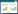中国科学院微生物研究所、中国微生物学会主办
文章信息
- 李举谋, 石焜, 张志钧, 许建和, 郁惠蕾
- LI Jumou, SHI Kun, ZHANG Zhijun, XU Jianhe, YU Huilei
- 多酶级联反应的构建及其在双官能团功能化学品合成中的应用
- Construction of multi-enzyme cascade reactions and its application in the synthesis of bifunctional chemicals
- 生物工程学报, 2023, 39(6): 2158-2189
- Chinese Journal of Biotechnology, 2023, 39(6): 2158-2189
- 10.13345/j.cjb.221001
-
文章历史
- Received: December 14, 2022
- Accepted: February 6, 2023
化学品在我们日常生活中应用非常广泛,目前工业化生产大多是采用化学法合成,但是化学法合成存在环境污染大和催化条件严苛等限制条件。近些年来,越来越多的生物学家尝试利用生物法合成化学品,但是由于一些化学品结构较为复杂,往往需要多酶级联催化廉价易得底物合成目标化学品。所以,系统性地总结多酶级联反应的构建策略显得尤为必要。同时,为了进一步地了解目前多酶级联催化合成化学品的研究现状和实际应用情况,本文结合本课题组的一些研究方向和工作,阐述了多酶级联催化反应体系在6种双官能团功能化学品,包括ω-氨基脂肪酸、烷基内酰胺、α, ω-二元羧酸、α, ω-二胺、α, ω-二醇、ω-氨基醇合成中的应用。
1 多酶级联反应多酶级联反应是指将两种甚至更多的酶结合起来生产目标化合物[1-4],这种方法已被证明有利于提高原子效率、避免不稳定或有毒的中间体、无需中间产物分离提取和减少溶剂消耗和废物生成量,能最大化地节省反应器体积与反应时间[5-7],有时还能实现化学催化无法实现的反应[3, 8]。
1.1 多酶级联反应的类型根据反应环境的不同,可分为体内、体外和混合级联反应[9-10] (图 1)。体外级联反应是以纯酶、冻干酶粉、粗酶液等催化剂形式进行反应,其操作较为简单,便于对反应参数进行调节和优化,时空产率相对更高[11],其应用已非常广泛。最典型的例子是2015年笔者所在课题组[12]和曼彻斯特大学Turner团队[13]几乎同时发表的双酶借氢级联法由醇制胺新路线,该路线依赖醇脱氢酶(alcohol dehydrogenase, ADH)和胺脱氢酶(amine dehydrogenase, AmDH)的串联操作,使很多不同结构的芳香醇和脂肪醇能够实现一锅胺化,并且得到高达97%的转化率和99% ee的对映体选择性。这种辅因子自给型氧化还原级联反应具有很高的原子经济性,只需用铵盐作为氨基供体,而且产生的唯一副产物是水,产物分离大大简化。相对于体外级联,体内多酶级联反应是构建人工细胞工厂,将所需要的元件酶在细胞内进行共表达,其优点在于无需单独添加所需的元件酶,可减少发酵和酶制备的成本;同时在胞内的酶稳定性更高,可利用胞内自身的辅因子再生系统。目前已有多个体内多酶级联反应成功应用的案例[14-18],例如以甘氨酸和醛为底物合成α-官能化有机酸[14]以及烯烃的氧化和氨基官能化[16]。但是体内级联方式也存在一些缺点,如胞内的代谢网络过于复杂,干扰因素较多,难以精准调控;多个基因共表达操作步骤繁琐,且多个基因在单细胞内共表达会导致代谢负担过大、表达效果差,使得最终合成的时空产率相对更低。为了结合体外级联反应和体内级联反应的优点,同时避免两者的缺点,2018年,新加坡国立大学Liu等[19]提出了采用整细胞与无细胞提取液进行偶联反应的新概念,通过表达醇脱氢酶和NAD(P)H氧化酶的整细胞偶联胺脱氢酶和葡萄糖脱氢酶粗酶液进行一锅级联反应,将细胞内的NAD+和细胞外的NADH辅因子循环再生系统相互隔离,避免两种辅因子循环相互干扰以提高其利用效率。

|
| 图 1 体外(ⅰ)、体内(ⅱ)和混合(ⅲ)级联反应的比较 Fig. 1 Comparison of in vitro (ⅰ), in vivo (ⅱ), and hybrid (ⅲ) multienzyme cascade reactions. Product D is generated from starting material A using the highly selective biocatalysts cat i−cat iii. |
| |
另一方面,根据多酶级联催化反应路线的不同,可以将其分为4类:即线性级联、平行级联、正交级联和循环级联(图 2)。(1) 线性级联(图 2A):底物经过一步或连续多步转化合成目标产物。这种级联路线的优势在于可以避免有毒、不稳定和易爆中间体的储存和处理,同时有助于节省时间并减少多步合成中间产物分离的步骤[20]。另外中间产物在下一步反应的转化也有利于可逆反应的平衡移动和解除中间产物对酶的抑制。(2) 平行级联(图 2B):平行级联可能是生物催化氧化还原反应最常见的级联类型,产物的形成与同时进行的第二个平行反应相结合。一个典型的例子是氧化还原酶依赖的NAD(P)H的辅因子循环[21],另一个例子是自给式氢化物穿梭级联合成两种有应用价值的产物[22]。(3) 正交级联(图 2C):相较于平行级联,此级联方式进一步将副产物转化为其他无应用价值的产物,但是可进一步促进平衡移动。例如在转氨酶催化反应中通过引入乳酸脱氢酶/葡萄糖脱氢酶来进一步转化氨基供体丙氨酸产生的副产物丙酮酸,促使平衡向转氨方向移动[23-24]。(4) 循环级联(图 2D):循环级联的主要特征是生成的产物被转化为底物。例如,通过转氨酶或胺脱氢酶的去外消旋化,即将外消旋混合物转化为光学纯的单一对映异构体[25-26]。然而在实际应用中,通常是以目标产物为导向的多种不同级联方式的自由组合。
|
| 图 2 多酶级联催化反应的4种类型 Fig. 2 Four types of enzymatic cascade reactions. A: Linear cascade. B: Parallel cascade. C: Orthogonal cascade. D: Cyclic cascade. |
| |
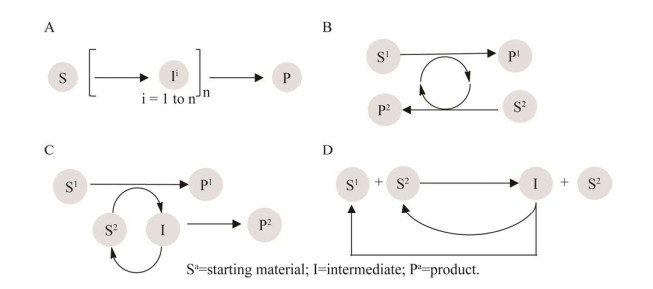
构建级联反应的策略是“路线设计-元件招募-系统测试-重构优化”循环[10] (图 3),首先是级联路线设计,可根据自然代谢途径获得灵感,或通过生物催化逆合成分析来设计路线,即根据目标产物和酶的催化功能,逆向分析所需的底物和酶元件;随后,通过筛选获得所需催化功能的元件酶,同时可通过理性设计或者定向进化等方法进一步提高元件酶的催化性能和稳定性;在确定了元件酶和级联路线后,系统测试分析反应体系的条件,如温度、pH、辅底物及催化剂浓度等,通过色谱等分析方法测定级联催化过程的底物和产物的浓度变化,研究级联反应的瓶颈问题,如多酶协同作用、交叉反应、热力学平衡和中间反应物对酶的抑制作用等;最后,在解决影响级联反应整体效率的问题后,为进一步降低成本,针对性地对多个不同的元件酶进行共表达及人工细胞工程的重构,如大肠杆菌(Escherichia coli)菌群构建,用于协同催化合成目标产物,并对级联反应的参数进一步优化,最终解决瓶颈问题,降低催化成本,提升级联反应的合成效率。
|
| 图 3 多酶级联体系的“路线设计-元件招募-系统测试-重构优化”循环策略 Fig. 3 "Cascade design-enzymes recruitment-system test-reconstruction and optimization" cycle of multi-enzyme cascade development. |
| |
多酶级联路线主要是根据现有的自然代谢反应途径或通过逆合成分析进行设计。Korman等[27]利用糖酵解和甲羟戊酸途径创建了一个由27种来自于不同物种的元件酶组成的体外酶级联,由葡萄糖合成单萜类化合物,在5 d内柠檬烯和蒎烯的滴度分别为12.5 g/L和14.9 g/L,产率超过88%。这大大超过了利用全细胞代谢生产柠檬烯滴度的最高纪录,证明了这一体外级联反应路线的潜力。开发体外多酶级联的另一种方法是从头构建自然界中不存在的合成途径,利用该方法可以任意定制合成所需的新型化合物。然而,在没有现成路线的情况下,设计合成目标分子的级联反应可能是一个挑战。在有机合成化学中,通常采用逆向合成来设计目标化合物的合成路线。因此,逆向合成意味着从目标化合物分子开始,确定要形成的化学键,并相应地确定前体和中间物,该策略同样适用于多酶生物催化反应。目前,已经成功开发了一些计算工具,如RetroBioCats[28]或myExperiment- RetroPath[29]算法,有助于利用所有已知的可用的酶催化反应以及级联路线的设计。中国科学院天津工业生物技术研究所马延和团队成功构建了包括11步反应的非天然固碳与淀粉合成途径[30]。具体来说,首先根据碳原子个数将该途径分为4个不同的模块(C1、C3、C6和Cn模块);随后在计算途径设计软件/网站的指导下,通过选择和组装来自31个生物体的62种酶构成11个核心催化反应,并将热力学上最有利(∆Gcascade < 0)的多个反应组装在一起;最后优化途径,定向进化瓶颈酶,使淀粉的最终产率达到410 mg/(L·h)。同时,还有多个逆向合成设计多酶级联合成新途径的案例,如人类免疫缺陷病毒(human immunodeficiency virus, HIV)逆转录酶的核苷类似物和抑制剂didanosine[31]或islatravir[32]的合成。
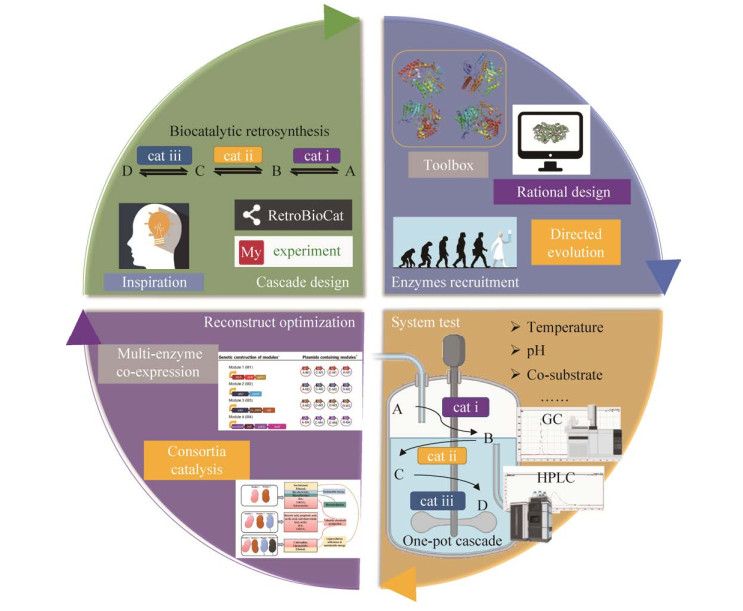
1.2.2 元件酶招募整个级联反应的效率取决于每个元件酶,元件酶既要具有高催化活性和更好的兼容性,又需要保持较强的底物特异性以避免交叉反应和副产物的产生,因此获得符合级联反应要求的元件酶对整个级联反应的成败至关重要。目前元件酶筛选常用的策略主要有6种(图 4)。
|
| 图 4 获取元件酶的常用策略 Fig. 4 Common strategies for recruiting enzymes. |
| |
(1) 从土壤微生物中富集培养:通过从土壤微生物中富集培养挖掘所需的元件酶已经被证明是一种有效的策略,在培养基中加入特定的碳源或者氮源筛选具有特定催化功能的菌株,经过几轮扩增培养后,筛选出的菌株进一步用优化的生长培养基培养,并进行生物催化活性测定实验(图 5)。Shin等[33]研究小组已经从不同环境的土壤样品中筛选获得目标微生物,并被用于手性拆分外消旋胺,如苏云金芽孢杆菌(Bacillus thuringiensis JS64, BtTA),肺炎克雷伯氏菌(Klebsiella pneumonia JS2F, KpTA)和河流弧菌(Vibrio fluvialis JS17, VfTA)是最早对α-甲基苄胺和仲丁胺进行(S)-选择性全细胞生物转化的微生物,其(R)-对映体的产率 > 95%。
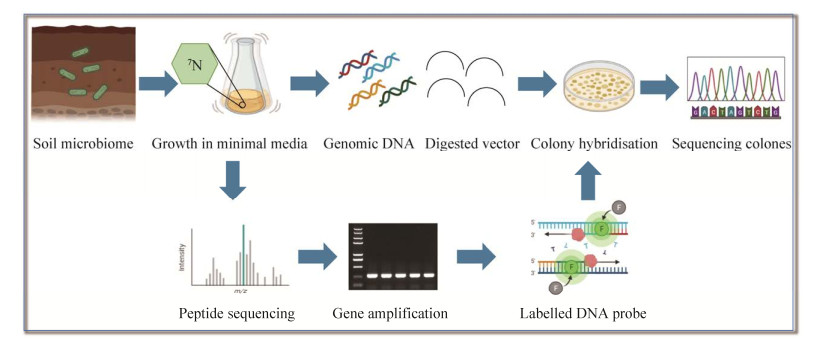
|
| 图 5 富集培养筛选示意图 Fig. 5 The screening diagram for enrichment culture. |
| |
(2) 从特定基因组文库挖掘目标酶:宏基因组的样品来源非常广泛,从深海喷口、海洋表面和火山温泉到共生哺乳动物宿主,以产生特定的功能。宏基因组基因挖掘通常有两种方法:“功能驱动”和“序列驱动”。其中“功能驱动”法已被用于获取可用于工业应用的生物学数据,也已经有其他工业酶通过“功能驱动”的方法被发现。Yeh等[34]利用此方法发现了稳定性更高的新型内切葡聚糖酶,Bayer等[35]以肉桂腈等6种不同的腈的混合物为底物,从超基因组文库中筛选出新的腈水解酶基因nit1,可用于生产精细化工产品1, 5-二甲基-2-哌啶酮。Ward等[36]利用“序列驱动”的方法从人类宿主口腔共生微生物菌群中发现新的转氨酶基因,具体步骤为首先从样品中提取DNA,然后利用高通量测序方法进行测序,再使用pfam独立工具注释推测的DNA ω-转氨酶(I型折叠)蛋白质,鉴定完的序列在E. coli BL21中进行异源表达。在元基因组文库中,异源基因的表达仍然是鉴定基因功能的瓶颈。
