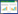中国科学院微生物研究所、中国微生物学会主办
文章信息
- 支睿, 卢艳波, 王敏, 李国辉, 邓禹
- ZHI Rui, LU Yanbo, WANG Min, LI Guohui, DENG Yu
- 生物可降解塑料单体二元羧酸的生物合成研究进展
- Recent progress in the biosynthesis of dicarboxylic acids, a monomer of biodegradable plastics
- 生物工程学报, 2023, 39(5): 2081-2094
- Chinese Journal of Biotechnology, 2023, 39(5): 2081-2094
- 10.13345/j.cjb.220944
-
文章历史
- Received: November 24, 2022
- Accepted: February 28, 2023
2. 山东渤海实业集团有限公司 山东省油脂油料精深加工技术重点实验室, 山东 滨州 256500;
3. 江南大学生物工程学院, 江苏 无锡 214122
2. Shandong Provincial Key Laboratory of Fat & Oil Deep-Processing, Shandong Bohi Industry Co., Ltd., Binzhou, 256500, Shandong, China;
3. School of Biotechnology, Jiangnan University, Wuxi 214122, Jiangsu, China
全球能源、资源当下面临严峻的挑战[1],传统的石油化工生产逐渐不能满足日益增长的需求,同时,随着社会环保意识的增强、碳中和概念的提出以及代谢工程和合成生物学的飞速发展,生物制造得到广泛关注,有望在未来成为替代传统制造方法的可行方案。有别于金属和纸类,塑料在生产上具有耗能低、材质上耐腐蚀、耐用等优点,经过几百年的发展,已经成为工业生产和日常生活中不可或缺的聚合物之一。然而,大量产生的塑料垃圾难以回收处理[2],对环境和人类健康造成了极大的破坏。生产能够被微生物(细菌、真菌、藻类)降解成为对环境无污染的物质[3]的“生物可降解塑料”已经成为未来塑料行业发展的必由之路。
二元羧酸是生物可降解材料中的重要组成部分,二元羧酸既能通过全生物方法合成,又可以作为底物被微生物降解。同时,二元羧酸在医药、服装、香料等行业也有广泛的应用[4]。然而,部分二元羧酸如中长链二元羧酸或环状二元羧酸的全生物生产目前仍不能满足工业生产的要求。
因此,本文聚焦生物可降解塑料中已应用以及有应用潜力的二元羧酸,包括直链二元羧酸(丁二酸、己二酸、十二烷二元羧酸)和环状二元羧酸(2-吡喃酮-4, 6-二元羧酸、呋喃-2, 5-二元羧酸),介绍其在生物可降解塑料中的应用、生物法合成机理以及通过代谢工程和合成生物学手段合成这些二元羧酸所取得的最新进展以及挑战。
1 直链二元羧酸 1.1 己二酸己二酸(adipic acid, AA)是商业化学品中最重要的脂肪族二元羧酸之一[5],全球的己二酸年产值超过300万t,并以3%–5%年增长率逐年上升。目前,己二酸可作为单体用于生产如聚丁二酸己二酸丁二酯[poly(butylene succinate-co-butylene adipate, PBSA]、聚对苯二甲酸己二酸丁二酯[poly(butylene adipate-co-terephthalate, PBAT)][6]等生物可降解塑料。PBSA是一种乳白至浅黄色颗粒,熔点为80−110 ℃,该聚合物具有较高的强度,较优的生物可降解性以及可加工性,被广泛应用于制造农用地膜、有机垃圾袋以及线缆等[7]。
利用全生物法生产己二酸目前主要有3种路径:逆/顺β-氧化途径结合ω-氧化途径、逆己二酸降解途径以及α-酮庚二酸途径。Clomburg等[8]通过在大肠杆菌(Escherichia coli)中过表达硫解酶(bktB)、3-羟基乙酰辅酶A脱氢酶/脱水酶(fadB)、硫解酶(ydiI)、醇脱氢酶(yjgB)、反式烯酰辅酶A (ter)、ω-羧化酶(alkBGT)、醇脱氢酶以及醛脱氢酶(chnD, chnE)的编码基因,构建逆β氧化结合ω-氧化的己二酸合成途径(图 1A),删除与己二酸合成途径竞争碳流的部分基因(fadA, tesB),以甘油为碳源合成了170 mg/L己二酸;假丝酵母(Candida spp.)可以以脂肪酸作为碳源,通过ω-氧化途径结合β-氧化途径生产二元羧酸。Beardslee等[9]以假丝酵母为底盘,删除了底物谱较宽(C4−C20)的酰基辅酶A氧化酶(POX4)防止其继续降解己二酸,保留了底物谱较窄(大于C6)的酰基辅酶A氧化酶(POX5)。最终,采用两阶段发酵策略,利用脂肪酸(椰油)作为碳源,己二酸产量达到了50 g/L。
|
| 图 1 己二酸合成途径 Fig. 1 Metabolic pathway for the production of adipic acid. A:逆/顺β-氧化途径结合ω-氧化途径. B:逆己二酸降解途径图. C:α-酮庚二酸途径 A: Combination of β/β reversal- and ω-oxidation pathway. B: Reverse adipate degradation pathway. C: α-ketopimelate pathway. |
| |
