基酸结构域(又称为细胞周期蛋白盒)、破坏框、脯氨酸/谷氨酸/丝氨酸/苏氨酸(pro-glu-ser-thr, PEST)序列等[3]。这些结构与特征调控周期蛋白与CDK结合、周期蛋白的泛素化降解、G1期周期蛋白更新等过程[3]。此外,不同种类的周期蛋白只在细胞周期特定时期表达和调节特定CDK。随着细胞周期的进展,周期蛋白产生效应后立即降解[21]。但有趣的是CDK的浓度不会随着周期进程变化而波动。因此,在细胞周期进程中其活性的激活则是细胞周期各阶段进程能顺利进行的关键。此外,周期蛋白的降解是由后期促进复合物(anaphase-promoting complex/cyclosome, APC/C)在M期后期至G1期后期的活动,以及SCF (Skp1-Cul1-F-box)-Skp2 (S-phase kinase-associated protein 2)复合物在G1期后期至M期早期的活动所致[22-23]。另外,在胚胎干细胞中周期蛋白的表达模式明显与体细胞不同[24]。例如小鼠胚胎干细胞具有更高且持续的cyclinA/E蛋白质表达水平,且在整个细胞周期中cyclinA/E的相关激酶CDK2被持续性激活[25]。表明周期蛋白在细胞周期进程中的表达模式不是一成不变,且在不同细胞类型中表达存在差异。因此,在探究不同细胞类型周期进程中,应注意对细胞周期蛋白表达模式的确认及验证,见图 1A。
|
| 图 1 Cyclin-CDK活性调控过程 Fig. 1 The regulation process of cyclin-CDK activity. A: Cyclin ubiquitination and degradation. Cyclin is ubiquitinated and degraded by APC/C-Cdc20 or SCF-Skp2[22-23]. B: Cyclin-CDK phosphorylation modification, CAK mediates Thr161 phosphorylation, WEE kinase family mediates Thr14 and Tyr15 site phosphorylation, phosphorylated Cdc25 activates cyclin-CDK by removing Thr14 and Tyr15 phosphate groups, and feedback regulates Cdc25 and WEE kinase family activities[27, 30, 33]. C: CKIs ubiquitination degradation and activation, CKIs are degraded by SCF-Skp2 ubiquitination and degradation, DNA damage activates P53 to promote the expression of CKIs, and the TGF-β signaling pathway mediates SMAD4-SMAD2/3 binding to promote the expression of CKIs[7, 38]. |
| |
CDK与周期蛋白结合是其具有活性的必备条件,但仅有周期蛋白结合还不足以激活CDK,必须在CDK特定位点的磷酸化或去磷酸化才能使其活性得以激活。人类和小鼠体细胞周期研究中发现,WEE激酶家族是驱动CDK抑制位点磷酸化主要的蛋白质激酶家族,该家族主要由这3类蛋白质WEE1、膜相关酪氨酸/苏氨酸蛋白激酶1 (protein kinase membrane associated tyrosine/threonine 1, PKMYT1)和WEE1B构成,主要通过介导Tyr15和Thr14位点的磷酸化,降低CDK的活性[26-28]。WEE激酶家族所造成的CDK活性降低,致使大量周期蛋白-CDK无活性,从而导致该复合物在细胞周期下一阶段开始前大量积累[27]。而一种在进化上保守的磷酸酶细胞分裂周期25 (cell division cycle 25, Cdc25),可通过移除CDK上由WEE激酶介导的抑制性磷酸基团,重新激活周期蛋白-CDK[29-30]。有趣的是,重新活化的周期蛋白-CDK还可抑制WEE激酶的活性,并防止WEE激酶再一次灭活CDK[31]。同时,在人类体细胞中发现活化的周期蛋白-CDK还可磷酸化激活Cdc25的活性,使得周期蛋白-CDK可在短期内急剧增加,这表明了一种周期蛋白-CDK活性激活的反馈调控机制[32]。在CDK的激活中,除抑制位点磷酸化外,激活位点的磷酸化也是决定CDK活性激活的关键。在人类和小鼠体细胞中发现,CDK激活激酶(CDK-activating kinase, CAK)可通过磷酸化CDK的Thr161位点,激活CDK活性,从而确保细胞周期有序且精确地进行[33-34]。综上所述,CDK激活需要如下两种模式:通过Cdc25将WEE激酶介导的抑制性磷酸基团去除,称为抑制位点去磷酸化;通过CAK将CDK的激活位点磷酸化,称为激活位点磷酸化。而只有这两种磷酸化模式同时在CDK上进行,才可激活CDK的活性。因此,在细胞周期进程中对CDK特定位点磷酸化状态的探究就显得尤为关键,见图 1B。
1.3 CDK活性抑制剂在细胞周期中存在多种CKI,激酶4抑制因子(inhibitors of kinase 4, INK4)家族(p16INK4A、p15INK4B、p18INK4C和p19INK4D等)、抑制蛋白(CDK interaction protein/kinase inhibitor protein, CIP/ KIP)家族(p21Cip1、p27Kip1和p57Kip2等)以及核糖体蛋白抑制CDK (ribosomal protein-inhibiting CDKs, RPICs)[7]。这些CKI主要通过与周期蛋白-CDK相互作用,阻止周期蛋白与对应的CDK结合,从而抑制CDK活性,造成细胞周期进程停滞[35]。其中,INK4家族仅抑制cyclinD-CDK4/CDK6,而CIP/KIP家族则具有更广泛的特异性,可抑制cyclinD-CDK4/CDK6、cyclinE-CDK2、cyclinA-CDK2和cyclinB-CDK1等[35-36]。因此,为了保证在特定时期细胞周期正常有序进行,则需对这些抑制剂进行降解或抑制以及相应的激活。在多种人类恶性肿瘤(多发性骨髓瘤等)细胞中发现SCF-Skp2复合物可通过泛素化降解CKI,消除CKI对周期蛋白-CDK的抑制效果,促进细胞周期进程[37]。TGF-β (transforming growth factor-beta)信号通路被证明可通过促进SMAD2/3和SMAD4结合,抑制c-Myc促进p15INK4B表达[38]。并且SMAD2/3-SMAD4复合物还可直接与p15INK4B和p21Cip1相互作用促进其表达,从而抑制细胞周期进程[39]。另外,p107和E2F转录因子4/5 (adenovirus E2 promoter binding factor transcription factor 4/5, E2F4/5)也可作为SMAD的辅助因子,抑制c-Myc促进p15INK4B表达[40]。可见在细胞周期进程调控中SMAD家族起着重要作用。此外,DNA损伤可通过激活p53促进p21Cip1/WAF1的表达,抑制相关CDK的激活,阻碍细胞周期进程[41]。另外,CDK不仅可被CKI抑制,同时周期蛋白-CDK还可通过隔离CKI,促进相关CDK激活,例如在乳腺癌和人类B细胞淋巴瘤细胞周期研究中发现cyclinD-CDK4/6可通过与p27kip1特异性结合,隔离p27kip1对cyclinE-CDK2的抑制作用,促进CDK2的激活[42]。表明CDK与CKI可能存在一种反馈调节机制。综上所述,CDK活性激活需要将这些由泛素化介导的CKI在细胞周期特定时期内降解,才可保证细胞周期有序进行,见图 1C。
2 G1期cyclinD-CDK4/6复合物D型细胞周期蛋白(cyclin D1/D2/D3)与CDK4/6是驱动整个细胞周期启动的核心分子。胞外生长因子信号通过MAPK (mitogen-activated protein kinases)信号通路刺激cyclinD-CDK4/6启动细胞周期[43]。在细胞分裂过程中,激活的cyclinD-CDK4/6可通过使底物视网膜母细胞瘤蛋白(retinoblastoma protein, RB)磷酸化,促使RB与E2F1/2/3解离,激活E2F1/2/3的转录活性,从而协同DP1/2促进cyclinE及进入S期所需的酶和蛋白质翻译,进而保证G1-S期转换[19, 44]。此外,非磷酸化的RB可与转录因子E2F1/2/3紧密结合抑制转录因子的活性,从而诱导G1期停滞[45]。表明在G1-S期转换过程中磷酸化RB是关键事件。此外,有学者发现cyclinD-CDK4/6通过与RB C末端的一个α-螺旋配对,从而磷酸化RB,并且该螺旋不可被cyclinE、cyclinA和cyclinB识别,而且RB C末端螺旋的突变能阻止其被cyclinD-CDK4/6磷酸化,从而造成G1期停滞,表明cyclinD-CDK4/6磷酸化RB与其C端螺旋密切相关[46]。
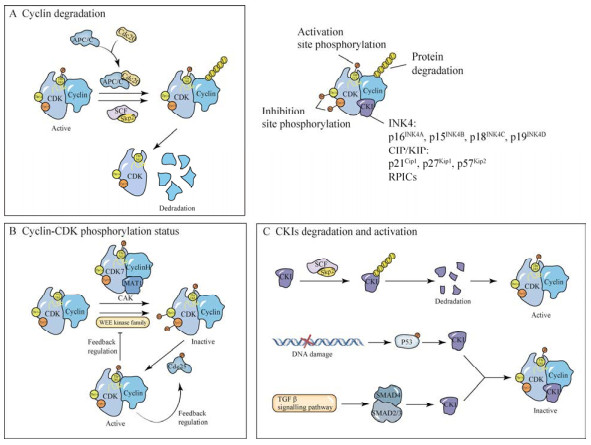
磷酸酶Cdc25A已在体细胞中被证明可通过去除特定位点磷酸化激活cyclinD-CDK4/ CDK6,促进G1-S期转换[47]。而G1期至有丝分裂期Cdc25A是一种不稳定的蛋白质,并且过于稳定的Cdc25A会造成G1-S期和G2-M期转换加速,致使基因组不稳定导致癌症及疾病发生[32]。而cyclinD-CDK4/CDK6可通过以ssTrCP依赖的方式降低Cdc25A的稳定性,保证G1-S期正常转换[32]。表明cyclinD-CDK4/ CDK6与G1期Cdc25A间可能存在一种调控负反馈回路,控制着G1-S期转换。此外,异常表达的cyclinD会导致DNA损伤、复制应激和检查点激酶1 (checkpoint kinase 1, CHK1)激活,致使细胞出现异常生长[48]。因此,关于cyclinD-CDK4/6降解机制的研究就显得尤为关键。研究发现,cyclinD羧基末端磷酸化可触发泛素化蛋白酶体降解途径,并且该途径受到泛素连接酶(E3)家族成员CRL4AMBRA1 (the cullin-4-based RING-type)调控[49]。AMBRA1 (activating molecule in beclin-1-regulated autophagy)主要负责cyclinD与CRL4的靶向连接,随后E3则将泛素蛋白链(ubiquitin, Ub)附着在cyclinD上,从而使cyclinD被泛素化降解[48-49]。另外,当AMBRA1耗竭或下调时cyclinD及Myc (n-Myc、c-Myc)家族蛋白水平升高,并且Myc家族蛋白质水平升高的同时还可上调cyclinD和cyclinE的表达,促进细胞周期的进展[48]。此外,AMBRA1水平的降低可能是对CDK4/6抑制剂脱敏的机制之一,其可通过增加cyclinD-CDK4/6和cyclinD-CDK2的形成,从而抵抗CDK4/6抑制剂的敏感性[50]。表明AMBRA1是G1-S期转换的关键调节因子,可控制G1-S期的转换,并有助于在DNA复制期间保持基因组的完整性减缓发育异常和肿瘤生长。cyclinD-CDK4/6机制调控过程如图 2A所示。
|