(3) 基于序列同源性挖掘目标酶:随着测序技术的发展,大量的基因数据被导入数据库,这使序列比对成为基因挖掘的有效方法。例如,以转氨酶VfTA为模板进行蛋白序列比对,成功挖掘到了与VfTA同源性38%的来源于紫罗兰色杆菌(Chromobacterium violaceum)的转氨酶CvTA,该酶在转氨催化过程中表现出和模板蛋白一样优异的催化性能和潜力[37-38]。以VfTA为先导序列,通过序列比对基因挖掘获得来源于脱氮副球菌(Paracoccus denitrificans)的转氨酶PdTA,与VfTA同源性为94%,随后利用PdTA和苏氨酸脱氨酶级联将L-苏氨酸(天然)转化为L-高丙氨酸(非天然),转化率高达91%[39]。
(4) 基于特征序列的基因挖掘:基于蛋白质的不同进化起源,一些特征序列对酶的催化功能起着关键作用,常用的挖掘策略有不同催化特性的酶多序列比对、进化树分析以及和计算相结合分析。以转氨酶为例,尽管(S)-选择性胺转氨酶[(S)-transaminases, (S)-TAs]已被大量报道和研究,但(R)-TAs报道较少。2010年,Höhne等[40]从蛋白质拓扑结构、晶体结构和转氨酶家族的生化信息推断(R)-TAs属于第Ⅳ类折叠,他们的研究假定D-氨基酸转移酶(D-amino acid transferase, DAT)、4-氨基-4-脱氧分支酸裂解酶(4-amino-4-deoxybranched acid lyase, ADCL)和L-支链氨基酸氨基转移酶(L-branched-chain amino acid transferase, BCAT)在进化上保守的氨基酸残基(或特征序列)基序是(R)-TAs的合理祖先,于是开发了一种计算机注释算法。该算法仔细分析了多个序列比对的基序,并从搜索的公共数据库中丢弃了错误标记的BCAT、DAT和ADCL。利用该方法从大约6 000个酶库中鉴定出21个(R)-TAs,其中17个酶被实验证实具有活力。选择7个酶进一步表征,发现它们具有极优异的(R)-选择性和催化效率[24]。基于他们的方法,多个研究小组后续又从各种原核生物和真核生物中发现了具有各种胺化和动力学拆分外消旋胺活性的有益基因[41-43]。本课题组通过寻找参与脂肪氧合酶核心催化功能以及区域选择性和立体选择性的保守性残基,如金属结合残基(HHHNI)、Coffa位点(异构体手性的决定性位点,(S)-脂氧合酶为Ala,(R)-脂氧合酶为Gly)以及决定异构体位置的残基,筛选到3个脂肪氧合酶,对亚油酸表现出较好的活性,比活性高达73.1 U/mg protein[44]。
(5) 基于蛋白结构推断酶的功能:随着蛋白质结构预测方法的发展(如AlphaFold[45]等),大量未知功能的酶结构被存入蛋白质数据库,将这些结构与它们的功能联系起来具有重要意义。Höhne等[46]研究了“鸟氨酸氨基转移酶(ornithine aminotransferase, OAT)-类似蛋白”集群中功能未知的晶体结构,OAT是依赖于吡哆醛-5′-磷酸(pyridoxal-5′-phosphate, PLP)的酶,属于PLP折叠I类,该家族中所有58个可用的3D结构都显示出相当大的相似性,但它们在活性部位的重要残基上有所不同,这些残基显然参与底物识别。随后,成功鉴定了结构和功能未知的来自硅酸杆菌(Silicibacter pomeroyi, SpTA)、浑球红假单胞菌(Rhodobacter sphaeroides KD131, RsTA)、鲁杰氏菌(Ruegeria sp. TM1040, RsTA)和百脉根根瘤菌(Mesorhizobium loti MAFF30399, MlTA)的4个转氨酶TAs,它们具有广泛的底物谱和高的底物特异性,其中SpTA已被广泛地应用于多酶级联催化不同底物合成脂肪胺[47-52]。同样,来源于炭疽杆菌(Bacillus anthracis, BaTA)的TA也根据已报道的结构用于特定催化丙酮酸盐等多个不同底物[53]。
(6) 利用酶分子工程进一步改造元件酶:酶的分子工程包括基于随机突变的定向进化、基于序列和结构分析的理性设计和两者结合的半理性设计,已被广泛证明是一种实用而有效的获取生物催化剂的策略[54]。在多酶级联催化过程中,需要多个酶协同工作,往往某个瓶颈酶就会成为限制整个级联反应效率的短板,所以亟需对其进行酶分子改造,以进一步提高级联反应的整体效率。Huffman等[32]设计的合成核苷类似物依斯拉韦的级联反应包括9种酶,其中5种是通过定向进化而获得的,以乙炔基甘油为原料合成的最后总收率为51% (图 6A)。如Hailes等[55]用平行级联酶促合成苄基异喹啉生物碱,该平行级联设计中结合了酪氨酸酶、酪氨酸脱羧酶、转氨酶和去甲肾上腺素合成酶。随后发现酪氨酸酶对底物3-F-L-酪氨酸接受度较差,所以为了提高该级联体系中酪氨酸酶对底物的催化活性,对酪氨酸酶进行定向进化改造,获得的最优突变体将产率从27%提高至89%。江南大学刘立明等[56]使用简单的苯甲醛和丙酮酸作为底物通过酶促-化学级联催化高效生产L-高苯丙氨酸,在筛选元件酶过程中发现苯丙氨酸脱氢酶(phenylalanine dehydrogenase, TiPheDH)为限速酶。因此,对TiPheDH进行改造以提高其催化效率(82%)和表达水平(254%),并在5 L反应器中实现L-高苯丙氨酸的高效合成(100.9 g/L, > 99% ee) (图 6B)。2021年,上海交通大学林双君等[57]以4-甲磺酰基苯甲醛为底物,通过偶联转酮醇酶(trans-ketohydrolases, TK)和ω-转氨酶(ω-transaminase, ω-TA),建立了一锅法高立体选择性地合成含两个手性中心的氟苯尼考氨基二醇中间体。通过结构导向的酶分子改造将TK的对映体选择性从(S, 93% ee)转化为(R, 95% ee),并逆转了转氨酶ATA117的对映选择性(从ES=9到ER=12)和对酮/醛底物的选择性,利用改造后的TK和TA进行级联催化反应,实现了(1R, 2R)-对甲磺酰基苯丝氨醇的生物合成(76% yield, 96% de, > 99% ee)。最近,李智等[58]建立了以SMO-StEH-Aldo-CvTA为催化剂,通过环氧化-水解-氧化-胺化反应合成(R)-苯乙醇胺的级联反应,通过定向进化改造瓶颈酶醇氧化酶,使其催化效率提高了3倍,使得产物(R)-苯乙醇胺的滴度达到34.6 mmol/L (> 99% ee) (图 6C)。

|
| 图 6 元件酶改造用于多酶级联反应 Fig. 6 Modified enzymes used in multi-enzyme cascade reactions. Description of catalyst abbreviation: Galactose oxidase (GOA), pantothenate kinase (PanK), deoxyribose 5-phosphate aldolase (DERA), phosphopentomutase (PPM), purine nucleoside phosphorylase (PNP), catalase (Cat), horseradish peroxidase (HRP), acetate kinase (AcK), aldolase (Ald), ene-reductase (ER), phenylalanine dehydrogenase (PheDH), styrene monooxygenase (SMO), epoxide hydrolase (EH), alditol oxidase (AldO), transaminase (TA). Those marked with light green underline are key enzymes for molecular modification. |
| |
当多酶级联反应中的元件酶确定之后,酶的比例和浓度也需要进一步地优化。通过平衡多种不同生物催化剂的活性,可以实现提高通量和减少酶用量。对于级联反应合成L-丙氨酸,调整酶的比例可以改善性能和提高产率[59],通过对缓冲体系和辅因子浓度的进一步优化,获得了95%以上的产率。另外通过对级联反应合成2′, 3′-环鸟苷-腺苷一磷酸中4种酶的浓度进行了调整,使得产物浓度增加了2倍[60]。酶的比例对级联反应的效率起着关键作用,影响着大部分的优化目标,如转化率、产量、速率、立体选择性和热稳定性等。
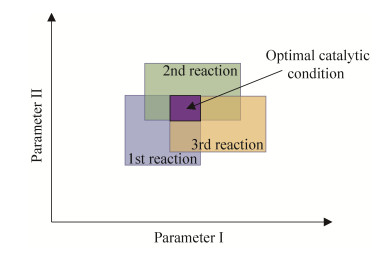
尽管酶的催化条件较温和,反应条件较为类似,但合成过程中每个步骤对最佳反应条件的要求可能存在差异。适用于多酶级联反应中所有反应步骤的重叠参数,定义为最优催化条件(图 7),该参数根据具体反应参数和酶种类的不同而变化,该参数的选择经常是一个挑战,尤其是当级联路线复杂,所涉及的中间体、酶元件或辅酶较多的情况下。可供优化的典型参数主要有溶剂系统[37, 61-63]、反应成分的浓度(底物[64-65]、盐[15, 52, 66]、助溶剂[23, 67]、辅酶[39, 68]、其他添加剂)、缓冲体系的pH值[69-70]、反应温度[71],以及辅酶再生系统[72]。如果级联反应中的多步反应同时进行的话,很有必要选择在多个酶反应的最适条件下进行。值得庆幸的是,大部分酶是在比较温和条件下进行反应,比如水溶剂,温度在20–37 ℃之间,pH值在6.0–8.0之间,这也降低了其自由度。当然,也有部分酶能耐受更极端的pH值、温度、压力、溶剂和盐浓度等环境[73]。酶具有较高的热稳定性对合成反应来说有很大的好处(例如由于较高的工艺温度可以达到较高的活性),但只有当整个级联反应的酶元件热稳定性都高时才能显示出明显的提升。同时也需要综合考虑反应体系中盐离子对酶的抑制作用,比如当羧酸还原酶和转氨酶级联转化脂肪酸合成脂肪胺时,由于羧酸还原酶需要添加二价阳离子Mg2+以提高其催化活性,但是过高的Mg2+浓度对转氨酶有明显的抑制作用[49]。
|
| 图 7 多个不同酶催化反应的最优参数条件 Fig. 7 Optimal catalytic conditions for multiple reactions involved in a multi-enzyme cascade. This optimal catalytic condition predefines the parameter ranges of the cascade. Usually there are more than two parameters under practical conditions. |
| |
热力学平衡对级联反应产率的影响同样不可忽视,尤其是当级联反应的最后一步为可逆反应时,需要提高反应平衡常数才能推动反应进行。最典型的例子是涉及转氨酶的级联反应,常用的平衡移动策略主要是“推”和“拉”[74]。“推”指的是添加过量的辅底物,如转氨酶催化反应中通常需要添加过量的氨基供体,同时也需要注意过量的氨基供体对转氨酶的酶活可能会存在抑制作用[75];“拉”主要指产物移除或副产物移除,如本课题组利用阳离子交换树脂吸附产物(R)-苯基甘氨醇以提高级联产率[76],或通过偶联转化副产物丙酮酸的乳酸脱氢酶(lactate dehydrogenase, LDH)[24, 77-78]或用于丙氨酸氨基供体循环的丙氨酸脱氢酶(alanine dehydrogenase, AlaDH)[79-80]以进一步降低副产物浓度。
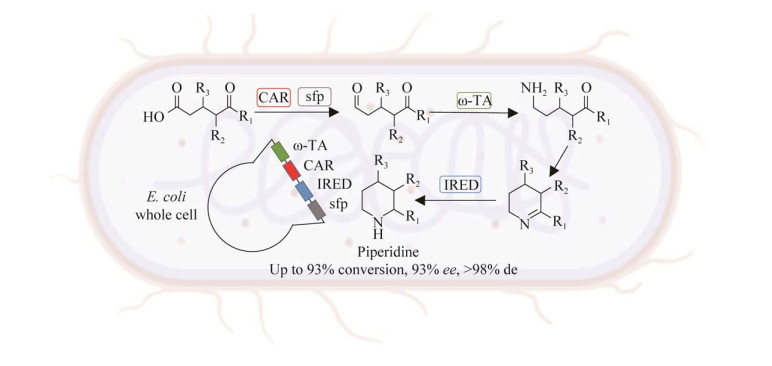
1.2.4 重构优化与无细胞催化剂相比,构建体内多酶级联(构建细胞工厂)系统优点明显,无需单独添加所需的各种酶元件,只需添加一个全细胞作为催化剂,操作步骤更简单,成本降低[81-82]。同时,细胞内酶的稳定性更高,可以避免酶的纯化、添加辅因子等繁琐费事的步骤[10]。如Turner等[83]在前期多酶级联工作的基础上,进一步将该级联反应中的4个核心酶,在10种不同的载体上进行共表达,成功筛选得到一个四酶共表达体系的产率最高,用于催化5个不同的酮酸底物合成哌啶,转化率为57%,其中对映体过量ee值高达93% (图 8)。随后,作者根据级联过程中中间产物的积累情况,判断每个元件酶的酶活,确定ω-转氨酶为限制性酶,并利用基因复制策略共表达了2次ω-转氨酶,使得胺产量大幅增加,最高达93%[84]。
|