Deng等[10]通过代谢物分析发现1株嗜热放线菌(Thermobifida fusca) B6可以通过逆己二酸降解途径天然产己二酸,该途径包括6种酶:Tfu_0875 (β-酮硫解酶)、Tfu_2399 (3-羟基辅酶A脱氢酶)、Tfu_0067 (3-脱氢己二酰辅酶A脱氢酶)、Tfu_1647 (5-羰基-2-戊二烯酰辅酶A还原酶)以及Tfu_2576–Tfu_2577 (己二酰辅酶A合成酶) [11]。将上述6个基因引入大肠杆菌,通过更换启动子提高限速酶Tfu_1647的表达量,删除ldhA、atoB和sucD基因减少副产物积累。最后,己二酸产量在5 L发酵罐上达到68 g/L。此外,大肠杆菌中天然存在苯乙酸降解途径[12],途径中3个酶PaaJ、PaaH以及PaaF与嗜热放线菌中的Tfu_0875、Tfu_2399以及Tfu_0067具有相同催化功能,Cheong等[13]通过在大肠杆菌中过表达paaJ、paaH、paaF基因,引入反式烯酰辅酶A还原酶基因(ter)和乙酰基转移酶基因(acot8),构建了类似的逆己二酸降解途径(图 1B)。为提高前体物质琥珀酰辅酶A在胞内的水平,过表达辅酶A转移酶(Cat1)基因。最终,以甘油作为碳源,琥珀酸作为补加物质,在发酵罐上得到2.5 g/L己二酸。Niu等[14]通过恶臭假单胞菌(Pseudomonas putida)中内源的木质素降解途径结合逆己二酸降解途径实现了己二酸的合成。以木质素衍生物二羟基苯甲酸和邻苯二酚为碳源,首先通过木质素降解途径合成3-酮己二酸,再通过辅酶A转移酶(PcalJ)的催化形成3-酮己二酰辅酶A,再经逆己二酸降解途径中的3-羟基辅酶A脱氢酶(3-hydroxycoenzyme A dehydrogenase) PaaH、3-脱氢己二酰辅酶A脱氢酶(PaaF)、5-羰基-2-戊二烯酰辅酶A还原酶(TER)催化合成己二酸。最终,以4-羟基苯甲酸和葡萄糖作为碳源,经过补料分批发酵,己二酸的产量达到2.5 g/L。
在产甲烷古菌的辅酶B合成途径中,α-酮戊二酸可以通过一系列碳链延伸反应形成α-酮己二酸、α-酮庚二酸以及α-酮辛二酸[15-16],而α-酮庚二酸可以通过脱羧酶催化形成己二酸半醛,进而再通过转氨酶催化形成6-氨基己酸。Turk等[17]从该途径出发,选择大肠杆菌为底盘,通过过表达高柠檬酸合成酶(NifV)、高柠檬酸脱水酶、顺高乌头酸脱水酶、苏式异高柠檬酸脱氢酶(AksD、AksE、AksF)、α-酮庚二酸脱羧酶(KdcA)以及转氨酶(Vfl)基因,构建了1条6-氨基己酸的合成路线。然而其副产物己二酸的产量(0.32 g/L)远远高于目标产物6-氨基己酸(0.02 g/L),可能是大肠杆菌中内源的半醛脱氢酶催化己二酸半醛更多的转化为了己二酸,但酶的具体信息未知(图 1C)。
利用假丝酵母(Candida)中的ω-氧化途径结合β-氧化途径生产己二酸实现了工业化,但生物基己二酸的工业化仍然面临较大的挑战。首先,大多生物基生产己二酸普遍利用诱导型方法,诱导剂在发酵过程中的使用会大大提高生产成本[18];同时,由于己二酸合成途径普遍较长,因此实现从底物(葡萄糖、甘油等)到己二酸的合成需经多步反应,中间体较难分析,大大增加了代谢改造的难度。近些年来,通过利用模块化[19-20]、全细胞催化[21-22]、动态调控[23]等方法生产二元羧酸取得了相当的进步,通过这些方法,应当能够进一步提高己二酸的产量。
1.2 丁二酸丁二酸(succinic acid, SA)是一种在工业生产中占有重要地位的二元羧酸,可作为多种生物可降解塑料的前体物质,例如聚丁二酸丁二酯[poly(butylene), PBS)]和聚丁二酸-己二酸丁二酯(PBSA)。PBS是一种白色半结晶型聚合物,结晶化温度为75 ℃,结晶度范围为30%−60%,熔点为115 ℃,具有可加工性强、柔性好以及可降解性强等优点[24]。丁二酸可通过化学方式和生物方式合成,因代谢途径简单、较易进行改造以及产量较高,生物基己二酸已经在近些年投入工业化生产[7]。
丁二酸作为三羧酸循环中的中间体,由于其合成途径相较于其他长链二元羧酸较易解析,加之易于利用葡萄糖、甘油以及乙酸等成本较低的底物进行直接合成[25-27],吸引了大量研究者的关注[28]。其生物合成途径主要有3种:利用三羧酸循环(tricarboxylic acid, TCA)、还原性TCA途径以及乙醛酸途径[29]。以曼氏产琥珀酸菌(Mannheimia succiniciproducens)中的途径为例,葡萄糖或甘油通过糖酵解途径或甘油代谢途径转化为磷酸烯醇式丙酮酸后,经过磷酸烯醇式丙酮酸羧化酶转化为草酰乙酸后进入还原性TCA途径(无氧),苹果酸经苹果酸脱氢酶转化为富马酸,最后经过富马酸还原酶形成丁二酸[30]。曼氏产琥珀酸菌是一种从牛胃中分离出的,嗜二氧化碳、兼性厌氧的细菌。Ahn等[31]在1株高产琥珀酸的曼氏产琥珀酸杆菌中过表达顺-反式异构酶基因(cis-trans isomerase, cti)以及信号肽基因(pelB),提升细胞膜中的反式非饱和脂肪酸含量,进一步提高了该菌株对丁二酸的耐受性。最终,以葡萄糖和甘油为碳源,通过分批补料发酵,丁二酸的产量、产率以及生产强度分别达到97.1 g/L、1.26 mol/mol葡萄糖和3.01 g/(L·h)。虽然曼氏产琥珀酸菌、产琥珀酸放线杆菌(Actinobacillus succinogenes)、产琥珀酸厌氧螺菌(Anaerobiospirillum succiniciproducens)等琥珀酸天然生产菌在琥珀酸产量、产率以及生产强度上具有较大的优势,但因遗传背景不清晰,缺乏相应的代谢改造工具。
而非天然产琥珀酸菌如大肠杆菌[32]、谷氨酸棒状杆菌(Corynebacterium glutamate) [33-34]、酿酒酵母(Saccharomyces cerevisiae) [35]、解脂耶氏酵母(Yarrowia lipolytica) [36]等,一般都会同时利用3条路径(有氧TCA循环、无氧TCA循环以及乙醛酸途径)积累琥珀酸[32]。Chung等[37]通过对1株谷氨酸棒杆菌进行转录组分析确定了1种氧化应激反应途径中的负反馈调节蛋白基因Ncgl0275,通过过表达该基因解除了琥珀酸的终产物抑制。随后,通过过表达内源的丙酮酸羧化酶基因(pycP458S)加强丙酮酸到草酰乙酸的转化、过表达源自曼氏产琥珀酸菌和内源的磷酸烯醇式丙酮酸羧基酶基因(pck, ppc)以增强磷酸烯醇式丙酮酸到草酰乙酸的转化。最终,通过两阶段发酵丁二酸的产量、产率以及生产强度分别达到152.2 g/L、1.1 g/g葡萄糖和1.1 g/(L·h)。
近些年生物法生产丁二酸已经实现工业化,后续的研究需要关注于生物基丁二酸生产在发酵扩大过程中可能出现的基因异质性现象。异质性现象是由于代谢负担、细胞个体差异性所导致的一种现象,该现象可能会使代谢产物的产量、产率以及生产强度大幅下降[38]。在工业生产过程中,这是一个容易被忽视但却十分重要的参数。通过两阶段发酵,基因元件控制或通过高通量筛选获得高产菌株[39-41]等策略,可以避免异质性的出现,从而使丁二酸生产菌株始终维持较好的生产性能。