| 图 2 G1期cyclin-CDK机制调控过程 Fig. 2 The regulation process of cyclin-CDK mechanism in G1-phase. A: Mechanistic regulation of cyclinD-CDK4/6. Growth factor signaling-mediated MAPK signaling and phosphorylated Cdc25A can activate cyclinD-CDK4/6 activity; DNA damage inhibits cyclinD-CDK4/6 activity by activating CKI; cyclinD is inhibited by CRL4AMBRA1 ubiquitination degradation, and lack of CRL4AMBRA1 increases the expression of Myc and cyclinD, promotes cyclinD binding to CDK4/6 and CDK2, and reduces sensitivity to CDK4/6 inhibitors[19, 43, 47-50]. B: RB-mediated gene transcriptional regulation. CyclinD-CDK4/6 and cyclinE-CDK2 phosphorylate RB bound to E2F, thereby promoting S-phase related genes and cyclinE translation[45, 46]. C: Mechanistic regulation of cyclinE-CDK2. CyclinE-CDK2 activity is activated by Myc, inhibited by DNA damage-activated CKI, and degraded by ubiquitination by SCF-Skp2[53, 55]. |
| |
在正常细胞周期中,CDK2通过与E型细胞周期蛋白(cyclinE1/E2)结合促进G1-S期转换[51]。并且E型细胞周期蛋白与CDK2构成的复合物主要通过使特定底物磷酸化控制细胞周期进程和DNA复制[51]。其中,cyclinE-CDK2通过催化RB磷酸化,致使RB失去对E2F的抑制作用,促进DNA复制相关基因转录和G1-S期转换[51-52]。此外,由cyclinD-CDK4/6激活介导的RB磷酸化失活,可通过促进E2F释放使cyclinE表达量升高,从而使cyclinE在G1期积累[44]。在G1期积累的cyclinE则通过激活CDK2促进细胞G1-S期转换,并且此时cyclinE的表达量达到峰值[44]。到S期结束时,cyclinE被SCF-Skp2复合物降解,cyclinE-CDK2活性被消除,直至下一个G1期开始[53]。cyclinE的C端截断和C端附近的错义突变可赋予其在体内的高稳定性[54]。cyclinE-CDK2的Thr380自磷酸化抑制cyclinE表达,破坏cyclinE的稳定性,而当Thr380突变为Ala后,cyclinE稳定性增加[54]。并且T380A突变可消除cyclinE的破坏性磷酸化,并阻止cyclinE被泛素化降解[54]。表明cyclinE-CDK2的激活与cyclinE的特异性位点自磷酸化和泛素依赖性降解间的机制联系。此外,Myc基因家族可通过刺激和抑制关键细胞周期调节因子的表达,在生长控制和细胞周期进程中发挥作用[55]。并且过表达的Myc可通过激活cyclinE-CDK2,促进G1-S期转换[55]。cyclinE-CDK2机制调控过程见图 2B。
4 S期cyclinA-CDK2复合物CyclinA-CDK2是S期的标志复合物。cyclinA表达的阻断及cyclinA-CDK2活性的抑制是对正常细胞中细胞周期阶段控制具有特异性的事件,是癌细胞凋亡网络的一部分[56]。研究发现,在S期cyclinA-CDK2可通过磷酸化细胞分裂周期6 (cell division cycle 6, Cdc6),使其从G1期的核定位移位到细胞质中,从而调控Cdc6在启动DNA复制过程的作用,阻止S期和G2期的DNA再复制[57]。另外,DNA复制的启动涉及细胞周期依赖性组装和蛋白质复合物的拆卸,包括起源识别复合物(origin recognition complex, ORC)和Cdc6 AAA(+)ATPases[58]。其中,ORC可与Cdc6结合形成包含6个AAA+亚基的环状复合物,并通过Cdc6将启动目标定位到染色体中的特定DNA序列[59]。并且Cdc6和Cdt1 (Cdc10-dependent transcript 1)可将微小染色体维持家族(minichromosome maintenance family, MCMs)招募到复制起点,并与ORC和Cdc45结合形成复制前复合物(pre-replication complex),共同驱动DNA生物合成[60]。另外,在缺乏起源活性的DNA上,Cdc6 ATPase促进Cdc6的解离,而具有起源活性的DNA则可下调Cdc6 ATPase稳定ORC-Cdc6-DNA复合物,促进MCM加载启动DNA复制[58]。表明特定的DNA序列可通过控制Cdc6 ATPases的速率,调控Cdc6从ORC-Cdc6-DNA复合物中解离的速率。此外,cyclinA-CDK2的磷酸化可导致染色质许可和DNA复制因子1 (chromatin licensing and DNA replication factor 1, Cdt1)降解,并且Cdt1的降解、Cdc6的移位以及联会蛋白(geminin)能防止MCM与染色质在S期结合,从而通过控制复制起点严格限制染色体在每个细胞周期中只复制一次[61]。综上所述,维持基因组稳定性需要每个细胞周期精确复制一次DNA,而这一过程可能是通过限制复制起点许可实现,使每个复制起点的触发限制为每个细胞周期一次。
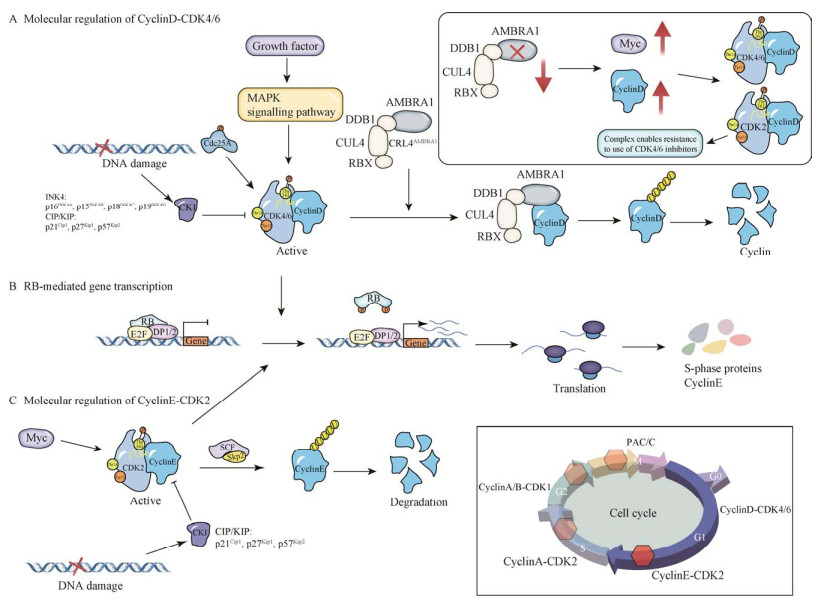
此外,正常的DNA复制过程中,cyclinA-CDK2激活受到Cdc25的调控,从而保证DNA的复制受到严格调控[62]。但随着DNA损伤信号ATM/ATR-Chk1/Chk2激酶的传导,使Cdc25暴露出一段降解基序,导致Cdc25被泛素化降解[62]。而Cdc25的降解则导致cyclinA-CDK2中的CDK2无法去除抑制性磷酸基团,致使其激活被抑制,细胞周期进程被阻止[63]。另外,cyclinA-CDK2可通过磷酸化抑制RB,促进E2F1/2/3和DP1/2复合物合成S期蛋白,从而促进S期DNA合成[64]。并且有序的S期进展还需要E2F及时失活,cyclinA-CDK2可通过与E2F稳定结合,从而指导E2F-DP1/2异构体的磷酸化,中和其DNA结合能力,致使其活性降低,保证有序的S期进展[65]。在S-G2期转换过程中,cyclinA2的定位从只在细胞核到既在细胞核又在细胞质的变化,并且只有在细胞质的cyclinA2-CDK2可通过Bora的磷酸化激活有丝分裂激酶PLK1 (polo-like kinase 1)[66]。表明细胞质中的cyclinA2可能通过触发PLK1的激活调控S-G2期转换,且细胞质存在的cyclinA2受到DNA损伤信号调控。而DNA损伤信号主要通过DNA依赖性蛋白激酶(DNA dependent protein kinase, DNA-PK)和ATM/ATR-Chk1/Chk2两条激酶途径磷酸化激活p53,促进CKIs (p21Cip、p27Kip1/2和p57Kip1/2)的表达,抑制cyclinA-CDK2活性造成S-G2期转换停滞[67]。因此,cyclinA2核质定位以及p53活性的变化,是细胞周期进程中至关重要的事件,是S-G2期转换的关键。核糖核酸还原酶(ribonucleotide reductase, RNR)催化用于DNA合成的脱氧核糖核苷酸三磷酸(deoxyribonucleotide triphosphates, dNTP)构建块的从头合成,且RNR是由两个大的RRM1亚基和两个小的RRM2亚基组成的异源四聚体[68]。在S-G2期,RRM1的Ser559可被cyclinA-CDK2磷酸化,并且RRM1的这种S559磷酸化可增强RNR活性,维持正常DNA复制过程中所需的dNTPs,确保基因组稳定[68]。此外,RRM1 S559磷酸化和ATR的联合靶向可触发致命的复制应激和深刻的抗肿瘤作用[68]。因此,RRM1的翻译后磷酸化可为精细调节RNR和癌症的治疗提供新策略。CyclinA-CDK2机制调控过程见图 3。
|
| 图 3 S期cyclinA-CDK2机制调控过程 Fig. 3 The regulatory process of cyclinA-CDK2 mechanism in S-phase. DNA damage activates P53 expression by activating DNA-PK and ATM/ATR-Chk1/Chk2 kinase pathways and promotes CKI expression; ATM/ATR-Chk1/Chk2 kinase pathway inhibits cyclinA-CDK2 activation by ubiquitinating and degrading Cdc25A; Activated cyclinA-CDK2 inhibits DNA biosynthesis by inhibiting the pre-replication complex; activated cyclinA-CDK2 phosphorylates RB and promotes the translation of DNA replication-related proteins in S-phase[60-62, 64, 67]. |
| |
CyclinH-CDK7是调控细胞周期进程的关键复合物,其遗传失活可导致细胞周期停滞,诱发成人干细胞衰竭导致的过早衰老等疾病的发生[69]。研究发现,cyclinH-CDK7-MAT1 (mating-type locus)组成的三聚体复合物CDK激活激酶(CDK-activating kinase, CAK),是激活周期蛋白-CDK活性的基础[34]。CAK通过使周期蛋白-CDK的T-loop发生磷酸化,从而激活特定的周期蛋白-CDK活性,调控细胞周期进程[70]。在后生动物中,cyclinH-CDK7-MAT1是当前被发现的CDK2和CDK1唯一已知的CAK,并且通过使CDK1和CDK2激活位点磷酸化调控细胞周期进程[71]。CDK2所结合的复合物cyclinA-CDK2的激活是促进S期进展的关键过程,抑制CDK7则阻止了cyclinA-CDK2的激活,延迟了S期的进展[72]。CDK1所结合的复合物cyclinA-CDK1和cyclinB-CDK1的激活是触发有丝分裂的关键过程,抑制CDK7则会阻止有丝分裂的进入并破坏cyclinB-CDK1的组装[72]。此外,CDK7除可以建立CDK1和CDK2的活性,还可通过维持CDK4和CDK6的活性,启动细胞周期起始[73]。当细胞退出静止状态时,CDK7与CDK4的激活磷酸化同时上升,并加速CDK4的激活[73]。表明CDK7及其结合的cyclinH-CDK7-MAT1三聚体是细胞周期进程的关键调控因素,主要通过激活相关周期蛋白-CDK的活性驱动细胞周期进程。
此外,CAK还与转录因子IIH (transcription factor IIH, TFIIH)介导的转录起始以及DNA修复有关,通过磷酸化RNA聚合酶Ⅱ (RNA polymerase Ⅱ, Pol Ⅱ)和相关转录因子(例如雌激素受体-α),调节基因表达[74]。当CAK与TFIIH结合时,CAK通过其C末端结构域(C-terminal domain, CTD)的高磷酸化激活Pol Ⅱ介导转录起始;而在没有TFIIH的情况下,CAK则通过控制CDK T-loop的磷酸化调控CDK活性[70]。另外,CDK7激活还可以独立于T-loop磷酸化发生,而这依赖于MAT1使CDK7的T-loop定位在其活性构象中[70]。这些结果表明CAK在细胞周期进程和基因转录调控中起着重要的作用。另外,M期促进因子(M phase-promoting factor, MPF)也被证明可通过诱导CDK7磷酸化,致使TFIIH相关的激酶和转录活动受到抑制,导致有丝分裂受到抑制[75]。综上所述,cyclinH-CDK7-MAT1组成的CAK是激活相关周期蛋白-CDK活性的基础,是细胞周期进程中关键的调控因子。