| 图 8 设计多酶级联共表达细胞用于合成手性环胺 Fig. 8 Design of a multi-enzyme cascade and its co-expression in a whole cell system for the synthesis of chiral cyclic amines. The enzymes involved are CAR (carboxylic acid reductase), sfp (phosphopantetheinyl transferase), ω-TA (ω-transaminase) and IRED (imine reductase). |
| |
然而,当级联路线中酶的数量超过4个时,如果将这些酶在同一个细胞中进行共表达,会导致细胞代谢负担过大和氧化还原不平衡等问题[85],从而使得部分酶不能正常表达或者表达量很低,从而影响最终的产率。大量研究已尝试构建人工全细胞菌群[14, 16, 48, 52],即将复杂的级联反应分为多个具有不同功能的模块,同一个模块所需的酶基因在一个细胞中共表达,然后多个细胞菌群一起协同高效催化合成具有高附加值的化学品。菌群模块化构建原则包括以下几点:(1) 每个细胞模块呈现氧化还原中性,如辅酶循环在一个细胞中;(2) 避免不同菌群中辅酶的干扰;(3) 减少蛋白质表达负担,以确保每个酶都能正常表达;(4) 每个菌群分工明确。
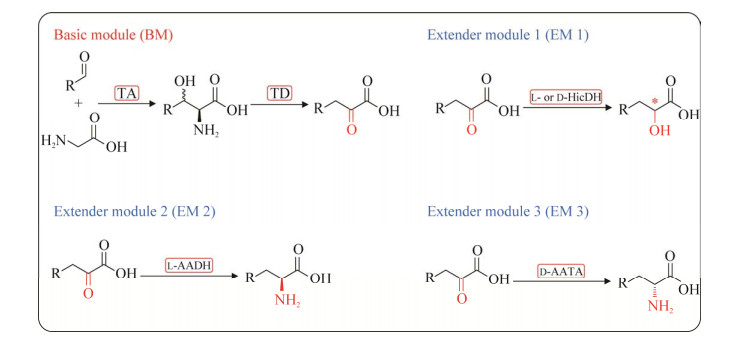
江南大学刘立明团队构建了多模块菌群催化平台,用于由简单的非手性甘氨酸和醛类合成α-氨基酸和α-羟基酸[14] (图 9)。作者设计的4个不同的功能化模块:基础模块(basic module, BM)包含苏氨酸醛缩酶和苏氨酸脱氨酶,用于催化底物甘氨酸和醛类合成对应的酮酸;扩展模块1 (extension modules 1, EM1)包含L-或D-羟基异己酸脱氢酶和甲酸脱氢酶用于将α-酮酸还原为α-羟基酸;扩展模块2 (EM2)包含L-氨基酸脱氢酶和甲酸脱氢酶,用于将α-酮酸还原为L-α-氨基酸;扩展模块3 (EM3)包含D-氨基酸转氨酶、甲酸脱氢酶、谷氨酸消旋酶和谷氨酸脱氢酶用于将α-酮酸转氨成D-α-氨基酸。随后,通过级联BM与EM1模块以重置手性-OH合成手性的α-羟基酸,级联BM和EM2或EM3重置手性-NH2用于合成手性α-氨基酸。在成功招募到各个模块所需的高效特异性元件酶后,对其中的限制性酶苏氨酸脱氨酶进行分子改造,使其活力提高了18倍。再将单个模块的所有酶在一个质粒中共表达,选取了4种兼容且具有不同抗性的质粒,共构建了64种不同的组合,筛选出产率最高的菌群组合形式。最终成功应用于催化9种不同的醛,包括芳香族、杂芳族、杂环族和脂肪族醛,高效合成45种不同的产物[α-酮酸、不同手性的α-羟基酸和α-氨基酸(R, S)],并将体系进一步扩大至100 mL规模,进而对7种有价值的化学品进行分离鉴定。
|
| 图 9 模块化串联式生物催化平台合成α-功能化有机酸 Fig. 9 Synthesis of α-functionalized organic acids by the proposed modular cascaded biocatalysis platform. |
| |
辅酶是一大类有机辅助因子的总称,是酶催化氧化还原反应、基团转移和异构化反应的必需因子,它们在酶催化反应中承担传递电子、原子或基团的功能。常见的辅酶有硫胺素、烟酰胺、核黄素、吡哆醛及其衍生物、三磷酸腺苷(adenosine triphosphate, ATP)、泛酸和叶酸等,大部分辅酶如NADPH等价格较为昂贵。为了进一步降低成本,多酶级联催化反应过程中通常需要实现辅酶的再生,其中烟酰胺类辅酶和ATP的再生应用最为广泛。
1.3.1 烟酰胺类辅酶再生烟酰胺类辅酶主要包括氧化型辅酶NAD+、NADP+及相应的还原态辅酶NADH和NADPH 4种。烟酰胺类辅酶价格相对昂贵,且稳定性差,所以为了降低经济成本和提高目标产物的产率,通常需要构建辅酶循环系统。同时,对于一些存在平衡反应步骤的级联体系,构建辅酶再生系统也有助于促进反应平衡的正向移动。
根据所需辅酶类型的不同,又分为再生还原型辅酶(图 10A)和再生氧化型辅酶(图 10B)两类。常用于还原型辅酶NAD(P)H再生的酶主要有醇脱氢酶(alcohol dehydrogenase, ADH)[86]、葡萄糖脱氢酶(glucose dehydrogenase, GDH)[87-89]、甲酸脱氢酶(formate dehydrogenase, FDH)[90-92]、亚磷酸脱氢酶(phosphite dehydrogenase, PDH)[93-95]等。在实际应用中,需要根据辅酶循环效率和整个级联反应体系的适配性,从而选择最优的辅酶循环系统。目前常用的氧化型辅酶NAD(P)+再生体系主要有乳酸脱氢酶(lactate dehydrogenase, LDH)[96]、NAD(P)H氧化酶[(NAD(P)H oxidase, NOX][97-98]、谷氨酸脱氢酶(glutamate dehydrogenase, GluDH)[99]、羰基还原酶(carbonyl reductase, KRED)[100]等。
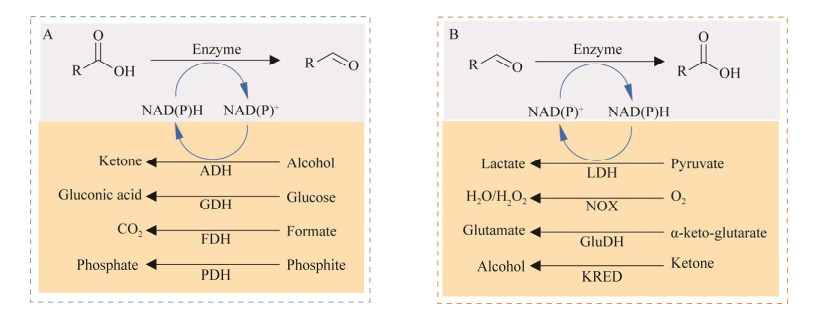
|
| 图 10 辅酶再生系统 Fig. 10 Reduced (A) and oxidized (B) coenzyme regeneration system. A:还原型辅酶再生系统. B:氧化型辅酶再生系统 |
| |
由于级联体系中通常涉及多个辅酶再生系统,存在相互竞争关系,常用的解决策略是选择辅酶偏好性不同的辅酶再生系统。在2018年,笔者所在课题组人工设计了一种新型的多酶级联反应催化环烷烃的C‒H键胺化体系(图 11)[15]。该级联反应系统主要由3种酶组成,分别是单加氧酶P450BM3突变体、来自腔肠链霉菌(Streptomyces coelicolo)的醇脱氢酶ScADH和来自西伯里克西果菌(Exiguobacterium sibiricum)的亮氨酸脱氢酶突变体EsLeuDH,在该体系中,作者将第一步羟基化反应所需的双辅酶偏好性的葡萄糖脱氢酶更换成单一NADP+偏好性的木糖醇脱氢酶,以避免其对后续借氢级联步骤的干扰。最终反应48 h后,产物环己胺的滴度达到12.8 mmol/L,产物选择性高达87.4%。Sattler等[101]在一锅多酶级联合成6-氨基己酸的反应中,也运用了同样的策略,从环己醇到己内酯合成过程所用的醇脱氢酶和单加氧酶为NADP(H)依赖性,而从己内酯到6-氨基己酸合成过程所用的醇脱氢酶为NAD+依赖性,从而有效地避免了两个反应的辅酶竞争和干扰。
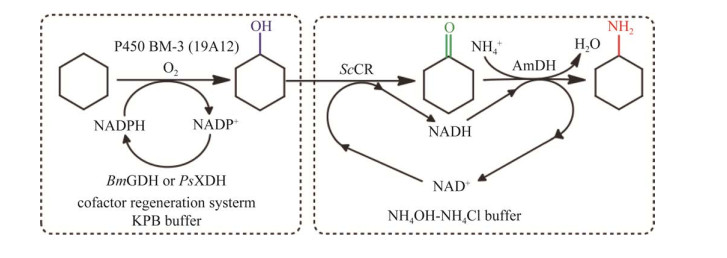
|
| 图 11 “一锅两步”策略将环己烷生物胺化生成环己胺[15] Fig. 11 A "two stage in one pot" strategy catalyzing the bioamination of cyclohexane into cyclohexylamine[15]. The enzymes involved are cytochrome P450 monoxygenase (P450 BM-3), xylitol dehydrogenase (PsXDH), glucose dehydrogenase (BmGDH), alcohol dehydrogenase (ScCR) and amine dehydrogenase (AmDH). |
| |
由于ATP价格昂贵,无法在多酶催化过程中大量添加,因此需要构建ATP再生体系。目前用于ATP再生的激酶主要有3-磷酸-甘油酸激酶(3-phosphate-glycerate kinase, 3-PGK)、乙酸激酶(acetate kinase, AK)、丙酮酸激酶(pyruvate kinase, PK)、肌酸激酶(creatine kinase, CK)、多磷酸激酶(polyphosphate kinase, PPK)、腺苷酸激酶(adenylate kinase, ADK)和氨基甲酸激酶(carbamate kinase, CBK)等(图 12A)。其中PK催化所需的辅底物价格较高,CBK的稳定性差,应用较少;而AK由于具有辅底物成本低和稳定性高等优点,应用较多。例如,默克和Codexis公司合作设计一锅9酶3步法合成抗艾滋病药物islatravir[32]的案例中,就是利用AK进行ATP再生。ADK由于辅底物也是ADP,无需其他辅底物,也常用于ATP再生,且常和多磷酸激酶(PPK2-Ⅱ)或多磷酸AMP磷酸转移酶(polyphosphate AMP phosphotransferase, PPT)进行级联用于ADP/AMP循环(图 12B)。

|
| 图 12 ATP再生常用激酶 Fig. 12 Commonly used kinases for ATP regeneration. A: Kinases commonly used in ATP regeneration and the corresponding substrates. B: The application of PPK/ADK/PPT in ATP regeneration when polyphosphate is used as the substrate. 3PGK: 3-phosphoglyceratekinase; AK: Acetate kinase; PK: Pyruvate kinase; PEP: Phosphoenolpyruvate; CK: Creatine kinase; ADK: Adenylate kinase; CBK: Carbamate kinase; PPK: Polyphosphate kinase; PPT: AMP phosphotransferase. A:ATP再生常用激酶及对应的底物. B:当以多聚磷酸为底物时,PPK/ ADK/PPT在ATP再生中的应用 |
| |
PPK稳定性最好,所需的辅底物多聚磷酸盐成本最低,来源广泛。PPK主要分为PPK1和PPK2两大类(表 1)。其中PPK1合成ATP方向的Keq低,热稳定性好,已有多个不同来源的PPK1用于体外ATP再生。例如,来源于E. coli的PPK1用于再生ATP,以从甲氧戊酸合成阿莫菲二烯[102]以及从丁二酸和甘氨酸合成5-氨基乙酰丙酸[103]。来源于细长热合胞球菌(Thermosynechococcus elongatus, TePPK)的PPK1酶热稳定性好,已成功应用于合成D-氨基酸二肽[104]和谷胱甘肽[105]。
| Catalyst | Source | Reaction | Coproduct | References |
| PPK1 | Escherichia coli K-12 | ADP+(Pi)n=ATP+(Pi)n−1 | Amorphadiene | [102] |
| 5-aminolevulinic acid | [103] | |||
| nucleotide triphosphates | [111] | |||
| PPK1 | Thermosynechococcus elongatus HB27 | ADP+(Pi)n=ATP+(Pi)n−1 | D-amino acid dipeptide | [104] |
| glutathione | [105] | |||
| PPK2-I | Sinorhizobium meliloti 1021 | ADP+(Pi)n=ATP+(Pi)n−1 | Acyl-coA regeneration, | [109] |
| S-adenosylmethionine regeneration | [112] | |||
| aldehydes | [108] | |||
| PPK2-I | Rhodobacter sphaeroides | ADP+(Pi)n=ATP+(Pi)n−1 | L-theanine | [106, 113] |
| PPK2-Ⅱ | Acinetobacter johnsonii | AMP+(Pi)n=ADP+(Pi)n−1 | Acyl-coA regeneration, | [109] |
| S-adenosylmethionine regeneration | [112] | |||