1.3 十二烷二元酸十二烷二元酸(dodecanedioic acid, DDCA)是一种长链二元羧酸,可用于制造长链脂肪族聚合物(aliphatic long-chain polyester, ALCPE)。长链脂肪族聚合物含有长链脂肪族(碳原子数大于12)单体,因此在晶体结构、理化性质等方面与聚乙烯(polyethylene, PE)类似,聚乙烯在高分子量下呈现为乳白色蜡状颗粒或粉末,熔点为85–110 ℃,主要用于制造薄膜、管道、容器等。不同于聚乙烯,该聚合物几乎拥有全反式构象,其分子排列与单斜晶系或正斜晶系单元格一致,因此赋予其更高的熔点以及结晶能力[42-43],同时,其化学结构含有一定数量的酯键,因此长链脂肪族聚合物具有较聚乙烯更好的降解性以及与其他极性聚合物结合的相容性[44]。
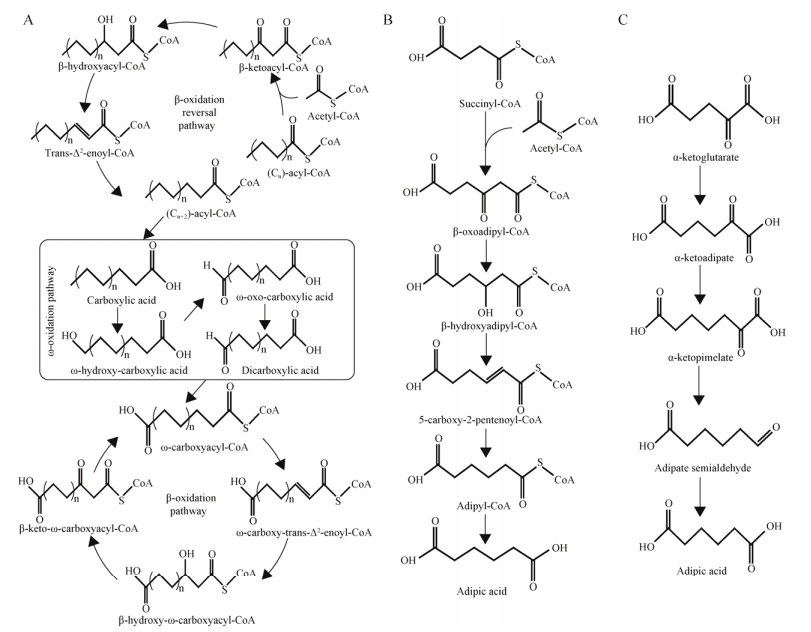
十二烷二元酸可以通过ω-氧化途径以及脂肪酸合成途径产生(图 2),并且其生物基生产多依赖于利用长链脂肪酸如非洲植物斑鸠菊油[45]、棕榈仁油以及椰子油[46]。热带假丝酵母(Candida tropicalis)天然的ω-氧化途径,Picataggio等[47]敲除编码酰基辅酶A氧化酶的基因(pox4, pox5),以十二烷为唯一碳源,令十二烷二元酸的产量、产率以及生产强度在15 L发酵罐分别达到140 g/L、0.8 g/g十二烷脂肪酸和0.9 g/(L·h)。
|
| 图 2 结合脂肪酸合成途径和ω-氧化途径的十二烷二元羧酸合成途径 Fig. 2 Metabolic pathway for the production of dodecanedioic acid that combines fatty acid synthesis pathway and ω-oxidation pathway. |
| |
为降低长链二元羧酸的生产成本,利用便宜易得的底物来进行生产势在必行。以葡萄糖为碳源,Bowen等[48]结合大肠杆菌中的脂肪酸合成途径以及ω-氧化途径,实现了十二烷二元酸的合成。在该过程中,葡萄糖经过糖酵解等途径形成乙酰辅酶A,经Ⅱ型脂肪酸合成酶复合体合成十二烷酰基ACP,再经过高选择性的酰基ACP硫解酶形成十二烷酸,通过细胞色素P450单加氧酶催化形成ω-十二烷羟基酸,再经醇脱氢酶形成ω-十二烷醛基酸,最后经醛脱氢酶催化后形成十二烷二元酸。Bowen等[48]通过过表达酰基ACP硫解酶基因(fatB2)、细胞色素P450单加氧酶基因(CPR2mut)、醇脱氢酶基因(alkJ)以及醛脱氢酶基因(puuC)构建了该途径。最终,通过发酵条件优化,十二烷二元酸的产量、产率以及生产强度分别达567 mg/L、0.038 g/g葡萄糖和0.026 g/(L·h)。
2 环状二元羧酸 2.1 2-吡喃酮-4, 6-二羧酸2-吡喃酮-4, 6-二羧酸(2-pyrone-4, 6-dicarboxylic acid, PDC)因其结构上与对苯二甲酸类似,有极大潜力在未来作为其替代产品。而对苯二甲酸是目前应用最广泛的塑料制品聚乙烯对苯二甲酸(polyethylene terephthalate, PET)的单体之一,PET呈颗粒状,熔点为250–255 ℃,大量用于制作纤维、电子电器等。与对苯二甲酸不同的是,PDC结构上的极性类苯环结构可以赋予聚合材料以较强的粘合性、硬度与延展性。更重要的是,PDC是多种土壤细菌的芳香族化合物降解途径中间体,因此可以预见相比于同类聚合物,PDC聚合材料的生物可降解性将大大加强。目前PDC的生产无法通过化学方法合成[49],只有通过生物法才可以实现PDC的合成。
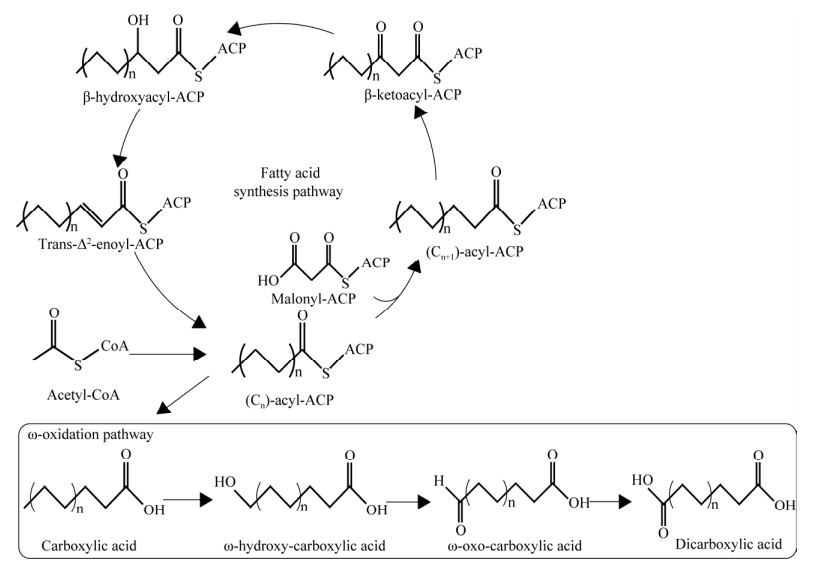
鞘氨醇单胞菌SYK-6 (Sphingobium sp.) SYK-6是一种从纸浆废液中获得,可以将木质素中低分子量物质通过内源途径降解的革兰氏阴性菌[50-52],通过这种降解途径,鞘氨醇单胞菌SYK-6可以将多种木质素衍生物转化为多种高值化合物,而PDC就是其中一种。该菌能将木质素中的低分子量物质通过一系列酶催化反应转化为香草醛、香兰酸以及丁香酸这3个重要的中间体。从这3种中间体出发,香草醛和香兰酸通过香草酸脱甲基酶催化形成原儿茶酸,再经原儿茶酸-4, 5-双氧酶催化形成4-羧基粘康酸-6-半醛,该中间体经过1个非酶催化的反应转化为其半缩醛形式之后,通过4-羧基-2-羟基粘康酸-6-半醛脱氢酶催化最终形成2-吡喃酮-4, 6-二羧酸;区别于上述2种化合物的转化过程,丁香酸首先通过丁香酸脱甲基酶形成没食子酸甲酯,再通过没食子酸甲酯-3, 4-双加氧酶催化最终形成2-吡喃酮-4, 6-二羧酸(图 3A)。目前,已报道的可用于生产PDC宿主主要有恶臭假单胞菌(Pseudomonas putida) [53]、大肠杆菌(Escherichia coli) [54-55]、嗜芳烃新鞘氨醇菌(Novosphingobium aromaticivorans) [56]以及本氏烟草(Nicotiana benthamiana) [49]。
|
| 图 3 2-吡喃酮-4, 6-二元羧酸合成途径 Fig. 3 Metabolic pathway for the production of 2-pyrone-4, 6-dicarboxylic acid. A:鞘氨醇单胞菌SYK-6 (Sphingobium sp. SYK-6)内源木质素降解途径. B:莽草酸途径. 3-MGA:没食子酸甲酯;CHMOD:4-羧基-2-羟基-6-甲氧基-6-氧代己-2, 4-二烯酸酯;DAHP:7-磷酸-d-阿拉伯庚酮糖;DHQ:3-脱氢奎尼酸;DHS:3-脱氢莽草酸;CA:分支酸;pHBA:4-羟基苯甲酸;PCA:原儿茶酸;CHMS:4-羧基-2-羟基-顺式, 顺式-粘康酸-6-半醛 A: Native-occurring lignin degradation pathway of Sphingobium sp. SYK-6. B: Shikimate pathway. 3-MGA: 3-methygallate; CHMOD: 4-carboxy-2-hydroxy-6-methoxy-6-oxohexa-2, 4-dienoate; DAHP: 2-dehydro-3-deoxy-d-arabinoheptonate-7-phosphate; DHQ: 3-dehyroquinate; DHS: 3-dehydroshikimate; CA: Chorismate; pHBA: p-hydroxybenzoic acid; PCA: Protocatechuic acid; CHMS: 4-carboxy-2-hydroxy-cis, cis-muconate-6-semialdehyde. |