6 G2期与M期中的cyclinB-CDK1复合物正常细胞周期进程中,B型细胞周期蛋白(cyclinB1/B2/B3)通常通过与CDK1结合触发有丝分裂[76]。在G2期,只有cyclinB积累到一定阈值才会触发G2-M期转换,但cyclinB被破坏则会造成G2-M期转换停滞,细胞周期进程紊乱[77]。在M期,cyclinB的表达则会导致有丝分裂中期向后期转换停滞,造成细胞周期进程停滞。机制调控过程见图 4。
|
| 图 4 G2期与M期cyclinB-CDK1机制调控过程 Fig. 4 The regulatory process of cyclinB-CDK1 and PAC/C mechanism in G2 and M phases. A: Mechanistic regulation of cyclinB-CDK1 in G2-phase. DNA damage mediates p53 expression by activating DNA-PK and ATM/ATR-Chk1/Chk2 kinase pathways, promoting Gadd45 to inhibit cyclinB-CDK1 activation; RASSF10 inhibits cyclinB-CDK1 activation by promoting Gadd45; WEE kinase family inhibits the activation of cyclinB-CDK1; Cdc25B/C binds to 14-3-3 to activate cyclinB-CDK1, and the activated cyclinB-CDK1 can feedback-regulate the activity of Cdc25B/C and 14-3-3 complex by activating PIK1, promote M-phase entry[87-89, 91]. B: CyclinB-CDK1 mechanism regulation in M-phase. When Cdc20 is released from MCC and binds to APC/C, it promotes the ubiquitination and degradation of securin and cyclinB, thereby promoting the transition from metaphase to anaphase; When MCC factors are tightly bound, APC/C activity is inhibited, resulting in arrest of metaphase to anaphase transition[92-94]. |
| |
在细胞周期进程调控过程中,cyclinB穿梭于细胞核与细胞质之间,并在不同结构中聚集。在间期,cyclinB-CDK1穿梭于细胞核和细胞质并聚集在中心体,促进核层的分解和核膜破裂(nuclear envelope breakdown, NEBD);在前期,cyclinB-CDK1聚集在细胞核及有丝分裂纺锤体;在中期,cyclinB-CDK1聚集在纺锤体中间重叠的微管,调控姐妹染色单体分离[78-79]。在中期结束时cyclinB-CDK1从纺锤体消失,进入后期后细胞质中的cyclinB-CDK1也逐渐消失[80]。表明cyclinB-CDK1的时空变化调节可能调控着有丝分裂进程。此外,CDK1激活磷酸化需要cyclinB-CDK1转移到细胞核中才能进行,阐明在有丝分裂开始时控制cyclinB1-CDK1空间易位的介质的作用对于细胞周期进程调控至关重要[77]。另外,cyclinB核输入的增加是由核输入机制的变化驱动,既不需要Plk1调控也不需要抑制核输出[78]。因此,cyclinB-CDK1的激活和其快速的核输入之间的内在联系协调着有丝分裂进入时细胞核和细胞质的重组。
6.2 G2-M期转换中的cyclinB-CDK1CyclinB-CDK1与Greatwall (Gwl)是M期促进因子(M-phase promoting factor, MPF)的关键组分,可促进M期启动[81]。研究发现,虽然过量的cyclinB-CDK1可诱导核膜破裂,但抑制纺锤体组装,Greatwall则可逆转这种现象发生并减少核膜破坏所需的cyclinB-CDK1数量,形成具有对齐染色体的纺锤体[81]。表明Greatwall与cyclinB-CDK1存在反馈调控作用。在没有Greatwall的情况下,即使cyclinB-CDK1完全活跃,MPF在细胞质中也检测不到,而当Greatwall被添加时,MPF的活性也随之恢复,从而触发有丝分裂[81]。表明MPF活性激活需要cyclinB-CDK1与Greatwall共同调节。此外,Greatwall在有丝分裂开始时可使抑制CDK活性的蛋白磷酸酶PP2A/B55失活,从而使CDK的活性在分裂期达到最大[82]。而在G2-M期Greatwall可被CDK1磷酸化激活,产生一个双稳态分子开关,使cyclinB-CDK1完全激活[82]。并且Greatwall对PP2A: B55的抑制,可使缺乏cyclinB-CDK1的细胞进入有丝分裂并对大多数与有丝分裂相关的蛋白质进行磷酸化[83]。表明Greatwall与cyclinB-CDK1存在反馈调控作用,并且两者相互协同在细胞周期进程调控中发挥着关键作用。
此外,cyclinB-CDK1活性受到一类p53调节的DNA损伤诱导蛋白家族Gadd45 (growth arrest and DNA damage 45)调控,包括Gadd45a、Gadd45b和Gadd45g等[84]。在DNA损伤时,Gadd45a、Gadd45b和Gadd45g通过抑制cyclinB1-CDK1活性造成G2-M期停滞、生长抑制和细胞凋亡[85]。此外,这3种Gadd45蛋白质在细胞暴露于紫外线照射后可协作激活S期和G2-M期检查点,其中Gadd45b和Gadd45a对cyclinB-CDK1活性的抑制涉及复合物的破坏,而Gadd45g没有破坏复合物[85]。Ras相关域蛋白家族(Ras association domain family, RASSF)是由许多肿瘤抑制基因编码,并被证明可抑制肿瘤细胞增殖[86]。其中,RASSF10可通过促进GADD45a的核积累,抑制cyclinB-CDK1形成,诱导有丝分裂停滞[87]。并且敲除NPM或GADD45a,可导致RASSF10介导的G2-M期停滞受损[87]。另外,在G2-M期选择性抑制NFkappaB可使G2-M期特异性基因cyclinB1/B2、Plk1和Cdc25B的转录被抑制,延迟有丝分裂进入[88]。
在G2-M期转换中,Cdc25家族(Cdc25A/ B/C)起着重要作用。Cdc25家族可通过消除由WEE激酶家族介导的CDK1 Thr14和Tyr15位点抑制性磷酸化以及与14-3-3结合的方式,激活cyclinB-CDK1活性,调控G2-M期转换[89-90]。同时,Cdc25A/B/C的活性也都受到DNA损伤信号诱导的ATM/ATR-Chk1/2激酶信号传导途径调控[91]。虽然cyclinB的积累可触发G2-M转换,但过早的cyclinB破坏则可触发有丝分裂退出。cyclinB的破坏主要受到异常激活的E3泛素连接酶APC/C和其共激活剂Cdc20调控[92]。因此,可通过降低Cdc20对APC/C的亲和力,以及催化有丝分裂检查点复合体(mitotic checkpoint complex, MCC) MAD2-BUBR1-BUB3-Cdc20的组装等方式阻止APC/C的异常激活,防止这种有丝分裂提前退出事件发生,从而使细胞周期有序进行[93-94]。
6.3 有丝分裂中期向后期转换中的cyclinB-CDK1在有丝分裂中期向后期转换过程中,APC/C被Cdc20激活,并且激活的APC/C促进分离酶抑制蛋白(securin)和cyclinB的泛素化降解启动后期[95]。在有丝分裂早期,姐妹染色单体被黏连蛋白复合物固定[96]。姐妹染色单体的分离由有丝分裂后期分离酶(Separin)触发,分离酶则通过其抑制剂securin和cyclinB的降解被激活,从而促进中后期的转换[96]。表明securin与cyclinB是抑制分离酶活性的关键因子,在中后期转换中起着关键作用。综上所述,cyclinB在G2期表达水平的维持可促进G2-M期转换,但进入有丝分裂期后,高水平的cyclinB则会通过抑制分离酶活性,造成中期向后期转换停滞。因此cyclinB需要在特定时期表达和降解,从而推进细胞周期进程的有序进行。
7 CDK抑制剂在癌症和疾病中的应用 7.1 CDK4/6抑制剂当前已有多类高特异性CDK4/6抑制剂被批准用于癌症及疾病的治疗,包括帕博西尼(palbociclib)、瑞博西尼(ribociclib)、阿贝西利(abemaciclib)和曲拉西利(trilaciclib)等[97]。但在临床研究中发现癌症及疾病对这些抑制剂有耐药性,并且许多耐药性病例缺乏分子研究基础。因此,解析这类抑制剂在癌症及疾病中的功能机制,将有助于提升治疗效果。AbuHammad等[98]在黑色素瘤中发现帕博西尼与蛋白精氨酸甲基转移酶5 (protein arginine methyltransferase 5, PRMT5)抑制剂GSK3326595联合使用可延缓耐药性的出现,并且提供的临床证据表明CDK4/6和PRMT5的联合抑制是一种有效且耐受性良好的治疗策略。Peng等[99]在结直肠癌(colorectal cancer, CRC)中发现,E26转化特异性变异转录因子5 (E26 transformation-specific variant transcription factor 5, ETV5)可通过抑制p21促进G1-S期转换,加速CRC的生长,并改变CRC对帕博西尼和迪那昔布(dinaciclib)的药物敏感性。Zhou等[100]发现在前列腺癌和乳腺癌细胞中,组蛋白去乙酰化酶5 (histone deacetylase 5, HDAC5)的缺失会损害RB对促癌基因的抑制并赋予癌症对帕博西尼的耐药性,而且这种影响被溴域和外端(bromodomain and extra-terminal, BET)-环磷酸腺苷反应元件结合蛋白(cyclic adenosine monophosphate response element binding protein, CBP)/p300双重抑制剂NEO2734克服。这些结果表明CDK4/6抑制剂与其他药物的联合治疗可有效地减轻耐药性的发生。
Zhang等[101]在乳腺癌中发现帕博西尼和吡罗替尼(pyrotinib)的联合治疗可通过降低pAKT和pHER3 (phosphorylated human epidermal growth factor receptor 3)活性,诱导G0-G1期停滞,增加细胞凋亡率。Kumarasamy等[102]在胰腺癌中发现帕博西尼与MEK抑制剂的联合治疗可使p27上调,增强体内肿瘤对帕博西尼的反应,提高帕博西尼对胰腺癌的治疗效果。阿贝西利是唯一一种在难治性转移性ER+乳腺癌中具有单一活性的CDK4/6抑制剂,能够有效穿过血脑屏障,并具有降低骨髓抑制的独特毒性特征[103]。与其他CDK4/6抑制剂相比,阿贝西利可能独立于cyclinD-CDK4/6-RB通路发挥作用,可为阿贝西利可能扩大的临床适应症和预测性生物标志物研究产生重要影响[103]。此外,除上述的CDK4/6抑制剂外,Therapeutics公司研发的一种小分子、短效的CDK4/6抑制剂曲拉西利,其具有骨髓保护作用,与癌症化疗联合使用时具有潜在的抗肿瘤功效与安全性[104]。曲拉西利能诱导骨髓中增殖的造血干细胞和祖细胞出现短暂的、可逆的G1期停滞,从而保护它们在化疗期间免受损害[104]。
综上所述,虽然当前CDK4/6抑制剂类药物已被证明可有效改善临床治疗效果,但内在或获得性耐药性的发展会限制这些治疗的功效。并且联合治疗比单独使用一类药物的表现更好,对机体的毒性更小,抗性更低。因此,在临床和转换研究中应重点关注CDK4/6抑制剂的敏感性/抗性机制和CDK4/6抑制剂的相关联合治疗策略,从而为癌症和疾病相关临床治疗药物、新的反应生物标志物的研发奠定基础[105]。