| PPK2-Ⅲ | Meiothermus ruber | ADP+(Pi)n=ATP+(Pi)n-1; AMP+(Pi)n=ADP+(Pi)n-1 |
Nucleotide-activated derivatives of glucose | [114] |
| aldehydes | [108] | |||
| PPK2-Ⅲ | Deinococcus proteolyticus | ADP+(Pi)n=ATP+(Pi)n−1; AMP+(Pi)n=ADP+(Pi)n−1 |
Aminoacyl prolines | [110] |
| PPK2-Ⅲ | Erysipelotrichaceae bacterium | ADP+(Pi)n=ATP+(Pi)n−1; AMP+(Pi)n=ADP+(Pi)n−1 |
Aldehydes | [115] |
由于PPK2合成ATP方向的平衡常数Keq高、催化效率高以及分子量小,所以应用更为广泛。PPK2又可以分为3小类:PPK2-Ⅰ、PPK2-Ⅱ和PPK2-Ⅲ。PPK2-Ⅰ的底物是ADP。例如,在γ-谷氨酰胺合成酶催化谷氨酸钠和乙胺盐酸盐合成L-茶氨酸的反应中,来源于浑球红假单胞菌(Rhodobacter sphaeroides)的PPK2-Ⅰ用于从聚磷酸盐再生ATP[106]。PPK2-Ⅱ的底物是AMP,由于PPK2-Ⅱ酶是AMP的磷酸化,它们必须与腺苷酸激酶(adenylate kinase, ADK)或其他PPKs一起工作以实现ATP再生(图 12B-Ⅱ, Ⅲ, Ⅳ),例如,来自约氏不动杆菌(Acinetobacter johnsonii)的ADK和PPK2-Ⅱ已被用于ATP再生[107]。2019年,Strohmeier等[108]在需要NADPH和ATP作为辅酶的羧酸还原酶催化胡椒酸体外还原反应中,利用PPK2-I和PPK2-Ⅱ催化由AMP再生ATP。同时,PPK2-Ⅱ独特的AMP再生特性在某些情况下非常有用,如利用羧酸-辅酶A连接酶将辅酶A (CoA)再生为酰基辅酶A (acyl-CoA)时需要ATP,释放AMP,其中A. johnsonii PPK2-I和窦根瘤菌(Meliloti Sinorhizobium) PPK2-Ⅱ协同催化可实现由AMP再生ATP[109]。PPK2-Ⅲ的底物既可以是ADP,也可以是AMP,所以PPK2-Ⅲ也能够通过实现从AMP合成ATP,其效果等同于PPK2-Ⅱ和PPK2-Ⅰ或ADK顺序级联催化AMP合成ATP (图 12B-Ⅴ, Ⅵ)。例如,来自溶蛋白芽球菌(Deinococcus proteolyticus)的PPK2-Ⅲ被用于将AMP再生为ATP,该酶被用于与酪氨酸合成酶偶联以合成氨基酰脯氨酸[110]。
2 多酶级联催化合成双官能团化学品的应用进展随着减少环境污染和对可持续性发展意识的提高,全球对双官能团化学品的需求逐渐增加。常见双官能团化学品有ω-氨基脂肪酸(ω-amino fatty acids, ω-AFAs)、烷基内酰胺(alkyl lactams, ALs)、α, ω-二元羧酸(α, ω-dicarboxylic acids, α, ω-DCAs)、α, ω-二胺(α, ω-diamines, α, ω-DMs)、α, ω-二醇(α, ω-diols, α, ω-DOs)和ω-氨基醇(ω-amino alcohols, ω-AOs) (图 13)。其中ω-AFAs、ALs、α, ω-DCAs和α, ω-DMs可作为尼龙的重要单体,α, ω-Dos是合成聚酯和聚氨酯的单体,ω-AOs在医药中间体以及香料、润肤剂和增塑剂中有着广泛的应用,因此它们都具有巨大的市场需求。以碳原子个数为6的化学品为例,6-氨基己酸和己内酰胺是尼龙6 (全球每年近700万t)的单体,己二酸和1, 6-己二胺是尼龙66的单体,1, 6-己二醇是合成聚酯、聚氨酯、黏合剂和不饱和聚酯的材料,6-氨基己醇由于同时具有羟基和氨基,具有醇和胺的通性,是一种用途广泛的医药中间体。
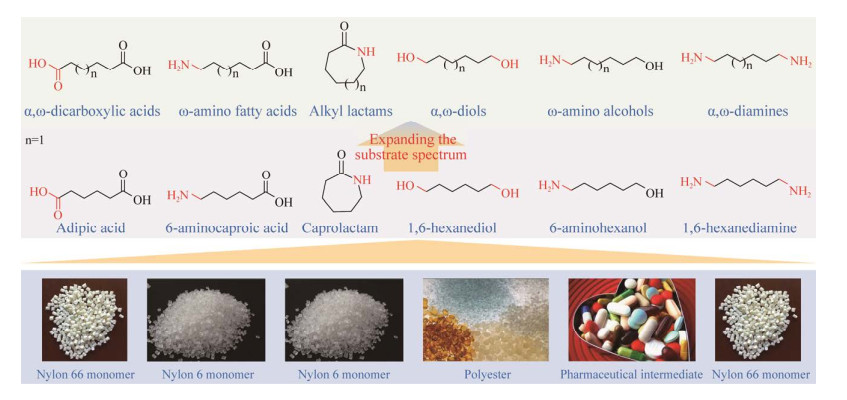
|
| 图 13 六种双官能团化学品结构式及其应用领域 Fig. 13 The structural formulae of six bi-functional chemicals and their applications. |
| |
脂肪族α, ω-二元羧酸(α, ω-DCAs)是一类广泛用于制备香料、尼龙、黏合剂和大环内酯类抗生素的化学品[116-117],主要通过化学法、生物发酵法、多酶级联催化法合成。其中化学合成法以环己烷为起始原料在苛刻条件下进行两步化学氧化合成[118],生物发酵法则是通过改造大肠杆菌内的代谢途径合成己二酸,主要以葡萄糖[119-124]、甘油[125]、纤维素[124]等为起始原料,其代谢合成能力最高达68 g/L[123]。相比于生物发酵法,多酶级联催化法可有效避免竞争性代谢途径、避免物理障碍和毒性问题,可以为非天然产物构建新合成途径。湖北大学李爱涛课题组成功设计了大肠杆菌菌群一锅法生物催化环烷烃合成α, ω-二元羧酸[126],相比于发酵法,该方法的α, ω-二元羧酸产物(C5–C8)滴度明显更高,如戊二酸(1.6–6.3 g/L vs. 0.82 g/L)[127]和辛二酸(1.1–7.3 g/L vs. 0.254 g/L)[128],己二酸产品滴度高达66 g/L,与文献报道的最高值(68 g/L)相当[123] (表 2)。
| Product | Substrates | Methods | Titer (g/L) | References |
| Glutaric acid | Glucose | Fermentation (growing cell) | 0.820 | [127] |
| Cyclopentane/ Cyclopentanol |
Biocatalysis (resting cell) | 1.60/ 6.30 |
[126] | |
| Adipic acid | Glucose | Fermentation (growing cell) | 68.0 | [123] |
| Cyclohexane/ Cyclohexanol/ ε-caprolactone |
Biocatalysis (resting cell) | 4.50/ 6.70/ 66.0 |
[126] | |
| Glucose | Fermentation (growing cell) | 6.39×10–4 | [122] | |
| Glucose | Fermentation (growing cell) | 2.76×10–2 | [125] | |
| Glucose | Fermentation (growing cell) | 2.59×10–3 | [128] | |
| Glucose | Fermentation (growing cell) | 2.23 | [124] | |
| Pimelic acid | Cycloheptane/ Cycloheptanol |
Biocatalysis (resting cell) | 3.20/ 7.70 |
[126] |
| Suberic acid | Glycerol | Fermentation (growing cell) | 0.254 | [128] |
| Cyclooctane/ Cyclooctanol |
Biocatalysis (resting cell) | 1.10/ 7.30 |
[126] | |
| Octanoic acid | Biocatalysis (resting cell) | 1.45 | [50] | |
| Nonanedioic acid | Nonanoic acid, hydroxynonanoic acid | Biocatalysis (resting cell) | 3.69 | [51] |
| Undecanedioic acid | Ricinoleic acid | Biocatalysis (resting cell) | 206 | [64] |
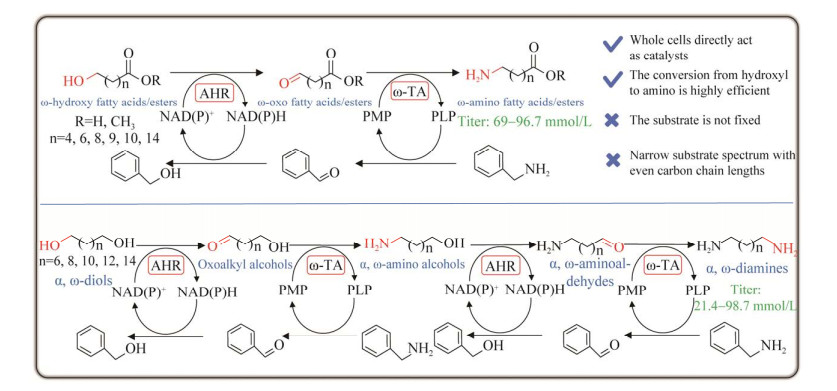
ω-氨基脂肪酸(ω-AFAs)是一类重要的脂肪酸衍生物,具有α-羧基和ω-氨基2个官能团,是一类重要的聚酰胺合成单体,可进一步缩合生成聚酰胺(polyamide),是尼龙(nylon)的重要单体,具有广泛的应用价值。目前,科研人员已经成功构建了多条生物催化级联路线用于合成ω-氨基脂肪酸和烷基内酰胺(表 3),主要集中于尼龙6、尼龙11和尼龙12单体的合成。
| Catalysts | Substrates | Products | Titer (mmol/L) | Function | References |
| ADH, BVMO, PFE, AlkJ, ω-TA |
11-hydroxystearic acid | 11-aminoundecanoic acid | 2.5 | Nylon 11 monomer | [129] |
| AlkJ, ω-TA | 12-hydroxydodecanoic acid | 12-aminododecanoic acid | 2.6 | Nylon 12 monomer | [129] |
| AHR, ω-TA | ω-hydroxy fatty acids (C=6, 8, 10, 11, 12, 16) |
ω-amino fatty acids (C=6, 8, 10, 11, 12, 16) |
> 84.7 | Nylon monomer | [47] |
| ADH, BVMO, Lip, NFO, TA, |
Ricinoleic acid | 11-aminoundecanoic acid |
232 | Nylon 11 monomer | [64] |
| AHR, ω-TA | ω-hydroxy dodecanoic acid methyl ester |
ω-amino dodecanoic acid methyl ester |
69.0 | Nylon 12 monomer | [47] |
| P450, ChnD, ω-TA (FDH, NOX) |
Nonanoic acid, 9-hydroxynonanoic acid |
9-aminononanoic acid | 4.1 | Nylon 9 monomer | [51] |
| AlkBGT, ω-TA, AlkL | Dodecanoic acid methyl ester | 12-aminododecanoic acid methyl ester |
0.55 | Nylon 12 monomer | [130] |
| BVMO, EndLP, AHR, ω-TA |
Fatty acid methyl esters (C=9, 11, 13) |
ω-amino fatty acids (C=8, 10, 12) |
29.1–46.3 | Nylon monomer | [48] |
| P450, AlkJ, ω-TA | Dodecanoic acid | 12-amino dodecanoic acid |
1.48 | Nylon 12 monomer | [131] |
| P450, ADH, ω-TA, AlaDH |
Dodecanoic acid | 12-amino dodecanoic acid |
4.83 | Nylon 12 monomer | [132] |
| P450, AlkJ, ω-TA | Dodecanoic acid | 12-amino dodecanoic acid |
0.6 | Nylon 12 monomer | [69] |
| P450, ChnD, ω-TA (FDH, NOX) |
Octanoic acid | 8-aminooctanoic acid | 8.6 | Nylon 8 monomer | [50] |
| Esterase, ADH, ω-TA, AlaDH |
ε-caprolactone | 6-aminohexanoic acid | 37.5 | Nylon 6 monomer | [101] |