| |
Otsuka等[53]将鞘氨醇菌SYK-6中的原儿茶酸-4, 5-双加氧酶和4-羧基-2-羟基粘康酸-6-半醛脱氢酶(LigAB, LigC)引入恶臭假单胞菌PpY1100中,以葡萄糖和原儿茶酸为碳源和补加物质,用LB培养基,使PDC产量在5 L发酵罐中达到10 g/L。为进一步降低底物成本,Nakajima等[54]过表达了大肠杆菌的莽草酸途径并结合鞘氨醇单胞菌中的木质素降解途径,构建了多种从葡萄糖直接生产PDC的合成路线(图 3B)。通过过表达3-脱氢奎尼酸合成酶(AroB)、3-脱氧-7-磷酸阿拉伯庚酮糖合成酶(AroFfbr)、3-脱氢莽草酸脱水酶(QutC)、分支酸丙酮酸裂解酶(UbiC)、4-羟基苯甲酸羟基酶(PobA)、原儿茶酸-4, 5-双加氧酶(LigAB)以及4-羧基-2-羟基粘康酸-6-半醛脱氢酶(LigC)基因,合成了173 mg/L PDC。Luo等[55]通过流量响应分析,以大肠杆菌为宿主,将qutC更换为源自苏云金芽孢杆菌(Bacillus thuringiensis)的asbF基因,将ligAB以及ligC基因更换为源自睾丸酮从毛单胞菌(Comamonas testosteroni) ATCC 11996的pmdABC基因[57]。同时,为加强PDC途径的碳流,过表达了3-脱氢莽草酸的转运蛋白ShiA基因,通过启动子替换和基因敲除等手段,PDC的产量在摇瓶水平达到2.21 g/L。最终,通过优化溶氧和pH等发酵条件,PDC的产量、产率以及生产强度在5 L发酵罐上分别达到16.72 g/L、0.201 g/g葡萄糖和0.172 g/(L·h),是目前已报道的PDC的最高产量。
吡喃酮-4, 6-二元羧酸同己二酸生物合成途径相似,其代谢途径也较长,但目前的研究多通过T7启动子表达其代谢途径,这可能会破坏途径基因协调表达的平衡[58],导致产量下降。后续或许可以通过更换不同强度诱导型启动子、不同强度的RBS位点或更换不同拷贝数的复制起点,从不同角度来更好地调控途径基因的表达模式,或通过进一步删除与目标产物竞争碳流或对其有反馈抑制的基因、辅因子工程等代谢工程手段,以提高PDC的产量。
2.2 呋喃-2, 5-二元羧酸呋喃-2, 5-二元羧酸(furan-2, 5-dicarboxylic acid, FDCA)因其有着广泛的应用范围被归为12种可用于生物基生产的平台化合物[59],可用于生产化学品、调料、聚合物、塑料、聚胺、聚酯的单体等。因FDCA在结构上(呋喃环)与对苯二甲酸的苯环相近,被认为有望在未来可以替代这种在塑料单体中广泛存在的石油化工基化学品。
在微生物中,FDCA可以通过5-羟甲基呋喃甲醛(5-hydroxymethyl furfural, HMF)降解途径产生。羟甲基呋喃是木质纤维素降解产生的副产物之一,有较强的毒性[60-61],而一些微生物如细菌[62-63]以及真菌[62-63]天然拥有将羟甲基呋喃甲醛脱毒(降解)的途径,FDCA是这类途径的中间产物之一[64]。在原核生物中,FDCA通过HMF降解形成通常有2条路径:在第1条路径中,HMF可以在周质空间中通过脱氢酶、氧化酶或者过氧化物酶完成到FDCA的转化,其中间体分别为5-羟甲基呋喃甲醛酸、5-甲酰-2-呋喃羧酸;在第2条路径中,HMF可以在细胞膜内通过上述3种酶的催化形成FDCA,其反应中间体为2, 5-呋喃双甲醛以及5-甲酰-2-呋喃羧酸(图 4)。真核生物降解HMF至FDCA的途径以及酶与原核生物基本相同,但降解位置有所不同,主要发生在细胞膜内或过氧化物酶体中[64]。
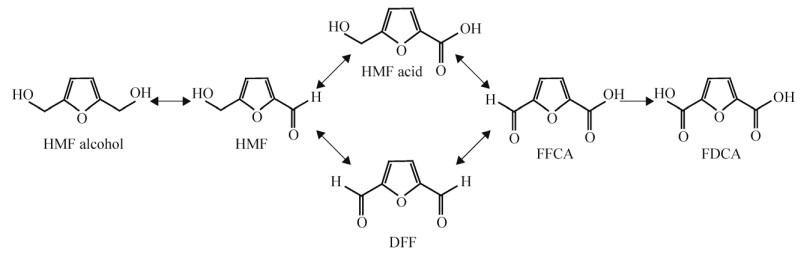
|
| 图 4 呋喃-2, 5-二元羧酸合成途径 Fig. 4 Metabolic pathway for the production of furan-2, 5-dicarboxylic acid. DFF:2, 5-呋喃双甲醛;FFCA:5-甲醛-2-呋喃羧酸 DFF: 2, 5-diformylfuran; FFCA: 5-formyl-2-furancarboxylic acid. |
| |
贪铜菌(Cupriavidus basilensis) HMF14是一种从土壤分离出的革兰氏阴性菌[61],是第1株被报道的天然拥有HMF降解途径的微生物[65],该降解途径基因簇hmfABCDE以及hmfFGH'H在表达后[60]可将HMF或呋喃甲醛完全降解用于菌体生长等细胞活动,而编码该降解途径氧化还原酶hmfH基因则与形成FDCA高度相关。Koopman等[66]将该基因引入恶臭假单胞菌S12中,以甘油和HMF作为碳源,通过在5 L发酵罐上优化甘油和HMF补加速率,使FDCA产量在144 h达到了30.1 g/L。为提高FDCA的产量、产率以及生产强度,Yuan等[67]将解鸟氨酸拉乌尔菌(Raoultella ornithinolytica) BF60作为宿主,引入HMF/呋喃甲醛氧化还原酶(HmfH)以及HMF氧化酶(HmfO),将它们置于不同强度启动子(Ptrc, Ptac)以及高中低拷贝数的质粒(pRSF、pCDF、pACYC)下进行组合以优化基因表达;随后通过替换不同强度启动子以及删除碳流竞争路径等手段,使FDCA产量达到了34.57 g/L。因为副产物HMF醇仍然存在,Yuan等[68]通过转录组分析,进一步进行基因改造使HMF醇的产量降低到了0.21 mmol/L。同时,根据转录组分析发现醛脱氢酶(aldehyde dehydrogenase, AldH)与催化HMF酸形成FDCA高度相关,通过过表达该基因,FDCA产量达到41.31 g/L。