7.2 CDK7相关抑制剂 7.2.1 CDK7特异性共价抑制剂THZ1细胞周期和转录控制的失调是肿瘤细胞的一般特征,这突出了使用CDK7抑制剂作为新型癌症治疗剂的潜力。当前已有多种CDK7特异性共价抑制剂被用于临床研究。其中,CDK7特异性共价抑制剂THZ1,是当前研究最多的CDK7特异性抑制剂。其通过抑制CDK7的表达,影响相关致病基因的转录活性以及癌细胞的周期进程,以达到治疗癌症及疾病的目的[106]。三阴性乳腺癌(triple-negative breast cancer, TNBC)是一种乳腺癌的特殊亚型,TNBC患者在治疗后通常表现出较差的预后和高复发率。Li等[107]在TNBC中发现高特异性的CDK7抑制剂BS-181和THZ1,均可下调CDK7介导的Pol Ⅱ磷酸化,其中THZ1的效力比BS-181高500倍。机制研究表明,TNBC细胞存活很大程度依赖于细胞中的B细胞淋巴瘤2 (B-cell lymphoma-2, BCL-2)/BCL-XL信号[107]。因此,研究人员将BCL-2/BCL-XL抑制剂ABT-263/ABT199与THZ1联合治疗,发现TNBC细胞出现生长抑制和凋亡[107]。表明CDK7可作为TNBC预后的候选生物标志物,并且CDK7和BCL-2/BCL-XL抑制剂的联合治疗可为改善TNBC治疗提供有价值的参考。胰腺导管腺癌(pancreatic ductal adenocarcinoma, PDAC)是一种具有高死亡率的致死性恶性肿瘤。Lu等[108]在多个PDAC临床模型中发现THZ1抑制CDK7可导致有丝分裂和核因子-κB (nuclear factor-kappaB, NF-κB)信号传导相关转录物的转录被抑制,从而调控癌细胞周期进程以达到治疗的目的。表明PDAC中抑制依赖CDK7的转录激活,可为针对高侵袭性PDAC的治疗提供积极的策略。此外,THZ1还可用于这些癌症治疗:结直肠癌[109]、上皮性卵巢癌[110]、胆囊癌[111]等。
7.2.2 其他CDK7特异性共价抑制剂除THZ1外,这些CDK7抑制剂也在疾病及癌症治疗和临床研究中得到应用,例如BS-181、ICEC0942、SY-1365、YKL-5-124、SY-5609和AT7519等[112-113]。类风湿性关节炎(rheumatoid arthritis, RA)是一种慢性炎症性疾病,可导致严重残疾。Hong等[114]在胶原蛋白诱导的关节炎(collagen-induced arthritis, CIA)小鼠模型中,发现BS-181可通过抑制CDK7抑制RA的炎症反应,表明CDK7具有显著的抗炎作用。Patel等[115]发现一种用于治疗癌症的口服生物CDK7抑制剂ICEC0942,其在BC和CRC的异种移植物中,具有显著的抗肿瘤作用。并且与他莫昔芬(tamoxifen)的联合治疗可使雌激素受体阳性肿瘤异种移植物生长被完全停滞[115]。表明ICEC0942可作为单一药物或与其他药物联合使用治疗癌症。Hu等[116]在对卵巢癌(ovarian cancer)和乳腺癌患者群体进行的临床试验中发现,SY-1365治疗降低了抗凋亡BCL2家族成员MCL1和BCL-XL的蛋白质水平,并且BCL-XL表达低的癌细胞对SY-1365更敏感。此外,SY-1365作为单一药剂在多种急性髓性白血病(acute myeloid leukemia, AML)和卵巢癌异种移植模型中也表现出显著的抗肿瘤作用,并且与BCL2抑制剂维奈托克(venetoclax)联合使用时,SY-1365诱导的生长抑制作用被增强[116]。表明在血液肿瘤和实体肿瘤的临床治疗中SY-1365拥有巨大潜力。Olson等[117]发现一种治疗以E2F失调为标志的癌症的CDK7抑制剂YKL-5-124,其与THZ1不同,不会导致Pol Ⅱ C末端结构域磷酸化发生变化,主要通过抑制E2F驱动的基因表达,从而导致G1-S期转换停滞,进而达到治疗的目的。Zhang等[118]在小细胞肺癌(small-cell lung cancer, SCLC)中发现YKL-5-124通过抑制CDK7表达,增强基因组不稳定性,破坏细胞周期进程,触发SCLC的抗肿瘤免疫力。表明CDK7抑制剂与抗肿瘤免疫存在内在联系,这可为由CDK7抑制剂和免疫疗法组成的联合治疗方案提供理论依据。
7.3 其他CDK抑制剂除了相对成熟的CDK4/6和CDK7抑制剂外,还有许多CDK抑制剂也被应用于癌症及非癌症疾病治疗上,但大多数仍处于临床前与早期临床阶段。例如,CDK2/7/9抑制剂seliciclib (CYC202)、CDK9抑制剂NVP-2、CDK2/9抑制剂fadraciclib (CYC065)等[119-120]。Seliciclib是一种CDK抑制剂,可竞争CDK2/7/9上的ATP结合位点,抑制CDK2/7/9活性[121]。在临床试验上seliciclib被发现可通过抑制CDK2/7/9活性,诱导肿瘤细胞凋亡,减少模型小鼠肿瘤生长[121]。昼夜节律系统和细胞周期在癌症中经常失调。Iurisci等[122]在格拉斯哥骨肉瘤(Glasgow osteosarcoma)中发现塞利西利(seliciclib)可通过抑制CDK1/2/7/9调控生物钟改善肿瘤的恶性状况。P276-00是一种CDK4抑制剂,可抑制CDK4表达[123]。Rathos等[123]在胰腺癌细胞中发现P276-00与吉西他滨(gemcitabine)联合治疗可抑制CDK4和Bcl-2表达,增强细胞凋亡,从而抑制肿瘤恶性增长。AT7519是CDK2靶向抑制剂[124]。Dolman等[124]在神经母细胞瘤中发现AT7519可通过抑制CDK2表达,改善小鼠的生存率与肿瘤的恶性增长状况,表明AT7519是一种极具前景的靶向药物,可用于治疗高危神经母细胞瘤患者。在过去50年中,AML的治疗效果一直在稳步改善[125]。CDK2和CDK9失活被证明可克服AML细胞分化停滞,表明靶向CDK2和CDK9可能是一种极具前途的AML治疗方法[125]。维奈托克(Venetoclax)是FDA批准的Bcl-2选择性抑制剂,Luedtke等[126]在慢性淋巴细胞白血病和AML中发现CDK9抑制剂voruciclib与维奈托克联合靶向CDK9可产生针对AML细胞和原发性患者样本的协同抗白血病活性。Fadraciclib (CYC065)是一种CDK2/9抑制剂,在AML中具有临床前疗效。Chantkran等[119]在AML中发现当fadraciclib与维奈托克或常规化疗药物阿糖胞苷或阿扎胞苷联合使用时显示出协同活性,fadraciclib和阿扎胞苷的联合使用具有最有利的治疗效果。Mandal等[127]发现CDK9抑制剂BAY1251152针对不同实体瘤的Ⅰ期临床试验显示出良好的抗肿瘤、靶向活性和可控的安全性。同时,为了提高有效性和靶点多样性并降低潜在的耐药性,将CDK9抑制剂与针对MYC、MCL-1和HSP90的抑制剂相结合,联合治疗将是研究者后续可关注的方向[127]。Richters等[128]在前列腺癌(Castration-resistant prostate cancer, CRPC)中发现口服KB-0742可通过抑制CDK9降低CRPC恶性生长,表明KB-0742可作为靶向CRPC具有前景的治疗药物。细胞因子干扰素γ (cytokine interferon gamma, IFNG)介导的适应性免疫抗性仍是癌症免疫治疗的主要问题[129]。Huang等[129]在胰腺癌中发现CDK抑制剂迪那昔布(dinaciclib)可通过抑制CDK1/2/5克服IFNG介导的胰腺癌适应性免疫抗性,表明CDK1/2/5活性对于IFNG介导的癌症免疫逃逸至关重要,可为克服IFNG引发的胰腺肿瘤免疫获得性耐药提供一种新策略。
8 问题与展望细胞周期进程是一种复杂的机制调控过程,涉及各类细胞周期有关的蛋白激酶和蛋白磷酸酶催化的磷酸化和去磷酸化过程。在这一过程中有许多细胞周期调控因子发挥着重要作用,包括周期蛋白、CDK、CKIs、WEE激酶家族、CDC家族和APC/C等,且这些调控因子在时间与空间上受到转录、翻译后修饰以及蛋白质降解等多种方式调节。其中,细胞周期进程运行机制的核心是由CDK和其对应的周期蛋白“轮动式”表达介导,并且涉及的上下游调节网络复杂且精细,只要任一环节出现问题,就会导致细胞分裂出现异常。而细胞分裂异常是所有恶性肿瘤细胞的特征,所以几乎所有关于癌症的研究都会涉及细胞周期[6]。因此,近十几年以来关于抗癌药物的研发多围绕CDK的药理学靶点进行,例如CDK4/6抑制剂(palbociclib、ribociclib、abemaciclib等)、CDK7抑制剂(THZ1、SY-1365等)等。并且CDK抑制剂类治疗药物在临床研究中可通过作为单一药物或与其他治疗药物及方法联合使用治疗癌症及疾病。而癌症的高耐药性、药物的靶向特异性以及潜在的副作用风险,一直以来是癌症治疗中CDK靶向与非靶向药物研发的重要挑战。因此,在设计治疗方案及药物研发时应充分考虑药物的耐药性、靶向特异性、潜在的副作用风险以及CDK在细胞周期进程调控的特殊性,从而开发减少或克服耐药性、低副作用的新型CDK靶向及非靶向药物。
细胞周期是由蛋白质的时间与空间上的精确调节驱动,且当前对细胞周期研究通常以细胞群体为目标,极少对单个分裂细胞进行探究,而对单个分裂细胞进行深入的探索则可能会获得许多意想不到的结果。随着诸如转录组、翻译组、蛋白质组以及单细胞分析技术等组学和测序手段的发展,使得细胞周期领域研究取得了长足的进展。其中,单细胞分析技术的出现给细胞周期研究带来了新的策略,利用单细胞RNA测序(scRNA-seq)可发现更多细胞周期依赖性基因,但这一测序手段主要在基因组和转录组水平对mRNA的丰度进行探究,无法对蛋白质的翻译后修饰、蛋白质动力学等相关表型特征进行直接且深入的表征。单细胞蛋白质组学(single-cell proteomics)的出现,则使从蛋白质水平进行直接表征变为可能,使单个细胞的蛋白质表达水平从基于mRNA丰度的推导转变为真实意义的测量[130]。而将单细胞蛋白质组与转录组的联合分析,则可系统性地在mRNA和蛋白质水平鉴定细胞周期相关蛋白质,从而为细胞周期时空蛋白质图谱的建立,提供更为精确的蛋白质及基因时空表达谱信息。此外,也可利用单细胞蛋白质基因组构建重要经济动物(例如羊、牛和猪等)的细胞周期蛋白质图谱,完善物种信息库,为动物经济性状的提升和种质资源的筛选提供积极的策略。同时,单细胞蛋白质组学也可应用于构建不同类型癌细胞的细胞周期蛋白质图谱,并根据不同类型癌细胞的特异性周期调控机制,筛选相关致癌与抑癌特异性细胞周期基因或蛋白质,从而为癌症治疗相关靶向药物的研发添砖加瓦。
| [1] |
MALUMBRES M. Cyclin-dependent kinases. Genome Biology, 2014, 15(6): 122. DOI:10.1186/gb4184
|
| [2] |
ÖRD M, MÖLL K, AGEROVA A, KIVI R, FAUSTOVA I, VENTA R, VALK E, LOOG M. Multisite phosphorylation code of CDK. Nature Structural & Molecular Biology, 2019, 26(7): 649-658. |
| [3] |
WOOD DJ, ENDICOTT JA. Structural insights into the functional diversity of the CDK-cyclin family. Open Biology, 2018, 8(9): 180112. DOI:10.1098/rsob.180112
|
| [4] |
RUSSO AA, JEFFREY PD, PAVLETICH NP. Structural basis of cyclin-dependent kinase activation by phosphorylation. Nature Structural & Molecular Biology, 1996, 3(8): 696-700.