| LA, AHR, ω-TA | ε-caprolactone | 6-aminohexanoic acid | 80.0 | Nylon 6 monomer | [47] |
| CalB, ADH, ω-TA | ε-caprolactone | 6-aminohexanoic acid | 42.0 | Nylon 6 monomer | [133] |
| CalB, ADH, ω-TA | δ-valerolactone | 5-aminovaleric acid | 12.0 | Nylon 5 monomer | [133] |
| CalB, ADH, ω-TA | γ-butyrolactone | γ-aminobutyric acid | 15.0 | Nylon 4 monomer | [133] |
| ω-TA, CHMO, LA, AHR |
Cycloalkyl-amines (C=4, 5, 6) |
ω-amino fatty acids (C=4, 5, 6) |
> 48.1 | Nylon monomer | [134] |
| ADH, CHMO, LA, AHR, ω-TA |
Cyclohexanol | 6-aminohexanoic acid | 97.2 | Nylon 6 monomer | [52] |
| CAR, sfp, ω-TA, GluDH (PPK2, FDH, PPA) |
Adipic acid | 6-aminohexanoic acid | 9.5 | Nylon 6 monomer | [135] |
| ω-TA, CHMO, LA, AHR, Cys, |
Cycloalkylamines (C=4, 5, 6) |
Alkyl lactams | 0.41–7.4 | Nylon monomer | [134] |
| ADH, CHMO, LA, AHR, ω-TA, CAR, Cys |
Cyclohexanol | Caprolactam | 2.84 | Nylon 6 monomer | [52] |
| ADH: Alcohol dehydrogenase; BVMO: Baeyer-Villiger monooxygenase; LA: Lactonase; AlaDH: Alanine dehydrogenase; ω-TA: ω-transaminase; CAR: Carboxylic acid reductase; sfp: Phosphopantetheinyl transferase; FDH: Formate dehydrogenase; PPK2: Polyphosphate kinase; PPA: Pyrophosphatase; PFE: Esterase; CHMO: Cyclohexanone monooxygenases; EndLP: Endogenous lipase; ALDH: Aldehyde dehydrogenase; NOX: NAD(P)H oxidase; Lip: Lipase; P450: Cytochrome P450 monooxygenase; NFO: NAD(P)H flavin oxidoreductase; AHR: Aldehyde reductase. | |||||
早在2014年,Sattler等[101]构建了多酶级联反应路径,以环己醇作为出发底物,在只消耗氧气和氨气的条件下合成尼龙6单体6-氨基己酸。该合成路线可以实现辅因子的自给自足,从环己醇生产ε-己内酯,随后进一步合成6-氨基己酸(图 14A),但是产率只有1%。研究发现6-羟基己酸对醇脱氢酶存在抑制作用,通过“羧基封端策略”,以最大限度地减少中间体6-羟基己酸的形成。利用此策略,前体ε-己内酯在存在甲醇的水性介质中被转化为相应的甲酯,而不是酸。同时,首次表明马肝酯酶可以催化ε-己内酯的选择性开环,并且明显偏好甲醇而不是水作为亲核试剂。当底物环己醇浓度为50 mmol/L时,尼龙单体的产率提高到了24%。该路线为生物酶法催化环己醇合成尼龙6单体开辟了先河,但是产率依然较低,可能是因为甲醇对单加氧酶有抑制作用。
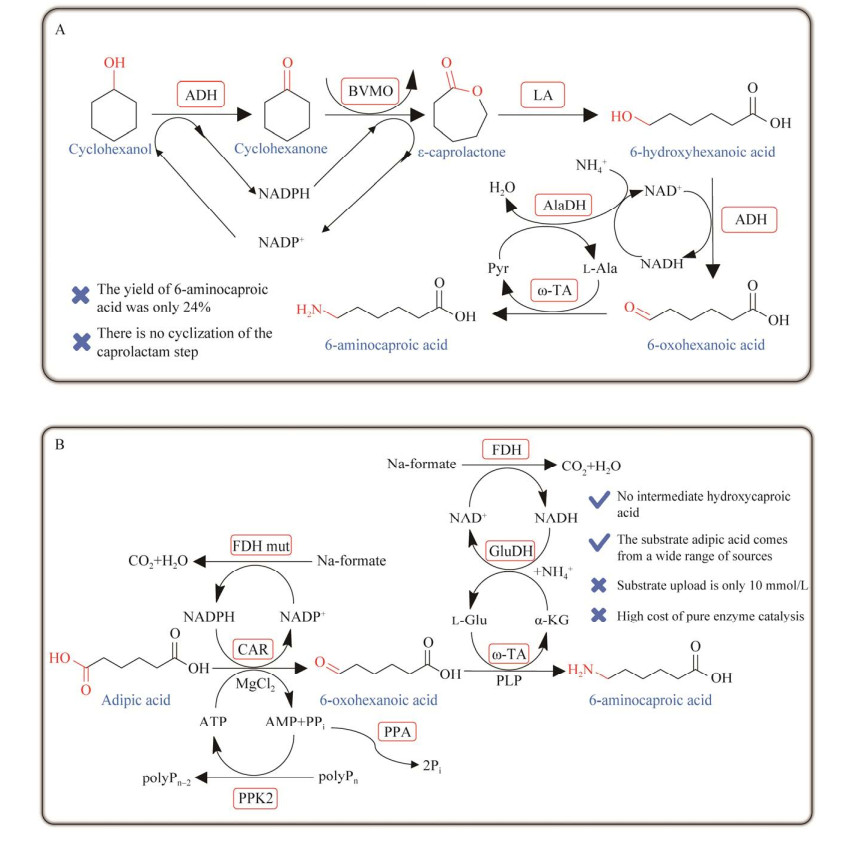
|
| 图 14 一锅法多酶级联催化不同底物合成6-氨基己酸 Fig. 14 One-pot multi-enzyme cascade biosynthesis of 6-aminohexanoic acid from different substrates. A: Multi-enzyme cascade catalysed synthesis of 6-aminohexanoic acid from cyclohexanol. ADH: Alcohol dehydrogenase; BVMO: Baeyer-Villiger monooxygenase; LA: Lactonase; AlaDH: Alanine dehydrogenase; ω-TA: ω-transaminase. B: Biotransformation of adipic acid to 6-aminohexanoic acid. CAR: Carboxylic acid reductase; FDH: Formate dehydrogenase; PPK2: Polyphosphate kinase; PPA: Pyrophosphatase; ω-TA: ω-transaminase; GluDH: Glutamate dehydrogenase. |
| |
2020年,Fedorchuk等[135]以己二酸为底物,建立了一锅酶法级联反应体系,利用羧酸还原酶(CARs)和转氨酶(TAs)催化10 mmol/L己二酸合成6-氨基己酸的产率高达95% (图 14B)。该途径中将CAR进行分子改造,使其对6-氨基己酸的催化效率显著提高,同时筛选大量的TAs,将6-氨基己酸到己二胺的路线也成功打通。但是己二胺的产率依然较低(30%),同时为了避免反应过程中产生副产物,所有酶制剂均采用纯酶的形式进行催化,导致操作流程复杂,生产成本过高,且底物上载量只有10 mmol/L,远远达不到工业化生产的要求。
烷基内酰胺作为尼龙(nylon)的重要单体,目前工业化合成尼龙单体主要是利用化学法以环己醇为底物经4步催化合成己内酰胺(图 15A),但是该化学合成路线具有反应条件苛刻、环境污染大、副产物多等缺点,在工业应用中存在限制[136]。生物法合成烷基内酰胺的常用策略是先合成ω-氨基脂肪酸,然后酶促ω-氨基脂肪酸环化合成烷基内酰胺。2021年,韩国建国大学Yun等[134]以环烷基胺为底物,构建了一个智能的辅酶/副产物循环体系,用于高效合成尼龙单体ω-氨基脂肪酸和烷基内酰胺(C=4, 5, 6) (图 15B)。由于最后一步的环化酶是限速步骤,作者通过筛选对ω-氨基脂肪酸催化效率高的环化酶并进一步改造,随后对该级联过程中的酶进行共表达,利用全细胞高效催化合成ω-氨基脂肪酸和烷基内酰胺,产率分别达到96%和74%。该级联路线具有不添加辅酶和产率高等优点,但是依然存在着底物环烷基胺价格昂贵等问题。

|
| 图 15 工业上化学法合成己内酰胺(A)和一锅法多酶级联催化环烷基衍生物合成ω-氨基脂肪酸和烷基内酰胺(B) Fig. 15 Chemical catalysts (A) and One-pot multi-enzyme cascade biosynthesis of ω-amino fatty acids and alkyl lactams from cycloalkyl derivatives (B). ω-TA: ω-transaminase; CHMO: Cyclohexanone monooxygenases; LA: Lactonase; AHR: Aldehyde reductase. |
| |
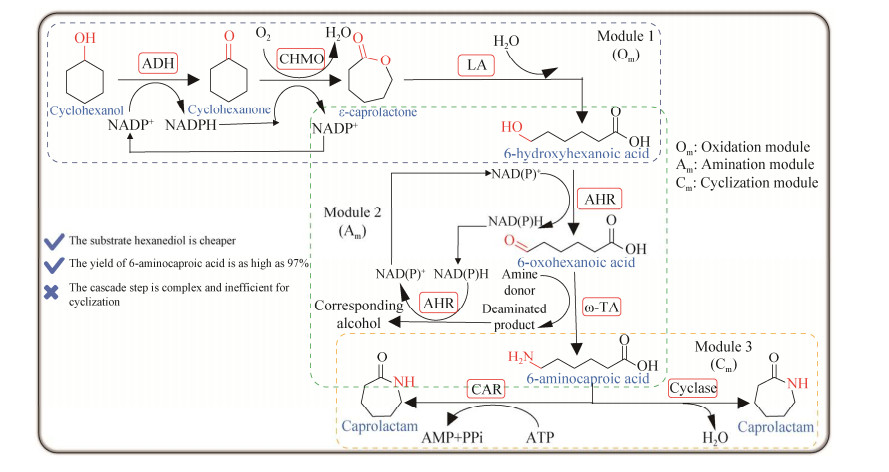
2021年,韩国建国大学Yun团队又开发了一种以环己醇为底物,采用基于单元的模块化策略,利用全细胞菌群催化环己醇生产6-氨基己酸和ε-己内酰胺(图 16)。该路线是一个具有高原子经济性和辅因子自给自足的多酶级联生物催化系统[52],无需额外添加辅酶。设计的每个模块对应一个大肠杆菌细胞,然后利用菌群协作催化100 mmol/L环己醇合成6-氨基己酸,6-氨基己酸得率超过97%,进一步偶联环化步骤可生成28%的己内酰胺。该路线具有底物价格低,6-氨基己酸产率高等优势,但是依然存在着级联步骤复杂和环化效率低等问题。
|
| 图 16 一锅法多酶级联模块化菌群催化环己醇合成6-氨基己酸和烷基内酰胺 Fig. 16 One-pot multi-enzyme cascade biosynthesis of 6-aminohexanoic acid and alkyl lactams from cyclohexanol using Escherichia coli-based concurrent cascade consortia. ADH: Alcohol dehydrogenase; ω-TA: ω-transaminase; CHMO: Cyclohexanone monooxygenases; LA: Lactonase; AHR: Aldehyde reductase; CAR: Carboxylic acid reductase. |
| |
除了上述尼龙单体,双官能团化学品还有α, ω-二醇(α, ω-DOs)和ω-氨基醇(ω-AOs)。α, ω-Dos可作为聚酯和聚氨酯的单体,ω-AOs在散装化学品以及香料、润肤剂和增塑剂中有着广泛的应用。近10年来多个研究团队致力于利用多酶级联催化反应合成α, ω-二醇和ω-氨基醇(表 4),并取得了一定的成果。
| Catalysts | Substrate | Product | Titer | Function | References |
| BVMO, EndLP, CAR, sfp, EndAHRs |
ω-fatty acid methyl esters (C=9, 11, 13) | α, ω-diol (C=8, 10, 12) | 8.3–27.0 mmol/L | Poly(ammonia) ester monomer |
[48] |
| P450, ADH, BVMO, Lac, CAR, PPT, AKR (GDH) |
Cyclohexane | 1, 6-hexanediol | 13 mmol/L | Polyesters, polyurethane |
[137] |
| P450, ADH, BVMO, Lac, CAR, sfp, AKR(GDH) |
Cyclohexanol | 1, 6-hexanediol | 16–18 mmol/L | Polyesters, polyurethane |
[137] |
| MFL, AAD, KDC, YqhD | Amino acid | Diol | 4.2‒276.4 mg/L | Monomers for polymer | [138] |
| CAR, sfp, AHR | ω-hydroxy fatty acids (C8–C16) |
α, ω-diol (C8–C16) |
3.01–9.70 mmol/L | Poly(ammonia) ester monomer |
[139] |
| P450, CAR, sfp, AHR | dodecanoic acid | 1, 12-dodecanediol | 6.9 mmol/L | Poly(ammonia) ester monomer |
[139] |
| BVMO, EndLP, AHR, ω-TA, CAR, sfp, EndAHRs |
ω-fatty acid methyl esters (C=9, 11, 13) | ω-amino alcohol (C=8, 10, 12) |
4.90–29.12 mmol/L | Fragrances, emollients and plasticizers |
[48] |
| EP, EH, ADH, ω-TA, AlaDH |
Terminal alkenes | 1, 2-amino alcohol | 3.2–17.2 mmol/L | β-adrenergic receptor agonists |