HMF,呋喃-2, 5-二元羧酸生物合成途径的重要底物之一,是一种发酵抑制物[69],对菌株的生长可能会有一定影响。未来或许可以通过细胞膜工程手段等方式,提高菌株对其的耐受性以及代谢途径中间体的传递效率。另外,目前呋喃-2, 5-二元羧酸的生物合成途径还较为单一,产量、产率以及生产强度也尚未达到工业化的标准,亟需通过更多的合成生物学以及代谢工程手段以进一步提高。
3 总结与展望本文总结了在生物可降解塑料中已有广泛应用的丁二酸、己二酸以及具有潜在应用前景的十二烷二元羧酸、呋喃-2, 5-二元羧酸以及2-吡喃酮-4, 6-二元羧酸的生物合成途径以及代谢改造进展(表 1)。除丁二酸已经工业化,可以作为单体生产生物可降解塑料以外,目前仅有十二烷二元羧酸以及己二酸的生物合成水平较高,有较大潜力作为单体生产对应的生物可降解塑料。但十二烷二元羧酸生物合成的底物价格相对较高,而利用葡萄糖等较便宜底物进行生物合成产量尚未达到工业化水平,仍需进一步进行改造。而己二酸生物合成目前仍需要诱导表达才能够达到较高的产量。总的来说,除丁二酸外,其他的二元羧酸的全生物合成方法由于途径复杂、改造困难等原因,亟需对底盘宿主、代谢途径以及发酵策略开展更有效地改造,来显著提高产量、产率以及生产强度。同时,也需要对下游提取工艺进行优化,提高产品纯度,更好地作为单体应用于生物可降解塑料的合成。
| Product | Titer (g/L) | Yield (g/g) | Productivity (g/(L·h)) | Host | Reference |
| Succinic acid | 97.1 | 0.80 | 3.0 | M. succiniciproducens | [31] |
| 152.2 | 1.10 | 1.1 | C. glutamicum | [37] | |
| 134.2 | 0.82 | 21.3 | M. succiniciproducens | [70] | |
| Adipic acid | 50.0 | 0.70 | 0.4 | Candida spp. | [9] |
| 57.6 | n.r. | 0.4 | E. coli | [18] | |
| 68.0 | 0.40 | 0.9 | E. coli | [11] | |
| Dodecanedioic acid | 140.0 | 0.80 | 0.9 | C. tropicalis | [47] |
| 0.6 | 0.04 | 0.03 | E. coli | [48] | |
| 2-pyrone-4, 6-dicarboxylic acid | 10.0 | n.r. | 0.3 | S. paucimobilis | [53] |
| 16.7 | 0.20 | 0.2 | E. coli | [55] | |
| Furan-2, 5-dicarboxylic acid | 34.6 | 0.20 | 0.3 | R. ornithinolytica | [67] |
| 41.3 | 1.00 | 0.3 | R. ornithinolytica | [68] | |
| n.r.: Not reported. | |||||
| [1] |
ZHANG QN, SONG MZ, XU YY, WANG WC, WANG Z, ZHANG LQ. Bio-based polyesters: recent progress and future prospects. Progress in Polymer Science, 2021, 120: 101430. DOI:10.1016/j.progpolymsci.2021.101430
|
| [2] |
RHODES CJ. Plastic pollution and potential solutions. Science Progress, 2018, 101(3): 207-260. DOI:10.3184/003685018X15294876706211
|
| [3] |
PELLIS A, HERRERO ACERO E, GARDOSSI L, FERRARIO V, GUEBITZ GM. Renewable building blocks for sustainable polyesters: new biotechnological routes for greener plastics. Polymer International, 2016, 65(8): 861-871. DOI:10.1002/pi.5087
|
| [4] |
LI WN, SHEN XL, WANG J, SUN XX, YUAN QP. Engineering microorganisms for the biosynthesis of dicarboxylic acids. Biotechnology Advances, 2021, 48: 107710. DOI:10.1016/j.biotechadv.2021.107710
|
| [5] |
BART JCJ, CAVALLARO S. Transiting from adipic acid to bioadipic acid. 1, petroleum-based processes. Industrial & Engineering Chemistry Research, 2015, 54(1): 1-46.
|
| [6] |
POLEN T, SPELBERG M, BOTT M. Toward biotechnological production of adipic acid and precursors from biorenewables. Journal of Biotechnology, 2013, 167(2): 75-84. DOI:10.1016/j.jbiotec.2012.07.008
|
| [7] |
LETCHER TM. Plastic Waste and Recycling: Environmental Impact, Societal Issues, Prevention, and Solutions. Amsterdam: Academic Press, 2020.