|
| [5] |
XIE ZL, HOU SZ, YANG XX, DUAN YJ, HAN JH, WANG Q, LIAO CZ. Lessons learned from past cyclin-dependent kinase drug discovery efforts. Journal of Medicinal Chemistry, 2022, 65(9): 6356-6389. DOI:10.1021/acs.jmedchem.1c02190
|
| [6] |
LIU J. Cell cycle on the crossroad of tumorigenesis and cancer therapy. Trends in Cell Biology, 2022, 32(1): 30-44. DOI:10.1016/j.tcb.2021.07.001
|
| [7] |
BURY M. New insights into CDK regulators: novel opportunities for cancer therapy. Trends in Cell Biology, 2021, 31(5): 331-344. DOI:10.1016/j.tcb.2021.01.010
|
| [8] |
MARTIN MP, ENDICOTT JA, NOBLE MEM. Structure-based discovery of cyclin-dependent protein kinase inhibitors. Essays in Biochemistry, 2017, 61(5): 439-452. DOI:10.1042/EBC20170040
|
| [9] |
SHI ZF, TIAN L, QIANG TT, LI JY, XING Y, REN XD, LIU C, LIANG CY. From structure modification to drug launch: a systematic review of the ongoing development of cyclin-dependent kinase inhibitors for multiple cancer therapy. Journal of Medicinal Chemistry, 2022, 65(9): 6390-6418. DOI:10.1021/acs.jmedchem.1c02064
|
| [10] |
FASSL A, GENG Y, SICINSKI P. CDK4 and CDK6 kinases: from basic science to cancer therapy. Science, 2022, 375(6577): eabc1495. DOI:10.1126/science.abc1495
|
| [11] |
WEDAM S, FASHOYIN-AJE L, BLOOMQUIST E, TANG SH, SRIDHARA R, GOLDBERG KB, THEORET MR, AMIRI-KORDESTANI L, PAZDUR R, BEAVER JA. FDA approval summary: palbociclib for male patients with metastatic breast cancer. Clinical Cancer Research, 2020, 26(6): 1208-1212. DOI:10.1158/1078-0432.CCR-19-2580
|
| [12] |
KWAPISZ D. Cyclin-dependent kinase 4/6 inhibitors in breast cancer: palbociclib, ribociclib, and abemaciclib. Breast Cancer Research and Treatment, 2017, 166(1): 41-54. DOI:10.1007/s10549-017-4385-3
|
| [13] |
ABDELDAYEM A, RAOUF YS, CONSTANTINESCU SN, MORIGGL R, GUNNING PT. Advances in covalent kinase inhibitors. Chemical Society Reviews, 2020, 49(9): 2617-2687. DOI:10.1039/C9CS00720B
|
| [14] |
TADESSE S, ANSHABO AT, PORTMAN N, LIM E, TILLEY W, CALDON CE, WANG S. Targeting CDK2 in cancer: challenges and opportunities for therapy. Drug Discovery Today, 2020, 25(2): 406-413. DOI:10.1016/j.drudis.2019.12.001
|
| [15] |
NING SB, WANG HW, ZENG C, ZHAO YJ. Prediction of allosteric druggable pockets of cyclin- dependent kinases. Briefings in Bioinformatics, 2022, 23(4): bbac290. DOI:10.1093/bib/bbac290
|
| [16] |
THIEL JT, DAIGELER A, KOLBENSCHLAG J, RACHUNEK K, HOFFMANN S. The role of CDK pathway dysregulation and its therapeutic potential in soft tissue sarcoma. Cancers, 2022, 14(14): 3380. DOI:10.3390/cancers14143380
|
| [17] |
CHOU J, QUIGLEY DA, ROBINSON TM, FENG FY, ASHWORTH A. Transcription-associated cyclin- dependent kinases as targets and biomarkers for cancer therapy. Cancer Discovery, 2020, 10(3): 351-370. DOI:10.1158/2159-8290.CD-19-0528
|
| [18] |
EVANS T, ROSENTHAL ET, YOUNGBLOM J, DISTEL D, HUNT T. Cyclin: a protein specified by maternal mRNA in sea urchin eggs that is destroyed at each cleavage division. Cell, 1983, 33(2): 389-396. DOI:10.1016/0092-8674(83)90420-8
|
| [19] |
ENGELAND K. Cell cycle regulation: P53-p21-RB signaling. Cell Death & Differentiation, 2022, 29(5): 946-960.
|
| [20] |
SUSANTI NMP, TJAHJONO DH. Cyclin-dependent kinase 4 and 6 inhibitors in cell cycle dysregulation for breast cancer treatment. Molecules, 2021, 26(15): 4462. DOI:10.3390/molecules26154462
|
| [21] |
SWAFFER MP, JONES AW, FLYNN HR, SNIJDERS AP, NURSE P. CDK substrate phosphorylation and ordering the cell cycle. Cell, 2016, 167(7): 1750-1761.e16. DOI:10.1016/j.cell.2016.11.034
|
| [22] |
KIMATA Y. APC/C ubiquitin ligase: coupling cellular differentiation to G1/G0 phase in multicellular systems. Trends in Cell Biology, 2019, 29(7): 591-603. DOI:10.1016/j.tcb.2019.03.001
|
| [23] |
WEI WY, AYAD NG, WAN Y, ZHANG GJ, KIRSCHNER MW, KAELIN WG Jr. Degradation of the SCF component Skp2 in cell-cycle phase G1 by the anaphase-promoting complex. Nature, 2004, 428(6979): 194-198. DOI:10.1038/nature02381
|
| [24] |
LIU LJ, MICHOWSKI W, KOLODZIEJCZYK A, SICINSKI P. The cell cycle in stem cell proliferation, pluripotency and differentiation. Nature Cell Biology, 2019, 21(9): 1060-1067. DOI:10.1038/s41556-019-0384-4
|
| [25] |
STEAD E, WHITE J, FAAST R, CONN S, GOLDSTONE S, RATHJEN J, DHINGRA U, RATHJEN P, WALKER D, DALTON S. Pluripotent cell division cycles are driven by ectopic Cdk2, cyclin A/E and E2F activities. Oncogene, 2002, 21(54): 8320-8333. DOI:10.1038/sj.onc.1206015
|
| [26] |
ZHU JY, CUELLAR RA, BERNDT N, LEE HE, OLESEN SH, MARTIN MP, JENSEN JT, GEORG GI, SCHÖNBRUNN E. Structural basis of wee kinases functionality and inactivation by diverse small molecule inhibitors. Journal of Medicinal Chemistry, 2017, 60(18): 7863-7875. DOI:10.1021/acs.jmedchem.7b00996
|
| [27] |
GHELLI LUSERNA DI RORÀ A, CERCHIONE C, MARTINELLI G, SIMONETTI G. A WEE1 family business: regulation of mitosis, cancer progression, and therapeutic target. Journal of Hematology & Oncology, 2020, 13(1): 1-17.