[16] |
| ObiH, TDC | Aldehydes | 1, 2-amino alcohol | 0.015–0.480 mmol/L | β-adrenergic receptor agonists |
[140] |
| EH, ADH, ω-TA | Epoxides | 1, 2-amino alcohol | 32.5–48.2 mmol/L | β-adrenergic receptor agonists |
[141] |
| TAL, tPAD1, FDH, SMO, EH, ADH, ω-TA |
L-henylalanine | 1, 2-amino alcohol | 12.2 mmol/L | β-adrenergic receptor agonists |
[142] |
| BVMO: Baeyer-Villiger monooxygenase; EndLP: Endogenous lipase; CAR: Carboxylic acid reductase; sfp: Phosphopantetheinyl transferase; EndAHRs: Endogenous aldehyde reductases; P450: Cytochrome P450 monoxygenase; ADH: Alcohol dehydrogenase; Lac: Lactonase; AKR: Aldo-keto reductase; GDH: Glucose dehydrogenas; MFL: Hydroxylase; AAD: L-amino acid deaminase; KDC: α-keto acid decarboxylase; YqhD: Aldehyde reductase; ω-TA: ω-transaminase; AlaDH: Alanine dehydrogenase; ObiH: Transaldolase; TDC: Tryptophan decarboxylase; EH: Epoxide hydrolase; TAL: Tyrosine ammonia lyase; tPAD1: Ferulic acid decarboxylase; FDH: Formate dehydrogenase; SMO: Styrene monooxygenase. | |||||
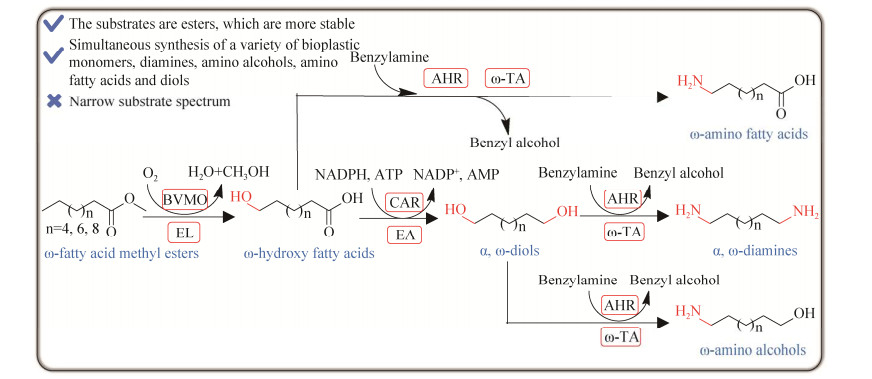
最近,Yun等[47]将胺化步骤作为菌群中的一个基本模块(胺化模块:Cell-Am)用于将底物的末端羟基转化为氨基,基于此报道了一种多细胞菌群催化合成生物塑料单体的多酶级联反应系统(图 17)[48]。除了胺化模块外,还有2个其他功能细胞模块,包括将脂肪酸甲酯(fatty acid methyl ester, FAME)转化为ω-羟基脂肪酸(ω-hydroxy fatty acid, ω-HFA)的羟基化模块(Cell-Hm)和将脂肪酸的羧基转化为羟基的还原模块(Cell-Rm)。随后以目标产物为导向,对这些细胞模块进行组装,配合不同参数的优化,一锅法催化不同碳链长度(C8、C10和C12)的脂肪酸甲酯合成ω-氨基脂肪酸、α, ω-二醇、ω-氨基醇和α, ω-二胺,其中12-氨基脂肪酸和1, 12-二醇的分离得率分别为66.5%和52.5%。
|
| 图 17 一锅法多酶级联催化ω-脂肪酸甲酯合成不同的生物塑料单体 Fig. 17 One-pot multi-enzyme cascade biosynthesis of various bioplastic monomers from ω-fatty acid methyl esters. BVMO: Baeyer-Villiger monooxygenase; EL: Endogenous lipase; CAR: Carboxylic acid reductase; EA: Endogenous aldehyde reductase; AHR: Aldehyde reductase; ω-TA: ω-transaminase. |
| |
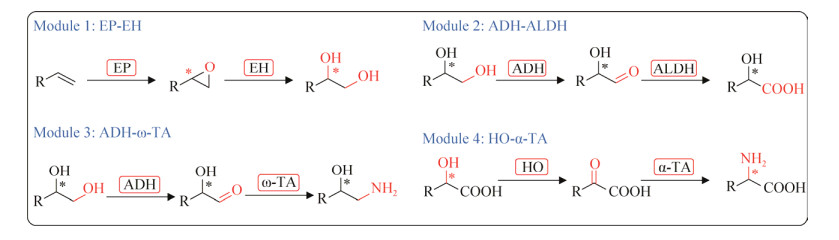
2016年,新加坡国立大学李智团队同样通过模块化级联策略对烯烃进行高度区域和对映选择性地多重氧化和氨基官能化[16] (图 18)。作者首先成功设计了4个不同功能的细胞模块,第1个模块包含环氧化物酶(epoxidase, EP)和环氧化物水解酶(epoxide hydrolase, EH),将末端烯烃水解为1, 2-二醇;第2个模块包含醇脱氢酶(alcohol dehydrogenase, ADH)和醛脱氢酶(aldehyde dehydrogenase, ALDH)用于将1, 2-二醇末端双氧化为α-羟基酸;第3个模块包含ADH、ω-转氨酶(ω-transaminase, ω-TA)和丙氨酸脱氢酶(alanine dehydrogenase, AlaDH)用于将1, 2-二醇氧化转氨为1, 2-氨基醇;第4个模块包含羟基酸氧化酶(hydroxy acid oxidase, HO)、α-转氨酶(α-transaminase, α-TA)、过氧化氢酶(catalase, CAT)和谷氨酸脱氢酶(glutamate dehydrogenase, GluDH),用于将α-羟基酸氧化转氨为α-氨基酸。将每个模块的酶都成功地在同一个质粒上共表达,且将模块中的基因克隆到其他3个不同的常用质粒上表达,比较其催化效率。将模块1和模块2进行组合和表达优化,催化120 mmol/L模式底物苯乙烯合成α-羟基酸,22 h内α-羟基酸的转化率为78% (98% ee)。以同样的方式将模块1和模块3进行组合,催化60 mmol/L苯乙烯合成1, 2-氨基醇,12 h内1, 2-氨基醇的转化率为70% (98% ee)。同样,将模块1_2和模块4进行组合和优化,催化60 mmol/L苯乙烯合成α-氨基酸,24 h内α-氨基酸的转化率为80%。
|
| 图 18 模块化级联生物催化对苯乙烯进行区域和对映选择性多重氧化和氨基官能化4个基本模块 Fig. 18 Four general basic enzyme modules of regio- and enantioselective multiple oxy- and amino-functionalizations of styrenes by modular cascade biocatalysis. EP: Epoxidase; EH: Epoxide hydrolase; ADH: Alcohol dehydrogenase; ALDH: Aldehyde dehydrogenase; ω-TA: ω-transaminase; HO: Hydroxy acid oxidase; and α-TA: α-transaminase. |
| |
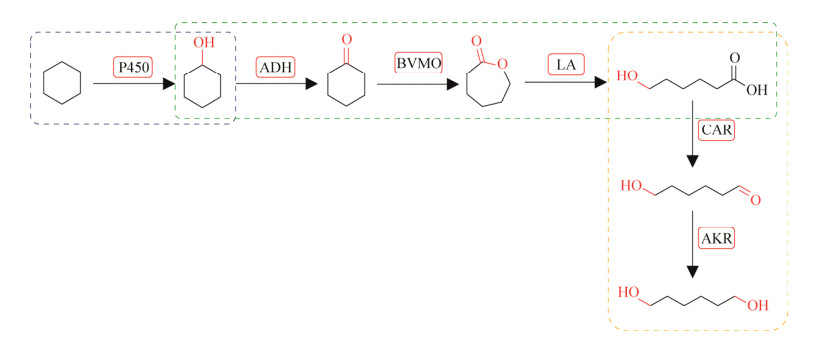
2020年,湖北大学李爱涛课题组从头设计了包含3个模块化菌群,实现一锅多酶级联催化环己烷合成1, 6-己二醇[137]。基于氧化还原自给自足,将参与生物催化级联的所有酶都分布在3个基本细胞模块中,每个模块中的酶都在一个细胞中共表达,然后3个细胞组成菌群协同催化合成1, 6-己二醇(图 19)。需要指出的是在模块3的最后一步催化过程中,不同于直接利用细胞的内源性醛脱氢酶将醛还原为醇,该处是利用恶臭假单胞菌(Pseudomonas putida)来源的醛酮还原酶PpAKR与葡萄糖脱氢酶(BmGDH)[88]介导的NADPH再生偶联将醛还原为醇。通过组合模块菌群2_3催化20 mmol/L环己醇高效合成16–18 mmol/L的1, 6-己二醇,组合模块菌群1_2_3催化30 mmol/L环己烷成功合成13 mmol/L的1, 6-己二醇,并进一步将底物谱拓展至不同碳原子数(C5–C8)的环烷烃。
|
| 图 19 从环己烷生产1, 6-己二醇的从头设计的生物催化级联路线的设计和模块化 Fig. 19 Design and modularization of a de novo biocatalytic cascade for 1, 6-hexanediol production from cyclohexane. P450: Cytochrome P450 monooxygenase; ADH: Alcohol dehydrogenase; BVMO: Baeyer-Villiger monooxygenase; LA: Lactonase; CAR: Carboxylic acid reductase; AKR: Aldo-keto reductase. |
| |
不同于上述多酶级联催化合成某个特定的二元醇,2022年西湖大学曾安平等[138]设计了一条4酶(氨基酸羟基酶、L-氨基酸脱氨酶、α-酮酸脱羧酶和乙醛还原酶)顺序级联催化路线催化转化6种不同的氨基酸(缬氨酸、亮氨酸、异亮氨酸、戊氨酸、正亮氨酸和苏氨酸),成功合成了10种不同的二醇,其中有6种二醇是利用生物法首次合成。
2.4 多酶级联催化合成α, ω-二胺尼龙是我们日常生活中最基本和最广泛使用的材料之一,2020年全球年产量已达到970万t,α, ω-二胺[143]和α, ω-二元羧酸是合成聚酰胺(也称为尼龙)的单体[123],如己二胺和己二酸作为尼龙66的单体。表 5总结了目前多酶级联催化不同底物合成α, ω-二胺的研究进展。
| Catalysts | Substrate | Product (α, ω-diamine) | Titer (mmol/L) | Function | References |
| ADH, ω-TA, AlaDH | α, ω-diol (C=10, 12) | C=10, 12 | > 49.5 | Nylon monomer | [144] |
| AHR, ω-TA | α, ω-diol (C=8, 10, 12, 14, 16) |
C=8, 10, 12, 14, 16 | 21.4–98.7 | Nylon monomer | [47] |
| BVMO, EndLP, AHR, ω-TA, CAR, sfp, EndAHRs |
Fatty acid methyl esters (C=9, 11, 13) |
C=8, 10, 12 | 4.2–21.5 | Nylon monomer | [48] |
| CAR, sfp, ω-TA (GluDH, PPK2, FDH, PPA) |
Adipic acid | C=6 | 3.0 | Nylon 66 monomer | [135] |
| ADH: Alcohol dehydrogenase; ω-TA: ω-transaminase; AlaDH: Alanine dehydrogenase; AHR: Aldehyde reductases; BVMO: Baeyer-Villiger monooxygenase; EndLP: Endogenous lipase; CAR: Carboxylic acid reductase; sfp: Phosphopantetheinyl transferase; EndAHRs: Endogenous aldehyde reductases; PPK2: Polyphosphate kinase; PPA: Pyrophosphatase; GluDH: Glutamate dehydrogenase; FDH: Formate dehydrogenase. | |||||
早在2012年,奥地利格拉茨大学的Kroutil等[144]成功构建了氧化还原自给自足的伯醇胺化反应路径,主要是利用醇脱氢酶和转氨酶级联将伯醇胺化,同时利用丙氨酸脱氢酶构建自给自足的辅酶再生系统,两次胺化即可将α, ω-二醇转化为α, ω-二胺,但是存在底物抑制和产物过氧化等问题。2018年,Yun等[47]创造性地发明了两步级联法将醇胺化产生ω-氨基脂肪酸和α, ω-二胺(图 20),与上述路线不同的是,该路线中作者引入了新颖的平行反义级联(氨基供体苄胺逆向脱氨最终转化为羟基)该路线中首先筛选获得来自集胞藻属(Synechocystis species)的NAD(P)+依赖性醛还原酶SsAHR和来源于硅酸杆菌(Silicibacter pomeroyi)的ω-转氨酶PMTA,利用SsAHR和PMTA在单细胞中共表达催化ω-羟基脂肪酸合成ω-氨基脂肪酸,并将底物谱拓展至C6–C16,底物浓度也提高至200 mmol/L,均取得了较高的胺产率。随后,利用此级联体系催化转化100 mmol/L的α, ω-二醇(C8–C14),经过2次胺化成功合成α, ω-二胺(C8–C14),产物二胺浓度最高达88 mmol/L。最后,通过结合内酯酶催化100 mmol/L ε-己内酯成功合成80 mmol/L的6-氨基己酸。