|
| [8] |
CLOMBURG JM, BLANKSCHIEN MD, VICK JE, CHOU A, KIM S, GONZALEZ R. Integrated engineering of β-oxidation reversal and ω-oxidation pathways for the synthesis of medium chain ω-functionalized carboxylic acids. Metabolic Engineering, 2015, 28: 202-212. DOI:10.1016/j.ymben.2015.01.007
|
| [9] |
BEARDSLEE T, PICATAGGIO S. Bio-based adipic acid from renewable oils. Lipid Technology, 2012, 24(10): 223-225. DOI:10.1002/lite.201200230
|
| [10] |
DENG Y, MAO Y. Production of adipic acid by the native-occurring pathway in Thermobifida fusca B6. Journal of Applied Microbiology, 2015, 119(4): 1057-1063. DOI:10.1111/jam.12905
|
| [11] |
ZHAO M, HUANG DX, ZHANG XJ, KOFFAS MAG, ZHOU JW, DENG Y. Metabolic engineering of Escherichia coli for producing adipic acid through the reverse adipate-degradation pathway. Metabolic Engineering, 2018, 47: 254-262. DOI:10.1016/j.ymben.2018.04.002
|
| [12] |
TEUFEL R, MASCARAQUE V, ISMAIL W, VOSS M, PERERA J, EISENREICH W, HAEHNEL W, FUCHS G. Bacterial phenylalanine and phenylacetate catabolic pathway revealed. Proceedings of the National Academy of Sciences of the United States of America, 2010, 107(32): 14390-14395. DOI:10.1073/pnas.1005399107
|
| [13] |
CHEONG S, CLOMBURG JM, GONZALEZ R. Energy- and carbon-efficient synthesis of functionalized small molecules in bacteria using non-decarboxylative claisen condensation reactions. Nature Biotechnology, 2016, 34(5): 556-561. DOI:10.1038/nbt.3505
|
| [14] |
NIU W, WILLETT H, MUELLER J, HE XY, KRAMER L, MA B, GUO JT. Direct biosynthesis of adipic acid from lignin-derived aromatics using engineered Pseudomonas putida KT2440. Metabolic Engineering, 2020, 59: 151-161. DOI:10.1016/j.ymben.2020.02.006
|
| [15] |
DREVLAND RM, JIA YH, PALMER DRJ, GRAHAM DE. Methanogen homoaconitase catalyzes both hydrolyase reactions in coenzyme B biosynthesis. Journal of Biological Chemistry, 2008, 283(43): 28888-28896. DOI:10.1074/jbc.M802159200
|
| [16] |
HOWELL DM, HARICH K, XU HM, WHITE RH. α-keto acid chain elongation reactions involved in the biosynthesis of coenzyme B (7-mercaptoheptanoyl threonine phosphate) in methanogenic archaea. Biochemistry, 1998, 37(28): 10108-10117. DOI:10.1021/bi980662p
|
| [17] |
TURK SCHJ, KLOOSTERMAN WP, NINABER DK, KOLEN KPAM, KNUTOVA J, SUIR E, SCHÜRMANN M, RAEMAKERS-FRANKEN PC, MÜLLER M, de WILDEMAN SMA, RAAMSDONK LM, van der POL R, WU L, TEMUDO MF, van der HOEVEN RAM, AKEROYD M, van der STOEL RE, NOORMAN HJ, BOVENBERG RAL, TREFZER AC. Metabolic engineering toward sustainable production of nylon-6. ACS Synthetic Biology, 2016, 5(1): 65-73. DOI:10.1021/acssynbio.5b00129
|
| [18] |
ZHOU Y, ZHAO M, ZHOU SH, ZHAO YY, LI GH, DENG Y. Biosynthesis of adipic acid by a highly efficient induction-free system in Escherichia coli. Journal of Biotechnology, 2020, 314/315: 8-13. DOI:10.1016/j.jbiotec.2020.03.011
|
| [19] |
LI WN, MA L, SHEN XL, WANG J, FENG Q, LIU LX, ZHENG GJ, YAN YJ, SUN XX, YUAN QP. Targeting metabolic driving and intermediate influx in lysine catabolism for high-level glutarate production. Nature Communications, 2019, 10(1): 3337. DOI:10.1038/s41467-019-11289-4
|
| [20] |
QIN JF, KRIVORUCHKO A, JI BY, CHEN Y, KRISTENSEN M, ÖZDEMIR E, KEASLING JD, JENSEN MK, NIELSEN J. Engineering yeast metabolism for the discovery and production of polyamines and polyamine analogues. Nature Catalysis, 2021, 4(6): 498-509. DOI:10.1038/s41929-021-00631-z
|
| [21] |
WANG F, ZHAO J, LI Q, YANG J, LI RJ, MIN J, YU XJ, ZHENG GW, YU HL, ZHAI C, ACEVEDO-ROCHA CG, MA LX, LI AT. One-pot biocatalytic route from cycloalkanes to α, ω-dicarboxylic acids by designed Escherichia coli consortia. Nature Communications, 2020, 11(1): 5035. DOI:10.1038/s41467-020-18833-7
|
| [22] |
BRETSCHNEIDER L, HEUSCHKEL I, BÜHLER K, KARANDE R, BÜHLER B. Rational orthologous pathway and biochemical process engineering for adipic acid production using Pseudomonas taiwanensis VLB120. Metabolic Engineering, 2022, 70: 206-217. DOI:10.1016/j.ymben.2022.01.014
|
| [23] |
HAO TT, LI GH, ZHOU SH, DENG Y. Engineering the reductive TCA pathway to dynamically regulate the biosynthesis of adipic acid in Escherichia coli. ACS Synthetic Biology, 2021, 10(3): 632-639. DOI:10.1021/acssynbio.0c00648
|
| [24] |
RAFIQAH SA, KHALINA A, HARMAEN AS, TAWAKKAL IA, ZAMAN K, ASIM M, NURRAZI MN, LEE CH. A review on properties and application of bio-based poly(butylene succinate). Polymers (Basel), 2021, 13(9): 1436. DOI:10.3390/polym13091436
|
| [25] |
LI YJ, HUANG B, WU H, LI ZM, YE Q, ZHANG YHP. Production of succinate from acetate by metabolically engineered Escherichia coli. ACS Synthetic Biology, 2016, 5(11): 1299-1307. DOI:10.1021/acssynbio.6b00052
|
| [26] |
LI Q, HUANG B, WU H, LI ZM, YE Q. Efficient anaerobic production of succinate from glycerol in engineered Escherichia coli by using dual carbon sources and limiting oxygen supply in preceding aerobic culture. Bioresource Technology, 2017, 231: 75-84. DOI:10.1016/j.biortech.2017.01.051
|
| [27] |
LI Q, WU H, LI ZM, YE Q. Enhanced succinate production from glycerol by engineered Escherichia coli strains. Bioresource Technology, 2016, 218: 217-223. DOI:10.1016/j.biortech.2016.06.090
|
| [28] |
BOZELL JJ, PETERSEN GR. Technology development for the production of biobased products from biorefinery carbohydrates—the US department of energy's "top 10" revisited. Green Chemistry, 2010, 12(4): 539-554. DOI:10.1039/b922014c
|
| [29] |
WITTMANN C, LIAO J. Industrial Biotechnology: Products and Processes. Weinheim: Wiley-VCH Verlag GmbH & Co. KgaA, 2017.