|
| [28] |
SCHMIDT M, ROHE A, PLATZER C, NAJJAR A, ERDMANN F, SIPPL W. Regulation of G2/M transition by inhibition of WEE1 and PKMYT1 kinases. Molecules, 2017, 22(12): 2045. DOI:10.3390/molecules22122045
|
| [29] |
RUDOLPH J. Inhibiting transient protein-protein interactions: lessons from the Cdc25 protein tyrosine phosphatases. Nature Reviews Cancer, 2007, 7(3): 202-211. DOI:10.1038/nrc2087
|
| [30] |
LUCENA R, ALCAIDE-GAVILÁN M, ANASTASIA SD, KELLOGG DR. Wee1 and Cdc25 are controlled by conserved PP2A-dependent mechanisms in fission yeast. Cell Cycle, 2017, 16(5): 428-435. DOI:10.1080/15384101.2017.1281476
|
| [31] |
ENDERS GH. Gauchos and ochos: a Wee1-Cdk tango regulating mitotic entry. Cell Division, 2010, 5(1): 1-7. DOI:10.1186/1747-1028-5-1
|
| [32] |
DOZIER C, MAZZOLINI L, CÉNAC C, FROMENT C, BURLET-SCHILTZ O, BESSON A, MANENTI S. CyclinD-CDK4/6 complexes phosphorylate CDC25A and regulate its stability. Oncogene, 2017, 36(26): 3781-3788. DOI:10.1038/onc.2016.506
|
| [33] |
CLARKE PR. Cyclin-dependent kinases: CAK-handed kinase activation. Current Biology, 1995, 5(1): 40-42. DOI:10.1016/S0960-9822(95)00013-3
|
| [34] |
GREBER BJ, PEREZ-BERTOLDI JM, LIM K, IAVARONE AT, TOSO DB, NOGALES E. The cryoelectron microscopy structure of the human CDK-activating kinase. Proceedings of the National Academy of Sciences of the United States of America, 2020, 117(37): 22849-22857. DOI:10.1073/pnas.2009627117
|
| [35] |
LI Q, JIANG BS, GUO JY, SHAO H, DEL PRIORE IS, CHANG Q, KUDO R, LI ZQ, RAZAVI P, LIU B, BOGHOSSIAN AS, REES MG, RONAN MM, ROTH JA, DONOVAN KA, PALAFOX M, REIS-FILHO JS, de STANCHINA E, FISCHER ES, ROSEN N, et al. INK4 tumor suppressor proteins mediate resistance to CDK4/6 kinase inhibitors. Cancer Discovery, 2022, 12(2): 356-371. DOI:10.1158/2159-8290.CD-20-1726
|
| [36] |
STAROSTINA NG. Multiple degradation pathways regulate versatile CIP/KIP CDK inhibitors. Trends in Cell Biology, 2012, 22(1): 33-41. DOI:10.1016/j.tcb.2011.10.004
|
| [37] |
CHEN Q, XIE WL, KUHN DJ, VOORHEES PM, LOPEZ-GIRONA A, MENDY D, CORRAL LG, KRENITSKY VP, XU WM, MOUTOUH-DE PARSEVAL L, WEBB DR, MERCURIO F, NAKAYAMA KI, NAKAYAMA K, ORLOWSKI RZ. Targeting the p27 E3 ligase SCFSkp2 results in p27- and Skp2-mediated cell-cycle arrest and activation of autophagy. Blood, 2008, 111(9): 4690-4699. DOI:10.1182/blood-2007-09-112904
|
| [38] |
FENG XH, LIANG YY, LIANG M, ZHAI WG, LIN X. Direct interaction of c-myc with Smad2 and Smad3 to inhibit TGF-β-mediated induction of the CDK inhibitor p15 Ink4B. Molecular Cell, 2016, 63(6): 1089. DOI:10.1016/j.molcel.2016.08.027
|
| [39] |
GOMIS RR, ALARCÓN C, HE W, WANG QQ, SEOANE J, LASH A, MASSAGUÉ J. A FoxO-Smad synexpression group in human keratinocytes. Proceedings of the National Academy of Sciences of the United States of America, 2006, 103(34): 12747-12752. DOI:10.1073/pnas.0605333103
|
| [40] |
CHEN CR, KANG YB, SIEGEL PM, MASSAGVÉ J. E2F4/5 and p107 as smad cofactors linking the TGFβ receptor to c-myc repression. Cell, 2002, 110(1): 19-32. DOI:10.1016/S0092-8674(02)00801-2
|
| [41] |
SCHADE AE, FISCHER M, DECAPRIO JA. RB, p130 and p107 differentially repress G1/S and G2/M genes after p53 activation. Nucleic Acids Research, 2019, 47(21): 11197-11208. DOI:10.1093/nar/gkz961
|
| [42] |
ZHANG WQ, BERGAMASCHI D, JIN BQ, LU X. Posttranslational modifications of p27kip1 determine its binding specificity to different cyclins and cyclin-dependent kinases in vivo. Blood, 2005, 105(9): 3691-3698. DOI:10.1182/blood-2003-07-2558
|
| [43] |
MIN MW, RONG Y, TIAN CZ, SPENCER SL. Temporal integration of mitogen history in mother cells controls proliferation of daughter cells. Science, 2020, 368(6496): 1261-1265. DOI:10.1126/science.aay8241
|
| [44] |
RUBIN SM, SAGE J, SKOTHEIM JM. Integrating old and new paradigms of G1/S control. Molecular Cell, 2020, 80(2): 183-192. DOI:10.1016/j.molcel.2020.08.020
|
| [45] |
KENT LN, LEONE G. The broken cycle: E2F dysfunction in cancer. Nature Reviews Cancer, 2019, 19(6): 326-338. DOI:10.1038/s41568-019-0143-7
|
| [46] |
TOPACIO BR, ZATULOVSKIY E, CRISTEA S, XIE S, TAMBO CS, RUBIN SM, SAGE J, KOIVOMAGI M, SKOTHEIM JM. Cyclin D-Cdk4, 6 drives cell-cycle progression via the retinoblastoma protein's C-terminal helix. Molecular Cell, 2019, 74(4): 758-770.e4. DOI:10.1016/j.molcel.2019.03.020
|
| [47] |
NAGAHARA H, EZHEVSKY SA, VOCERO-AKBANI AM, KALDIS P, SOLOMON MJ, DOWDY SF. Transforming growth factor β targeted inactivation of cyclin E: cyclin-dependent kinase 2(Cdk2) complexes by inhibition of Cdk2 activating kinase activity. Proceedings of the National Academy of Sciences of the United States of America, 1999, 96(26): 14961-14966. DOI:10.1073/pnas.96.26.14961
|
| [48] |
MAIANI E, MILLETTI G, NAZIO F, HOLDGAARD SG, BARTKOVA J, RIZZA S, CIANFANELLI V, LORENTE M, SIMONESCHI D, DI MARCO M, D'ACUNZO P, DI LEO L, RASMUSSEN R, MONTAGNA C, RACITI M, DE STEFANIS C, GABICAGOGEASCOA E, RONA G, SALVADOR N, PUPO E, et al. AMBRA1 regulates cyclin D to guard S-phase entry and genomic integrity. Nature, 2021, 592(7856): 799-803. DOI:10.1038/s41586-021-03422-5
|
| [49] |
CHAIKOVSKY AC, LI C, JENG EE, LOEBELL S, LEE MC, MURRAY CW, CHENG R, DEMETER J, SWANEY DL, CHEN SH, NEWTON BW, JOHNSON JR, DRAINAS AP, SHUE YAN TING, SEOANE JA, SRINIVASAN P, HE A, YOSHIDA A, HIPKINS SQ, MCCREA E, et al. The AMBRA1 E3 ligase adaptor regulates the stability of cyclin D. Nature, 2021, 592(7856): 794-798. DOI:10.1038/s41586-021-03474-7
|
| [50] |
SIMONESCHI D, RONA G, ZHOU N, JEONG YT, JIANG SW, MILLETTI G, ARBINI AA, O'SULLIVAN A, WANG AA, NITHIKASEM S, KEEGAN S, SIU Y, CIANFANELLI V, MAIANI E, NAZIO F, CECCONI F, BOCCALATTE F, FENYÖ D, JONES DR, BUSINO L, et al. CRL4AMBRA1 is a master regulator of D-type cyclins. Nature, 2021, 592(7856): 789-793. DOI:10.1038/s41586-021-03445-y
|
| [51] |
CHU C. Cyclin E in normal physiology and disease states. Trends in Cell Biology, 2021, 31(9): 732-746. DOI:10.1016/j.tcb.2021.05.001
|
| [52] |
CHUNG M. Transient hysteresis in CDK4/6 activity underlies passage of the restriction point in G1. Molecular Cell, 2019, 76(4): 562-573.e4. DOI:10.1016/j.molcel.2019.08.020
|
| [53] |
NAKAYAMA K. Targeted disruption of Skp2 results in accumulation of cyclin E and p27Kip1, polyploidy and centrosome overduplication. The EMBO Journal, 2000, 19(9): 2069-2081. DOI:10.1093/emboj/19.9.2069
|
| [54] |
WON KA, REED SI. Activation of cyclin E/CDK2 is coupled to site-specific autophosphorylation and ubiquitin-dependent degradation of cyclin E. The EMBO Journal, 1996, 15(16): 4182-4193. DOI:10.1002/j.1460-2075.1996.tb00793.x
|
| [55] |
ZAJAC-KAYE M. Myc oncogene: a key component in cell cycle regulation and its implication for lung cancer. Lung Cancer, 2001, 34: S43-S46. DOI:10.1016/S0169-5002(01)00343-9
|
| [56] |
LIVIO M, VALERIE W. Intrinsic S phase checkpoint enforced by an antiproliferative oncosuppressor cytokine. Cancer Gene Therapy, 2021, 29(7): 897-900.
|
| [57] |
PETERSEN BO. Phosphorylation of mammalian CDC6 by cyclin A/CDK2 regulates its subcellular localization. The EMBO Journal, 1999, 18(2): 396-410. DOI:10.1093/emboj/18.2.396
|
| [58] |
SPECK C, STILLMAN B. Cdc6 ATPase activity regulates ORC·Cdc6 stability and the selection of specific DNA sequences as origins of DNA replication. Journal of Biological Chemistry, 2007, 282(16): 11705-11714. DOI:10.1074/jbc.M700399200
|
| [59] |
HOSSAIN M. Multiple, short protein binding motifs in ORC1 and CDC6 control the initiation of DNA replication. Molecular Cell, 2021, 81(9): 1951-1969.e6. DOI:10.1016/j.molcel.2021.03.003
|
| [60] |
LIU ZK, LI J, CHEN J, SHAN QN, DAI HJ, XIE HY, ZHOU L, XU X, ZHENG SS. MCM family in HCC: MCM6 indicates adverse tumor features and poor outcomes and promotes S/G2 cell cycle progression. BMC Cancer, 2018, 18(1): 1-10. DOI:10.1186/s12885-017-3892-2
|
| [61] |
SUGIMOTO N. Cdt1 phosphorylation by cyclin A-dependent kinases negatively regulates its function without affecting geminin binding. Journal of Biological Chemistry, 2004, 279(19): 19691-19697. DOI:10.1074/jbc.M313175200
|
| [62] |
GOTO H, NATSUME T, KANEMAKI MT, KAITO A, WANG SJ, GABAZZA EC, INAGAKI M, MIZOGUCHI A. Chk1-mediated Cdc25A degradation as a critical mechanism for normal cell-cycle progression. Journal of Cell Science, 2019, 132(2): jcs223123.
|
| [63] |
KATICH SC, ZERFASS-THOME K, HOFFMANN I. Regulation of the Cdc25A gene by the human papillomavirus type 16 E7 oncogene. Oncogene, 2001, 20(5): 543-550. DOI:10.1038/sj.onc.1204130
|
| [64] |
HALABAN R. Melanoma cell autonomous growth: the Rb/E2F pathway. Cancer Metastasis Reviews, 1999, 18(3): 333-343. DOI:10.1023/A:1006396104073
|
| [65] |
XU M, SHEPPARD KA, PENG CY, YEE AS, PIWNICA-WORMS H. Cyclin A/CDK2 binds directly to E2F-1 and inhibits the DNA-binding activity of E2F-1/DP-1 by phosphorylation. Molecular and Cellular Biology, 1994, 14(12): 8420-8431.
|
| [66] |
SILVA CASCALES H, BURDOVA K, MIDDLETON A, KUZIN V, MÜLLERS E, STOY H, BARANELLO L, MACUREK L, LINDQVIST A. Cyclin A2 localises in the cytoplasm at the S/G2 transition to activate PLK1. Life Science Alliance, 2021, 4(3): e202000980. DOI:10.26508/lsa.202000980
|
| [67] |
HUANG RX, ZHOU PK. DNA damage response signaling pathways and targets for radiotherapy sensitization in cancer. Signal Transduction and Targeted Therapy, 2020, 5: 60. DOI:10.1038/s41392-020-0150-x
|
| [68] |
SHU Z, LI Z, HUANG HH, CHEN Y, FAN J, YU L, WU ZH, TIAN L, QI Q, PENG S, WEI CY, XIE ZQ, LI XB, FENG Q, SHENG H, LI GQ, WEI DP, SHAN CL, CHEN G. Cell-cycle-dependent phosphorylation of RRM1 ensures efficient DNA replication and regulates cancer vulnerability to ATR inhibition. Oncogene, 2020, 39(35): 5721-5733. DOI:10.1038/s41388-020-01403-y
|
| [69] |
GANUZA M, SÁIZ-LADERA C, CAÑAMERO M, GÓMEZ G, SCHNEIDER R, BLASCO MA, PISANO D, PARAMIO JM, SANTAMARÍA D, BARBACID M. Genetic inactivation of Cdk7 leads to cell cycle arrest and induces premature aging due to adult stem cell exhaustion. The EMBO Journal, 2012, 31(11): 2498-2510. DOI:10.1038/emboj.2012.94
|
| [70] |
PEISSERT S, SCHLOSSER A, KENDEL R, KUPER J, KISKER C. Structural basis for CDK7 activation by MAT1 and cyclin H. Proceedings of the National Academy of Sciences of the United States of America, 2020, 117(43): 26739-26748. DOI:10.1073/pnas.2010885117
|
| [71] |
BISTEAU X, PATERNOT S, COLLEONI B, ECKER K, COULONVAL K, de GROOTE P, DECLERCQ W, HENGST L, ROGER PP. CDK4 T172 phosphorylation is central in a CDK7-dependent bidirectional CDK4/CDK2 interplay mediated by p21 phosphorylation at the restriction point. PLoS Genetics, 2013, 9(5): e1003546. DOI:10.1371/journal.pgen.1003546
|
| [72] |
LAROCHELLE S. Requirements for Cdk7 in the assembly of Cdk1/cyclin B and activation of Cdk2 revealed by chemical genetics in human cells. Molecular Cell, 2007, 25(6): 839-850. DOI:10.1016/j.molcel.2007.02.003
|
| [73] |
SCHACHTER MM, MERRICK KA, LAROCHELLE S, HIRSCHI A, ZHANG C, SHOKAT KM, RUBIN SM, FISHER RP. A Cdk7-Cdk4 T-loop phosphorylation cascade promotes G1 progression. Molecular Cell, 2013, 50(2): 250-260. DOI:10.1016/j.molcel.2013.04.003
|
| [74] |
PATEL H, ABDULJABBAR R, LAI CF, PERIYASAMY M, HARROD A, GEMMA C, STEEL JH, PATEL N, BUSONERO C, JERJEES D, REMENYI J, SMITH S, GOMM JJ, MAGNANI L, GYŐRFFY B, JONES LJ, FULLER-PACE F, SHOUSHA SM, BULUWELA L, RAKHA EA, et al. Expression of CDK7, cyclin H, and MAT1 is elevated in breast cancer and is prognostic in estrogen receptor-positive breast cancer. Clinical Cancer Research, 2016, 22(23): 5929-5938. DOI:10.1158/1078-0432.CCR-15-1104
|
| [75] |
AKOULITCHEV S, REINBERG D. The molecular mechanism of mitotic inhibition of TFIIH is mediated by phosphorylation of CDK7. Genes & Development, 1998, 12(22): 3541-3550.