|
| 图 20 一种新的“平行反义”级联反应催化合成α, ω-二胺 Fig. 20 Enzymatic synthesis of α, ω-diamines via a novel "parallel anti-sense" cascade reaction. AHR: Aldehyde reductase; ω-TA: ω-transaminase. |
| |
与传统发酵生产相比,多酶级联催化合成化学品优势非常明显,功能可控性强,反应条件易控制,产品滴度更高。随着多种不同酶催化剂及其新功能的不断发现,越来越多的多酶级联体系被成功构建。但是目前大多数多酶级联反应仍处于实验室阶段,底物上载量低,催化体系小,合成效率无法达到大规模生产的需求。其中主要的原因在于所需元件酶的稳定性和催化效率等仍需进一步提高,如BVMO单加氧酶和P450羟化酶等往往成为级联反应的限速酶。缺乏级联反应路线中的关键酶,尤其是合成机制复杂尚未报道的酶元件也是多酶级联反应的一个关键瓶颈。当级联反应体系过于复杂时,酶的表达调控困难,催化体系的兼容性更具挑战,反应体系中多个底物、辅因子对酶催化效率的影响更大。
自2018年阿诺德教授因在定向进化方面的杰出工作获诺贝尔奖以来,全世界多个实验室都在尝试利用定向进化等技术手段以获得具备特定功能的酶元件,从而为多酶级联体系的构建提供更丰富的工具箱。2020年,AlphaFold的横空出世,通过酶的氨基酸序列精确预测蛋白三维结构,为酶的理性设计打开了新的大门。近些年来发展的辅酶再生技术日渐成熟,多酶融合蛋白技术以及菌群催化技术得到了进一步完善和应用,使多酶级联反应成本的进一步降低成为可能。未来应深入研究多酶催化反应动力学和热力学并建立对应的数学模型[87],将多酶级联路线设计与计算相结合,真正实现理论指导实践。
| [1] |
BARBER DM, ĎURIŠ A, THOMPSON AL, SANGANEE HJ, DIXON DJ. One-pot asymmetric nitro-mannich/hydroamination cascades for the synthesis of pyrrolidine derivatives: combining organocatalysis and gold catalysis. ACS Catalysis, 2014, 4(2): 634-638. DOI:10.1021/cs401008v
|
| [2] |
MUSCHIOL J, PETERS C, OBERLEITNER N, MIHOVILOVIC MD, BORNSCHEUER UT, RUDROFF F. Cascade catalysis-strategies and challenges en route to preparative synthetic biology. Chemical Communications, 2015, 51(27): 5798-5811. DOI:10.1039/C4CC08752F
|
| [3] |
SCHRITTWIESER JH, VELIKOGNE S, HALL M, KROUTIL W. Artificial biocatalytic linear cascades for preparation of organic molecules. Chemical Reviews, 2018, 118(1): 270-348. DOI:10.1021/acs.chemrev.7b00033
|
| [4] |
SPERL JM, SIEBER V. Multienzyme cascade reactions-status and recent advances. ACS Catalysis, 2018, 8(3): 2385-2396. DOI:10.1021/acscatal.7b03440
|
| [5] |
SANTACOLOMA PA, SIN G, GERNAEY KV, WOODLEY JM. Multienzyme-catalyzed processes: next-generation biocatalysis. Organic Process Research & Development, 2011, 15(1): 203-212.
|
| [6] |
RICCA E, BRUCHER B, SCHRITTWIESER JH. Multi-enzymatic cascade reactions: overview and perspectives. Advanced Synthesis and Catalysis, 2011, 353(13): 2239-2262. DOI:10.1002/adsc.201100256
|
| [7] |
OROZ-GUINEA I, GARCÍA-JUNCEDA E. Enzyme catalysed tandem reactions. Current Opinion in Chemical Biology, 2013, 17(2): 236-249. DOI:10.1016/j.cbpa.2013.02.015
|
| [8] |
BENÍTEZ-MATEOS AI, ROURA PADROSA D, PARADISI F. Multistep enzyme cascades as a route towards green and sustainable pharmaceutical syntheses. Nature Chemistry, 2022, 14(5): 489-499. DOI:10.1038/s41557-022-00931-2
|
| [9] |
LOPEZ-GALLEGO F, SCHMIDT-DANNERT C. Multi-enzymatic synthesis. Current Opinion in Chemical Biology, 2010, 14(2): 174-183. DOI:10.1016/j.cbpa.2009.11.023
|
| [10] |
FRANCE SP, HEPWORTH LJ, TURNER NJ, FLITSCH SL. Constructing biocatalytic cascades: in vitro and in vivo approaches to de novo multi-enzyme pathways. ACS Catalysis, 2017, 7(1): 710-724. DOI:10.1021/acscatal.6b02979
|
| [11] |
SIEDENTOP R, CLAAßEN C, ROTHER D, LÜTZ S, ROSENTHAL K. Getting the most out of enzyme cascades: strategies to optimize in vitro multi- enzymatic reactions. Catalysts, 2021, 11(10): 1183. DOI:10.3390/catal11101183
|
| [12] |
CHEN FF, LIU YY, ZHENG GW, XU JH. Asymmetric amination of secondary alcohols by using a redox-neutral two-enzyme cascade. ChemCatChem, 2015, 7(23): 3838-3841. DOI:10.1002/cctc.201500785
|
| [13] |
MUTTI FG, KNAUS T, SCRUTTON NS, BREUER M, TURNER NJ. Conversion of alcohols to enantiopure amines through dual-enzyme hydrogen-borrowing cascades. Science, 2015, 349(6255): 1525-1529. DOI:10.1126/science.aac9283
|
| [14] |
SONG W, WANG JH, WU J, LIU J, CHEN XL, LIU LM. Asymmetric assembly of high-value α-functionalized organic acids using a biocatalytic chiral-group- resetting process. Nature Communications, 2018, 9: 3818. DOI:10.1038/s41467-018-06241-x
|
| [15] |
YU HL, LI T, CHEN FF, LUO XJ, LI AT, YANG C, ZHENG GW, XU JH. Bioamination of alkane with ammonium by an artificially designed multienzyme cascade. Metabolic Engineering, 2018, 47: 184-189. DOI:10.1016/j.ymben.2018.02.009
|
| [16] |
WU SK, ZHOU Y, WANG TW, TOO HP, WANG DIC, LI Z. Highly regio- and enantioselective multiple oxy- and amino-functionalizations of alkenes by modular cascade biocatalysis. Nature Communications, 2016, 7: 11917. DOI:10.1038/ncomms11917
|
| [17] |
BOTH P, BUSCH H, KELLY PP, MUTTI FG, TURNER NJ, FLITSCH SL. Whole-cell biocatalysts for stereoselective C–H amination reactions. Angewandte Chemie International Edition, 2016, 55(4): 1511-1513. DOI:10.1002/anie.201510028
|
| [18] |
LUO zi wei, LEE SY. Biotransformation of p-xylene into terephthalic acid by engineered Escherichia coli. Nature Communications, 2017, 8: 15689. DOI:10.1038/ncomms15689
|
| [19] |
LIU J, LI Z. Enhancing cofactor recycling in the bioconversion of racemic alcohols to chiral amines with alcohol dehydrogenase and amine dehydrogenase by coupling cells and cell-free system. Biotechnology and Bioengineering, 2019, 116(3): 536-542. DOI:10.1002/bit.26896
|
| [20] |
BRUGGINK A, SCHOEVAART R, KIEBOOM T. Concepts of nature in organic synthesis: cascade catalysis and multistep conversions in concert. Organic Process Research & Development, 2003, 7(5): 622-640.