|
| [30] |
ZHU LW, TANG YJ. Current advances of succinate biosynthesis in metabolically engineered Escherichia coli. Biotechnology Advances, 2017, 35(8): 1040-1048. DOI:10.1016/j.biotechadv.2017.09.007
|
| [31] |
AHN JH, LEE JA, BANG J, LEE SY. Membrane engineering via trans-unsaturated fatty acids production improves succinic acid production in Mannheimia succiniciproducens. Journal of Industrial Microbiology and Biotechnology, 2018, 45(7): 555-566. DOI:10.1007/s10295-018-2016-6
|
| [32] |
SAWISIT A, JANTAMA K, ZHENG HB, YOMANO LP, YORK SW, SHANMUGAM KT, INGRAM LO. Mutation in galP improved fermentation of mixed sugars to succinate using engineered Escherichia coli AS1600A and AM1 mineral salts medium. Bioresource Technology, 2015, 193: 433-441. DOI:10.1016/j.biortech.2015.06.108
|
| [33] |
LITSANOV B, BROCKER M, BOTT M. Toward homosuccinate fermentation: metabolic engineering of Corynebacterium glutamicum for anaerobic production of succinate from glucose and formate. Applied and Environmental Microbiology, 2012, 78(9): 3325-3337. DOI:10.1128/AEM.07790-11
|
| [34] |
ZHU NQ, XIA HH, WANG ZW, ZHAO XM, CHEN T. Engineering of acetate recycling and citrate synthase to improve aerobic succinate production in Corynebacterium glutamicum. PLoS One, 2013, 8(4): e60659. DOI:10.1371/journal.pone.0060659
|
| [35] |
LI JJ, LI YK, CUI ZY, LIANG QF, QI QS. Enhancement of succinate yield by manipulating NADH/NAD+ ratio and ATP generation. Applied Microbiology and Biotechnology, 2017, 101(8): 3153-3161. DOI:10.1007/s00253-017-8127-6
|
| [36] |
CUI ZY, GAO CJ, LI JJ, HOU J, LIN CSK, QI QS. Engineering of unconventional yeast Yarrowia lipolytica for efficient succinic acid production from glycerol at low pH. Metabolic Engineering, 2017, 42: 126-133. DOI:10.1016/j.ymben.2017.06.007
|
| [37] |
CHUNG SC, PARK JS, YUN JE, PARK JH. Improvement of succinate production by release of end-product inhibition in Corynebacterium glutamicum. Metabolic Engineering, 2017, 40: 157-164. DOI:10.1016/j.ymben.2017.02.004
|
| [38] |
RUGBJERG P, SOMMER MOA. Overcoming genetic heterogeneity in industrial fermentations. Nature Biotechnology, 2019, 37(8): 869-876. DOI:10.1038/s41587-019-0171-6
|
| [39] |
CERONI F, BOO A, FURINI S, GOROCHOWSKI TE, BORKOWSKI O, LADAK YN, AWAN AR, GILBERT C, STAN GB, ELLIS T. Burden-driven feedback control of gene expression. Nature Methods, 2018, 15(5): 387-393. DOI:10.1038/nmeth.4635
|
| [40] |
DAHL RH, ZHANG FZ, ALONSO-GUTIERREZ J, BAIDOO E, BATTH TS, REDDING-JOHANSON AM, PETZOLD CJ, MUKHOPADHYAY A, LEE TS, ADAMS PD, KEASLING JD. Engineering dynamic pathway regulation using stress-response promoters. Nature Biotechnology, 2013, 31(11): 1039-1046. DOI:10.1038/nbt.2689
|
| [41] |
WEHRS M, TANJORE D, ENG T, LIEVENSE J, PRAY TR, MUKHOPADHYAY A. Engineering robust production microbes for large-scale cultivation. Trends in Microbiology, 2019, 27(6): 524-537. DOI:10.1016/j.tim.2019.01.006
|
| [42] |
STEMPFLE F, ORTMANN P, MECKING S. Which polyesters can mimic polyethylene?. Macromolecular Rapid Communications, 2013, 34(1): 47-50. DOI:10.1002/marc.201200611
|
| [43] |
LIU C, LIU F, CAI JL, XIE WC, LONG TE, TURNER SR, LYONS A, GROSS RA. Polymers from fatty acids: poly(ω-hydroxyl tetradecanoic acid) synthesis and physico-mechanical studies. Biomacromolecules, 2011, 12(9): 3291-3298. DOI:10.1021/bm2007554
|
| [44] |
ZHOU CF, WEI ZY, YU Y, SHAO SN, LENG XF, WANG YS, LI Y. Biobased long-chain aliphatic polyesters of 1, 12-dodecanedioic acid with a variety of diols: odd-even effect and mechanical properties. Materials Today Communications, 2019, 19: 450-458. DOI:10.1016/j.mtcomm.2019.05.005
|
| [45] |
AYORINDE FO, POWERS FT, STREETE LD, SHEPARD RL, TABI DN. Synthesis of dodecanedioic acid from Vernonia galamensis oil. Journal of the American Oil Chemists' Society, 1989, 66(5): 690-692. DOI:10.1007/BF02669953
|
| [46] |
CAO WF, LI HB, LUO JQ, YIN JX, WAN YH. High-level productivity of α, ω-dodecanedioic acid with a newly isolated Candida viswanathii strain. Journal of Industrial Microbiology and Biotechnology, 2017, 44(8): 1191-1202. DOI:10.1007/s10295-017-1948-6
|
| [47] |
PICATAGGIO S, ROHRER T, DEANDA K, LANNING D, REYNOLDS R, MIELENZ J, EIRICH LD. Metabolic engineering of Candida tropicalis for the production of long-chain dicarboxylic acids. Bio/Technology, 1992, 10(8): 894-898.
|
| [48] |
BOWEN CH, BONIN J, KOGLER A, BARBA-OSTRIA C, ZHANG FZ. Engineering Escherichia coli for conversion of glucose to medium-chain omega-hydroxy fatty acids and alpha, omega-dicarboxylic acids. ACS Synthetic Biology, 2016, 5(3): 200-206. DOI:10.1021/acssynbio.5b00201
|
| [49] |
LIN CY, VUU KM, AMER B, SHIH PM, BAIDOO EEK, SCHELLER HV, EUDES A. In-planta production of the biodegradable polyester precursor 2-pyrone-4, 6-dicarboxylic acid (PDC): stacking reduced biomass recalcitrance with value-added co-product. Metabolic Engineering, 2021, 66: 148-156. DOI:10.1016/j.ymben.2021.04.011
|
| [50] |
MASAI E, KATAYAMA Y, NISHIKAWA S, FUKUDA M. Characterization of Sphingomonas paucimobilis SYK-6 genes involved in degradation of lignin-related compounds. Journal of Industrial Microbiology and Biotechnology, 1999, 23(4): 364-373.