|
| [76] |
KRAUSE K, WASNER M, REINHARD W, HAUGWITZ U, LANGE-ZU DOHNA C, MÖSSNER J, ENGELAND K. The tumour suppressor protein p53 can repress transcription of cyclin B. Nucleic Acids Research, 2000, 28(22): 4410-4418. DOI:10.1093/nar/28.22.4410
|
| [77] |
PORTER LISA A, DONOGHUE DANIEL J. Cyclin B1 and CDK1:nuclear localization and upstream regulators. Progress in Cell Cycle Research, 2003, 5: 335-47.
|
| [78] |
GAVET O, PINES J. Activation of cyclin B1-Cdk1 synchronizes events in the nucleus and the cytoplasm at mitosis. Journal of Cell Biology, 2010, 189(2): 247-259. DOI:10.1083/jcb.200909144
|
| [79] |
PAPINI D. The Aurora B gradient sustains kinetochore stability in anaphase. Cell Reports, 2021, 37(6): 109818. DOI:10.1016/j.celrep.2021.109818
|
| [80] |
HUANG JY. The disappearance of cyclin B at the end of mitosis is regulated spatially in Drosophila cells. The EMBO Journal, 1999, 18(8): 2184-2195. DOI:10.1093/emboj/18.8.2184
|
| [81] |
HARA M, ABE Y, TANAKA T, YAMAMOTO T, OKUMURA E, KISHIMOTO T. Greatwall kinase and cyclin B-Cdk1 are both critical constituents of M-phase-promoting factor. Nature Communications, 2012, 3: 1059. DOI:10.1038/ncomms2062
|
| [82] |
GARCÍA-BLANCO N, VÁZQUEZ-BOLADO A, MORENO S. Greatwall-endosulfine: a molecular switch that regulates PP2A/B55 protein phosphatase activity in dividing and quiescent cells. International Journal of Molecular Sciences, 2019, 20(24): 6228. DOI:10.3390/ijms20246228
|
| [83] |
HÉGARAT N, CRNCEC A, SUAREZ PEREDO RODRIGUEZ MF, ECHEGARAY ITURRA F, GU Y, BUSBY O, LANG PF, BARR AR, BAKAL C, KANEMAKI MT, LAMOND AI, NOVAK B, LY T, HOCHEGGER H. Cyclin A triggers mitosis either via the greatwall kinase pathway or cyclin B. The EMBO Journal, 2020, 39(11): e104419.
|
| [84] |
JIN SQ, TONG T, FAN WH, FAN FY, ANTINORE MJ, ZHU XC, MAZZACURATI L, LI XX, PETRIK KL, RAJASEKARAN B, WU M, ZHAN QM. GADD45- induced cell cycle G2-M arrest associates with altered subcellular distribution of cyclin B1 and is independent of p38 kinase activity. Oncogene, 2002, 21(57): 8696-8704. DOI:10.1038/sj.onc.1206034
|
| [85] |
VAIRAPANDI M, BALLIET AG, HOFFMAN B, LIEBERMANN DA. GADD45b and GADD45g are cdc2/cyclinB1 kinase inhibitors with a role in S and G2/M cell cycle checkpoints induced by genotoxic stress. Journal of Cellular Physiology, 2002, 192(3): 327-338. DOI:10.1002/jcp.10140
|
| [86] |
IWASA H, HOSSAIN S, HATA Y. Tumor suppressor C-RASSF proteins. Cellular and Molecular Life Sciences, 2018, 75(10): 1773-1787. DOI:10.1007/s00018-018-2756-5
|
| [87] |
LAKSHMI CH NP. Molecular basis for RASSF10/NPM/RNF2 feedback cascade-mediated regulation of gastric cancer cell proliferation. Journal of Biological Chemistry, 2021, 297(2): 100935. DOI:10.1016/j.jbc.2021.100935
|
| [88] |
CUDE K, WANG YP, CHOI HJ, HSUAN SL, ZHANG HL, WANG CY, XIA ZG. Regulation of the G2-M cell cycle progression by the ERK5-NFκB signaling pathway. Journal of Cell Biology, 2007, 177(2): 253-264. DOI:10.1083/jcb.200609166
|
| [89] |
KOHAMA Y. Regulation of the stability and activity of CDC25A and CDC25B by protein phosphatase PP2A and 14-3-3 binding. Cellular Signalling, 2019, 54: 10-16. DOI:10.1016/j.cellsig.2018.11.017
|
| [90] |
FERENCOVA I, VASKOVICOVA M, DRUTOVIC D, KNOBLOCHOVA L, MACUREK L, SCHULTZ RM, SOLC P. CDC25B is required for the metaphase Ⅰ-metaphase Ⅱ transition in mouse oocytes. Journal of Cell Science, 2022, 135(6): jcs252924. DOI:10.1242/jcs.252924
|
| [91] |
SANCAR A, LINDSEY-BOLTZ LA, ÜNSAL-KAÇMAZ K, LINN S. Molecular mechanisms of mammalian DNA repair and the DNA damage checkpoints. Annual Review of Biochemistry, 2004, 73: 39-85. DOI:10.1146/annurev.biochem.73.011303.073723
|
| [92] |
LARA-GONZALEZ P. The G2-to-M transition is ensured by a dual mechanism that protects cyclin B from degradation by Cdc20-activated APC/C. Developmental Cell, 2019, 51(3): 313-325.e10. DOI:10.1016/j.devcel.2019.09.005
|
| [93] |
KAISARI S, MINIOWITZ-SHEMTOV S, SITRY-SHEVAH D, SHOMER P, KOZLOV G, GEHRING K, HERSHKO A. Role of ubiquitin-protein ligase UBR5 in the disassembly of mitotic checkpoint complexes. Proceedings of the National Academy of Sciences of the United States of America, 2022, 119(9): e2121478119. DOI:10.1073/pnas.2121478119
|
| [94] |
LARA-GONZALEZ P, KIM T, OEGEMA K, CORBETT K, DESAI A. A tripartite mechanism catalyzes Mad2-Cdc20 assembly at unattached kinetochores. Science, 2021, 371(6524): 64-67. DOI:10.1126/science.abc1424
|
| [95] |
SHEVAH-SITRY D, MINIOWITZ-SHEMTOV S, TEICHNER A, KAISARI S, HERSHKO A. Role of phosphorylation of Cdc20 in the regulation of the action of APC/C in mitosis. Proceedings of the National Academy of Sciences of the United States of America, 2022, 119(35): e2210367119. DOI:10.1073/pnas.2210367119
|
| [96] |
YU J, RAIA P, GHENT CM, RAISCH T, SADIAN Y, CAVADINI S, SABALE PM, BARFORD D, RAUNSER S, MORGAN DO, BOLAND A. Structural basis of human separase regulation by securin and CDK1-cyclin B1. Nature, 2021, 596(7870): 138-142. DOI:10.1038/s41586-021-03764-0
|
| [97] |
WANG RT. Clinical considerations of CDK4/6 inhibitors in triple-negative breast cancer. Biochimica et Biophysica Acta: BBA-Reviews on Cancer, 2021, 1876(2): 188590.
|
| [98] |
ABUHAMMAD S, CULLINANE C, MARTIN C, BACOLAS Z, WARD T, CHEN H, SLATER A, ARDLEY K, KIRBY L, CHAN KT, BRAJANOVSKI N, SMITH LK, RAO AD, LELLIOTT EJ, KLEINSCHMIDT M, VERGARA IA, PAPENFUSS AT, LAU P, GHOSH P, HAUPT S, et al. Regulation of PRMT5-MDM4 axis is critical in the response to CDK4/6 inhibitors in melanoma. Proceedings of the National Academy of Sciences of the United States of America, 2019, 116(36): 17990-18000. DOI:10.1073/pnas.1901323116
|
| [99] |
PENG Y, FENG HR, WANG CG, SONG ZJ, ZHANG YQ, LIU K, CHENG X, ZHAO R. The role of E26 transformation-specific variant transcription factor 5 in colorectal cancer cell proliferation and cell cycle progression. Cell Death & Disease, 2021, 12(5): 427.
|
| [100] |
ZHOU YK, JIN X, MA J, DING DL, HUANG ZL, SHENG HY, YAN YQ, PAN YQ, WEI T, WANG LG, WU HS, HUANG HJ. HDAC5 loss impairs RB repression of pro-oncogenic genes and confers CDK4/6 inhibitor resistance in cancer. Cancer Research, 2021, 81(6): 1486-1499. DOI:10.1158/0008-5472.CAN-20-2828
|
| [101] |
ZHANG K. CDK4/6 inhibitor palbociclib enhances the effect of pyrotinib in HER2-positive breast cancer. Cancer Letters, 2019, 447: 130-140. DOI:10.1016/j.canlet.2019.01.005
|
| [102] |
KUMARASAMY V, VAIL P, NAMBIAR R, WITKIEWICZ AK, KNUDSEN ES. Functional determinants of cell cycle plasticity and sensitivity to CDK4/6 inhibition. Cancer Research, 2021, 81(5): 1347-1360. DOI:10.1158/0008-5472.CAN-20-2275
|
| [103] |
CHONG QY. A unique CDK4/6 inhibitor: current and future therapeutic strategies of abemaciclib. Pharmacological Research, 2020, 156: 104686. DOI:10.1016/j.phrs.2020.104686
|
| [104] |
DHILLON S. Trilaciclib: first approval. Drugs, 2021, 81(7): 867-874. DOI:10.1007/s40265-021-01508-y
|
| [105] |
PIEZZO M, COCCO S, CAPUTO R, CIANNIELLO D, GIOIA GD, LAURO VD, FUSCO G, MARTINELLI C, NUZZO F, PENSABENE M, de LAURENTIIS M. Targeting cell cycle in breast cancer: CDK4/6 inhibitors. International Journal of Molecular Sciences, 2020, 21(18): 6479. DOI:10.3390/ijms21186479
|
| [106] |
WANG J, ZHANG RG, LIN ZY, ZHANG S, CHEN YB, TANG J, HONG JX, ZHOU XS, ZONG Y, XU YZ, MENG R, XU SB, LIU L, ZHANG T, YANG KY, DONG XR, WU G. CDK7 inhibitor THZ1 enhances antiPD-1 therapy efficacy via the p38α/MYC/PD-L1 signaling in non-small cell lung cancer. Journal of Hematology & Oncology, 2020, 13(1): 1-16.
|
| [107] |
LI B, NI CHONGHAILE T, FAN Y, MADDEN SF, KLINGER R, O'CONNOR AE, WALSH L, O'HURLEY G, MALLYA UDUPI G, JOSEPH J, TARRANT F, CONROY E, GABER A, CHIN SF, BARDWELL HA, PROVENZANO E, CROWN J, DUBOIS T, LINN S, JIRSTROM K, et al. Therapeutic rationale to target highly expressed CDK7 conferring poor outcomes in triple-negative breast cancer. Cancer Research, 2017, 77(14): 3834-3845. DOI:10.1158/0008-5472.CAN-16-2546
|
| [108] |
LU P, GENG J, ZHANG L, WANG Y, NIU NN, FANG Y, LIU F, SHI JJ, ZHANG ZG, SUN YW, WANG LW, TANG YJ, XUE J. THZ1 reveals CDK7-dependent transcriptional addictions in pancreatic cancer. Oncogene, 2019, 38(20): 3932-3945. DOI:10.1038/s41388-019-0701-1
|
| [109] |
ZHOU Y, LU LL, JIANG GM, CHEN ZJ, LI JX, AN PP, CHEN LK, DU J, WANG HS. Targeting CDK7 increases the stability of snail to promote the dissemination of colorectal cancer. Cell Death & Differentiation, 2019, 26(8): 1442-1452.
|
| [110] |
KIM J. CDK7 is a reliable prognostic factor and novel therapeutic target in epithelial ovarian cancer. Gynecologic Oncology, 2020, 156(1): 211-221. DOI:10.1016/j.ygyno.2019.11.004
|
| [111] |
HUANG CS, XU QC, DAI CL, WANG LY, TIEN YC, LI FX, SU Q, HUANG XT, WU J, ZHAO W, YIN XY. Nanomaterial-facilitated cyclin-dependent kinase 7 inhibition suppresses gallbladder cancer progression via targeting transcriptional addiction. ACS Nano, 2021, 15(9): 14744-14755. DOI:10.1021/acsnano.1c04570
|
| [112] |
KAZI A, CHEN LW, XIANG SY, VANGIPURAPU R, YANG H, BEATO F, FANG B, WILLIAMS TM, HUSAIN K, UNDERWOOD P, FLEMING JB, MALAFA M, WELSH EA, KOOMEN J, TREVINO J, SEBTI SM. Global phosphoproteomics reveal CDK suppression as a vulnerability to KRas addiction in pancreatic cancer. Clinical Cancer Research, 2021, 27(14): 4012-4024. DOI:10.1158/1078-0432.CCR-20-4781
|
| [113] |
MARINEAU JJ, HAMMAN KB, HU SH, ALNEMY S, MIHALICH J, KABRO A, WHITMORE KM, WINTER DK, ROY S, CIBLAT S, KE N, SAVINAINEN A, WILSILY A, MALOJCIC G, ZAHLER R, SCHMIDT D, BRADLEY MJ, WATERS NJ, CHUAQUI C. Discovery of SY-5609:a selective, noncovalent inhibitor of CDK7. Journal of Medicinal Chemistry, 2022, 65(2): 1458-1480. DOI:10.1021/acs.jmedchem.1c01171
|
| [114] |
HONG HH, ZENG YM, JIAN WX, LI L, LIN LY, MO YS, LIU ML, FANG SH, XIA Y. CDK7 inhibition suppresses rheumatoid arthritis inflammation via blockage of NF-κB activation and IL-1β/IL-6 secretion. Journal of Cellular and Molecular Medicine, 2018, 22(2): 1292-1301.
|
| [115] |
PATEL H, PERIYASAMY M, SAVA GP, BONDKE A, SLAFER BW, KROLL SHB, BARBAZANGES M, STARKEY R, OTTAVIANI S, HARROD A, ABOAGYE EO, BULUWELA L, FUCHTER MJ, BARRETT AGM, COOMBES RC, ALI S. ICEC0942, an orally bioavailable selective inhibitor of CDK7 for cancer treatment. Molecular Cancer Therapeutics, 2018, 17(6): 1156-1166. DOI:10.1158/1535-7163.MCT-16-0847
|
| [116] |
HU SH, MARINEAU JJ, RAJAGOPAL N, HAMMAN KB, CHOI YJ, SCHMIDT DR, KE N, JOHANNESSEN L, BRADLEY MJ, ORLANDO DA, ALNEMY SR, REN YX, CIBLAT S, WINTER DK, KABRO A, SPROTT KT, HODGSON JG, FRITZ CC, CARULLI JP, DI TOMASO E, et al. Discovery and characterization of SY-1365, a selective, covalent inhibitor of CDK7. Cancer Research, 2019, 79(13): 3479-3491. DOI:10.1158/0008-5472.CAN-19-0119
|
| [117] |
OLSON CM. Development of a selective CDK7 covalent inhibitor reveals predominant cell-cycle phenotype. Cell Chemical Biology, 2019, 26(6): 792-803.e10. DOI:10.1016/j.chembiol.2019.02.012
|
| [118] |
ZHANG H, CHRISTENSEN CL, DRIES R, OSER MG, DENG J, DISKIN B, LI F, PAN Y, ZHANG X, YIN Y, PAPADOPOULOS E, PYON V, THAKURDIN C, KWIATKOWSKI N, JANI K, RABIN AR, CASTRO DM, CHEN T, SILVER H, HUANG Q, et al. CDK7 inhibition potentiates genome instability triggering anti-tumor immunity in small cell lung cancer. Cancer Cell, 2020, 37(1): 37-54.e9. DOI:10.1016/j.ccell.2019.11.003
|
| [119] |
CHANTKRAN W, HSIEH YC, ZHELEVA D, FRAME S, WHEADON H, COPLAND M. Interrogation of novel CDK2/9 inhibitor fadraciclib (CYC065) as a potential therapeutic approach for AML. Cell Death Discovery, 2021, 7: 137. DOI:10.1038/s41420-021-00496-y
|
| [120] |
OLSON CM, JIANG BS, ERB MA, LIANG YK, DOCTOR ZM, ZHANG ZN, ZHANG TH, KWIATKOWSKI N, BOUKHALI M, GREEN JL, HAAS W, NOMANBHOY T, FISCHER ES, YOUNG RA, BRADNER JE, WINTER GE, GRAY NS. Pharmacological perturbation of CDK9 using selective CDK9 inhibition or degradation. Nature Chemical Biology, 2018, 14(2): 163-170. DOI:10.1038/nchembio.2538
|
| [121] |
MACCALLUM DE, MELVILLE J, FRAME S, WATT K, ANDERSON S, GIANELLA-BORRADORI A, LANE DP, GREEN SR. Seliciclib (CYC202, R-roscovitine) induces cell death in multiple myeloma cells by inhibition of RNA polymerase Ⅱ-dependent transcription and down-regulation of mcl-1. Cancer Research, 2005, 65(12): 5399-5407. DOI:10.1158/0008-5472.CAN-05-0233
|
| [122] |
IURISCI I, FILIPSKI E, REINHARDT J, BACH S, GIANELLA-BORRADORI A, IACOBELLI S, MEIJER L, LÉVI F. Improved tumor control through circadian clock induction by seliciclib, a cyclin-dependent kinase inhibitor. Cancer Research, 2006, 66(22): 10720-10728. DOI:10.1158/0008-5472.CAN-06-2086
|
| [123] |
RATHOS MJ, JOSHI K, KHANWALKAR H, MANOHAR SM, JOSHI KS. Molecular evidence for increased antitumor activity of gemcitabine in combination with a cyclin-dependent kinase inhibitor, P276-00 in pancreatic cancers. Journal of Translational Medicine, 2012, 10(1): 1-11. DOI:10.1186/1479-5876-10-1
|
| [124] |
DOLMAN MEM, POON E, EBUS ME, DEN HARTOG IJM, van NOESEL CJM, JAMIN Y, HALLSWORTH A, ROBINSON SP, PETRIE K, SPARIDANS RW, KOK RJ, VERSTEEG R, CARON HN, CHESLER L, MOLENAAR JJ. Cyclin-dependent kinase inhibitor AT7519 as a potential drug for MYCN- dependent neuroblastoma. Clinical Cancer Research, 2015, 21(22): 5100-5109. DOI:10.1158/1078-0432.CCR-15-0313
|
| [125] |
WANG LG, SHAO XJ, ZHONG TB, WU Y, XU AX, SUN XY, GAO HY, LIU YB, LAN TL, TONG Y, TAO X, DU WX, WANG W, CHEN YQ, LI T, MENG XB, DENG HT, YANG B, HE QJ, YING MD, et al. Discovery of a first-in-class CDK2 selective degrader for AML differentiation therapy. Nature Chemical Biology, 2021, 17(5): 567-575. DOI:10.1038/s41589-021-00742-5
|
| [126] |
LUEDTKE DA, SU YW, MA J, LI XY, BUCK SA, EDWARDS H, POLIN LS, KUSHNER J, DZINIC SH, WHITE K, LIN H, TAUB JW, GE YB. Inhibition of CDK9 by voruciclib synergistically enhances cell death induced by the Bcl-2 selective inhibitor venetoclax in preclinical models of acute myeloid leukemia. Signal Transduction and Targeted Therapy, 2020, 5: 17. DOI:10.1038/s41392-020-0112-3
|
| [127] |
MANDAL R, BECKER S, STREBHARDT K. Targeting CDK9 for anti-cancer therapeutics. Cancers, 2021, 13(9): 2181. DOI:10.3390/cancers13092181
|
| [128] |
RICHTERS DOYLE SK, FREEMAN DB, LEE C, LEIFER BS, JAGANNATHAN S, KABINGER F, KOREN JV, STRUNTZ NB, URGILES J, STAGG RA, CURTIN BH, CHATTERJEE D, MATHEA S, MIKOCHIK PJ, HOPKINS TD, GAO H, BRANCH JR, XIN H, WESTOVER L, et al. Modulating androgen receptor- driven transcription in prostate cancer with selective CDK9 inhibitors. Cell Chemical Biology, 2021, 28(2): 134-147.e114. DOI:10.1016/j.chembiol.2020.10.001
|
| [129] |
HUANG J, CHEN P, LIU K, LIU J, ZHOU BR, WU RL, PENG Q, LIU ZX, LI CF, KROEMER G, LOTZE M, ZEH H, KANG R, TANG DL. CDK1/2/5 inhibition overcomes IFNG-mediated adaptive immune resistance in pancreatic cancer. Gut, 2021, 70(5): 890-899. DOI:10.1136/gutjnl-2019-320441
|
| [130] |
MAHDESSIAN D, CESNIK AJ, GNANN C, DANIELSSON F, STENSTRÖM L, ARIF M, ZHANG C, LE T, JOHANSSON F, SCHUTTEN R, BÄCKSTRÖM A, AXELSSON U, THUL P, CHO NH, CARJA O, UHLÉN M, MARDINOGLU A, STADLER C, LINDSKOG C, AYOGLU B, et al. Spatiotemporal dissection of the cell cycle with single-cell proteogenomics. Nature, 2021, 590(7847): 649-654. DOI:10.1038/s41586-021-03232-9
|
 2023, Vol. 39
2023, Vol. 39