|
| [51] |
NISHIKAWA S, SONOKI T, KASAHARA T, OBI T, KUBOTA S, KAWAI S, MOROHOSHI N, KATAYAMA Y. Cloning and sequencing of the Sphingomonas (Pseudomonas) paucimobilis gene essential for the O demethylation of vanillate and syringate. Applied and Environmental Microbiology, 1998, 64(3): 836-842. DOI:10.1128/AEM.64.3.836-842.1998
|
| [52] |
NODA Y, NISHIKAWA S, SHIOZUKA K, KADOKURA H, NAKAJIMA H, YODA K, KATAYAMA Y, MOROHOSHI N, HARAGUCHI T, YAMASAKI M. Molecular cloning of the protocatechuate 4, 5-dioxygenase genes of Pseudomonas paucimobilis. Journal of Bacteriology, 1990, 172(5): 2704-2709. DOI:10.1128/jb.172.5.2704-2709.1990
|
| [53] |
OTSUKA Y, NAKAMURA M, SHIGEHARA K, SUGIMURA K, MASAI E, OHARA S, KATAYAMA Y. Efficient production of 2-pyrone 4, 6-dicarboxylic acid as a novel polymer-based material from protocatechuate by microbial function. Applied Microbiology and Biotechnology, 2006, 71(5): 608-614. DOI:10.1007/s00253-005-0203-7
|
| [54] |
NAKAJIMA M, NISHINO Y, TAMURA M, MASE K, MASAI E, OTSUKA Y, NAKAMURA M, SATO K, FUKUDA M, SHIGEHARA K, OHARA S, KATAYAMA Y, KAJITA S. Microbial conversion of glucose to a novel chemical building block, 2-pyrone-4, 6-dicarboxylic acid. Metabolic Engineering, 2009, 11(4/5): 213-220.
|
| [55] |
LUO ZW, KIM WJ, LEE SY. Metabolic engineering of Escherichia coli for efficient production of 2-pyrone-4, 6-dicarboxylic acid from glucose. Acs Synthetic Biology, 2018, 7(9): 2296-2307. DOI:10.1021/acssynbio.8b00281
|
| [56] |
PEREZ JM, KONTUR WS, ALHERECH M, COPLIEN J, KARLEN SD, STAHL SS, DONOHUE TJ, NOGUERA DR. Funneling aromatic products of chemically depolymerized lignin into 2-pyrone-4-6-dicarboxylic acid with Novosphingobium aromaticivorans. Green Chemistry, 2019, 21(6): 1340-1350. DOI:10.1039/C8GC03504K
|
| [57] |
BARRY KP, TAYLOR EA. Characterizing the promiscuity of LigAB, a lignin catabolite degrading extradiol dioxygenase from Sphingomonas paucimobilis SYK-6. Biochemistry, 2013, 52(38): 6724-6736. DOI:10.1021/bi400665t
|
| [58] |
PFLEGER BF, PITERA DJ, SMOLKE CD, KEASLING JD. Combinatorial engineering of intergenic regions in operons tunes expression of multiple genes. Nature Biotechnology, 2006, 24(8): 1027-1032. DOI:10.1038/nbt1226
|
| [59] |
WERPY T, PETERSEN G. Top value added chemicals from biomass volume Ⅰ – Results of screening for potential candidates from sugars and synthesis gas[EB/OL]. [2004-08-01]. https://www.osti.gov/biblio/15008859.
|
| [60] |
KOOPMAN F, WIERCKX N, de WINDE JH, RUIJSSENAARS HJ. Identification and characterization of the furfural and 5-(hydroxymethyl) furfural degradation pathways of Cupriavidus basilensis HMF14. Proceedings of the National Academy of Sciences of the United States of America, 2010, 107(11): 4919-4924. DOI:10.1073/pnas.0913039107
|
| [61] |
WIERCKX N, KOOPMAN F, BANDOUNAS L, de WINDE JH, RUIJSSENAARS HJ. Isolation and characterization of Cupriavidus basilensis HMF14 for biological removal of inhibitors from lignocellulosic hydrolysate. Microbial Biotechnology, 2010, 3(3): 336-343. DOI:10.1111/j.1751-7915.2009.00158.x
|
| [62] |
FELDMAN D, KOWBEL DJ, GLASS NL, YARDEN O, HADAR Y. Detoxification of 5-hydroxymethylfurfural by the Pleurotus ostreatus lignolytic enzymes aryl alcohol oxidase and dehydrogenase. Biotechnology for Biofuels, 2015, 8(1): 63. DOI:10.1186/s13068-015-0244-9
|
| [63] |
RAJESH RO, GODAN TK, RAI AK, SAHOO D, PANDEY A, BINOD P. Biosynthesis of 2, 5-furandicarboxylic acid by Aspergillus flavus ALPS-1: process optimization and intermediate product analysis. Bioresource Technology, 2019, 284: 155-160. DOI:10.1016/j.biortech.2019.03.105
|
| [64] |
RAJESH RO, GODAN TK, SINDHU R, PANDEY A, BINOD P. Bioengineering advancements, innovations and challenges on green synthesis of 2, 5-furan dicarboxylic acid. Bioengineered, 2020, 11(1): 19-38. DOI:10.1080/21655979.2019.1700093
|
| [65] |
YUAN HB, LI JH, SHIN HD, DU GC, CHEN J, SHI ZP, LIU L. Improved production of 2, 5-furandicarboxylic acid by overexpression of 5-hydroxymethylfurfural oxidase and 5-hydroxymethylfurfural/furfural oxidoreductase in Raoultella ornithinolytica BF60. Bioresource Technology, 2018, 247: 1184-1188. DOI:10.1016/j.biortech.2017.08.166
|
| [66] |
KOOPMAN F, WIERCKX N, de WINDE JH, RUIJSSENAARS HJ. Efficient whole-cell biotransformation of 5-(hydroxymethyl) furfural into FDCA, 2, 5-furandicarboxylic acid. Bioresource Technology, 2010, 101(16): 6291-6296. DOI:10.1016/j.biortech.2010.03.050
|
| [67] |
YUAN HB, LIU YF, LI JH, SHIN HD, DU GC, SHI ZP, CHEN J, LIU L. Combinatorial synthetic pathway fine-tuning and comparative transcriptomics for metabolic engineering of Raoultella ornithinolytica BF60 to efficiently synthesize 2, 5-furandicarboxylic acid. Biotechnology and Bioengineering, 2018, 115(9): 2148-2155. DOI:10.1002/bit.26725
|
| [68] |
YUAN HB, LIU YF, LV XQ, LI JH, DU GC, SHI ZP, LIU L. Enhanced 2, 5-furandicarboxylic acid (FDCA) production in Raoultella ornithinolytica BF60 by manipulation of the key genes in FDCA biosynthesis pathway. Journal of Microbiology and Biotechnology, 2018, 28(12): 1999-2008. DOI:10.4014/jmb.1808.08057
|
| [69] |
ZOU X, WANG YK, TU GW, ZAN ZQ, WU XY. Adaptation and transcriptome analysis of Aureobasidium pullulans in corncob hydrolysate for increased inhibitor tolerance to malic acid production. PLoS One, 2015, 10(3): e0121416. DOI:10.1371/journal.pone.0121416
|
| [70] |
AHN JH, SEO H, PARK W, SEOK J, LEE JA, KIM WJ, KIM GB, KIM KJ, LEE SY. Enhanced succinic acid production by Manheimia employing optimal malate dehydrogenase. Nature Communications, 2020, 11: 1970. DOI:10.1038/s41467-020-15839-z
|
 2023, Vol. 39
2023, Vol. 39