|
| [21] |
RODRIGUEZ C, LAVANDERA I, GOTOR V. Recent advances in cofactor regeneration systems applied to biocatalyzed oxidative processes. Current Organic Chemistry, 2012, 16(21): 2525-2541. DOI:10.2174/138527212804004643
|
| [22] |
YOU ZN, ZHOU K, HAN Y, YANG BY, CHEN Q, PAN J, QIAN XL, LI CX, XU JH. Design of a self-sufficient hydride-shuttling cascade for concurrent bioproduction of 7, 12-dioxolithocholate and L-tert- leucine. Green Chemistry, 2021, 23(11): 4125-4133. DOI:10.1039/D1GC01120K
|
| [23] |
KOSZELEWSKI D, LAVANDERA I, CLAY D, ROZZELL D, KROUTIL W. Asymmetric synthesis of optically pure pharmacologically relevant amines employing ω-transaminases. Advanced Synthesis & Catalysis, 2008, 350(17): 2761-2766.
|
| [24] |
SCHÄTZLE S, STEFFEN-MUNSBERG F, THONTOWI A, HÖHNE M, ROBINS K, BORNSCHEUER UT. Enzymatic asymmetric synthesis of enantiomerically pure aliphatic, aromatic and arylaliphatic amines with (R)-selective amine transaminases. Advanced Synthesis & Catalysis, 2011, 353(13): 2439-2445.
|
| [25] |
TRUPPO MD, TURNER NJ, DAVID ROZZELL J. Efficient kinetic resolution of racemic amines using a transaminase in combination with an amino acid oxidase. Chemical Communications, 2009(16): 2127-2129. DOI:10.1039/b902995h
|
| [26] |
YOON S, PATIL MD, SARAK S, JEON H, KIM GH, KHOBRAGADE TP, SUNG S, YUN H. Deracemization of racemic amines to enantiopure (R)- and (S)-amines by biocatalytic cascade employing ω-transaminase and amine dehydrogenase. ChemCatChem, 2019, 11(7): 1898-1902. DOI:10.1002/cctc.201900080
|
| [27] |
KORMAN TP, OPGENORTH PH, BOWIE JU. A synthetic biochemistry platform for cell free production of monoterpenes from glucose. Nature Communications, 2017, 8: 15526. DOI:10.1038/ncomms15526
|
| [28] |
FINNIGAN W, HEPWORTH LJ, FLITSCH SL, TURNER NJ. RetroBioCat as a computer-aided synthesis planning tool for biocatalytic reactions and cascades. Nature Catalysis, 2021, 4(2): 98-104. DOI:10.1038/s41929-020-00556-z
|
| [29] |
DELÉPINE B, DUIGOU T, CARBONELL P, FAULON JL. RetroPath2.0: a retrosynthesis workflow for metabolic engineers. Metabolic Engineering, 2018, 45: 158-170. DOI:10.1016/j.ymben.2017.12.002
|
| [30] |
CAI T, SUN HB, QIAO J, ZHU LL, ZHANG F, ZHANG J, TANG ZJ, WEI XL, YANG JG, YUAN QQ, WANG WY, YANG X, CHU HY, WANG Q, YOU C, MA HW, SUN YX, LI Y, LI C, JIANG HF, et al. Cell-free chemoenzymatic starch synthesis from carbon dioxide. Science, 2021, 373(6562): 1523-1527. DOI:10.1126/science.abh4049
|
| [31] |
BIRMINGHAM WR, STARBIRD CA, PANOSIAN TD, NANNEMANN DP, IVERSON TM, BACHMANN BO. Bioretrosynthetic construction of a didanosine biosynthetic pathway. Nature Chemical Biology, 2014, 10(5): 392-399. DOI:10.1038/nchembio.1494
|
| [32] |
HUFFMAN MA, FRYSZKOWSKA A, ALVIZO O, BORRA-GARSKE M, CAMPOS KR, CANADA KA, DEVINE PN, DUAN D, FORSTATER JH, GROSSER ST, HALSEY HM, HUGHES GJ, JO J, JOYCE LA, KOLEV JN, LIANG J, MALONEY KM, MANN BF, MARSHALL NM, MCLAUGHLIN M, et al. Design of an in vitro biocatalytic cascade for the manufacture of islatravir. Science, 2019, 366(6470): 1255-1259. DOI:10.1126/science.aay8484
|
| [33] |
SHIN JS, KIM BG. Kinetic resolution of α-methylbenzylamine with ω-transaminase screened from soil microorganisms: application of a biphasic system to overcome product inhibition. Biotechnology and Bioengineering, 1997, 55(2): 348-358. DOI:10.1002/(SICI)1097-0290(19970720)55:2<348::AID-BIT12>3.0.CO;2-D
|
| [34] |
YEH YF, CHANG SCY, KUO HW, TONG CG, YU SM, DAVID HO TH. A metagenomic approach for the identification and cloning of an endoglucanase from rice straw compost. Gene, 2013, 519(2): 360-366. DOI:10.1016/j.gene.2012.07.076
|
| [35] |
BAYER S, BIRKEMEYER C, BALLSCHMITER M. A nitrilase from a metagenomic library acts regioselectively on aliphatic dinitriles. Applied Microbiology and Biotechnology, 2011, 89(1): 91-98. DOI:10.1007/s00253-010-2831-9
|
| [36] |
JEFFRIES JWE, DAWSON N, ORENGO C, MOODY TS, QUINN DJ, HAILES HC, WARD JM. Metagenome mining: a sequence directed strategy for the retrieval of enzymes for biocatalysis. ChemistrySelect, 2016, 1(10): 2217-2220. DOI:10.1002/slct.201600515
|
| [37] |
MUTTI FG, KROUTIL W. Asymmetric bio-amination of ketones in organic solvents. Advanced Synthesis and Catalysis, 2012, 354(18): 3409-3413. DOI:10.1002/adsc.201200900
|
| [38] |
SIMON RC, GRISCHEK B, ZEPECK F, STEINREIBER A, BELAJ F, KROUTIL W. Regio- and stereoselective monoamination of diketones without protecting groups. Angewandte Chemie International Edition, 2012, 51(27): 6713-6716. DOI:10.1002/anie.201202375
|
| [39] |
PARK E, KIM M, SHIN JS. One-pot conversion of L-threonine into L-homoalanine: biocatalytic production of an unnatural amino acid from a natural one. Advanced Synthesis & Catalysis, 2010, 352(18): 3391-3398.
|
| [40] |
HÖHNE M, SCHÄTZLE S, JOCHENS H, ROBINS K, BORNSCHEUER UT. Rational assignment of key motifs for function guides in silico enzyme identification. Nature Chemical Biology, 2010, 6(11): 807-813. DOI:10.1038/nchembio.447
|
| [41] |
IGLESIAS C, PANIZZA P, RODRIGUEZ GIORDANO S. Identification, expression and characterization of an (R)-ω-transaminase from Capronia semiimmersa. Applied Microbiology and Biotechnology, 2017, 101(14): 5677-5687. DOI:10.1007/s00253-017-8309-2
|
| [42] |
JIANG JJ, CHEN X, ZHANG DL, WU QQ, ZHU DM. Characterization of (R)-selective amine transaminases identified by in silico motif sequence blast. Applied Microbiology and Biotechnology, 2015, 99(6): 2613-2621. DOI:10.1007/s00253-014-6056-1
|
| [43] |
SLABU I, GALMAN J, WEISE N, LLOYD R, TURNER N. Putrescine transaminases for the synthesis of saturated nitrogen heterocycles from polyamines. ChemCatChem, 2016, 8(6): 1038-1042. DOI:10.1002/cctc.201600075
|
| [44] |
QI YK, ZHENG YC, ZHANG ZJ, XU JH. Efficient transformation of linoleic acid into 13(S)-hydroxy- 9, 11-(Z, E)-octadecadienoic acid using putative lipoxygenases from Cyanobacteria. ACS Sustainable Chemistry & Engineering, 2020, 8(14): 5558-5565.
|
| [45] |
SENIOR AW, EVANS R, JUMPER J, KIRKPATRICK J, SIFRE L, GREEN T, QIN CL, ŽÍDEK A, NELSON AWR, BRIDGLAND A, PENEDONES H, PETERSEN S, SIMONYAN K, CROSSAN S, KOHLI P, JONES DT, SILVER D, KAVUKCUOGLU K, HASSABIS D. Improved protein structure prediction using potentials from deep learning. Nature, 2020, 577(7792): 706-710. DOI:10.1038/s41586-019-1923-7
|
| [46] |
STEFFEN-MUNSBERG F, VICKERS C, THONTOWI A, SCHÄTZLE S, TUMLIRSCH T, SVEDENDAHL HUMBLE M, LAND H, BERGLUND P, BORNSCHEUER UT, HÖHNE M. Connecting unexplored protein crystal structures to enzymatic function. ChemCatChem, 2013, 5(1): 150-153. DOI:10.1002/cctc.201200544
|
| [47] |
SUNG S, JEON H, SARAK S, AHSAN MM, PATIL MD, KROUTIL W, KIM BG, YUN H. Parallel anti-sense two-step cascade for alcohol amination leading to ω-amino fatty acids and α, ω-diamines. Green Chemistry, 2018, 20(20): 4591-4595. DOI:10.1039/C8GC02122H
|
| [48] |
YOO HW, JUNG H, SARAK S, KIM YC, PARK BG, KIM BG, PATIL MD, YUN H. Multi-enzymatic cascade reactions with Escherichia coli-based modules for synthesizing various bioplastic monomers from fatty acid methyl esters. Green Chemistry, 2022, 24(5): 2222-2231. DOI:10.1039/D1GC04532F
|
| [49] |
CITOLER J, DERRINGTON SR, GALMAN JL, BEVINAKATTI H, TURNER NJ. A biocatalytic cascade for the conversion of fatty acids to fatty amines. Green Chemistry, 2019, 21(18): 4932-4935. DOI:10.1039/C9GC02260K
|
| [50] |
CHONG GG, DING LY, QIU YY, QIAN XL, DONG YL, LI CX, LI AT, PAN J, XU JH. Building flexible Escherichia coli modules for bifunctionalizing n-octanol: the byproduct of oleic acid biorefinery. Journal of Agricultural and Food Chemistry, 2022, 70(34): 10543-10551. DOI:10.1021/acs.jafc.2c04329
|
| [51] |
CHONG GG, DING LY, QIU YY, QIAN XL, LI CX, PAN J, XU JH. All-carbon-atom refinery of oleic acid into bifunctional chemicals using artificial consortia of Escherichia coli strains. ACS Sustainable Chemistry & Engineering, 2022, 10(39): 13125-13132.
|
| [52] |
SARAK S, KHOBRAGADE TP, JEON H, PAGAR AD, GIRI P, LEE S, YUN H. One-pot biocatalytic synthesis of nylon monomers from cyclohexanol using Escherichia coli-based concurrent cascade consortia. Green Chemistry, 2021, 23(23): 9447-9453. DOI:10.1039/D1GC03056F
|
| [53] |
STEFFEN-MUNSBERG F, MATZEL P, SOWA MA, BERGLUND P, BORNSCHEUER UT, HÖHNE M. Bacillus anthracis ω-amino acid: pyruvate transaminase employs a different mechanism for dual substrate recognition than other amine transaminases. Applied Microbiology and Biotechnology, 2016, 100(10): 4511-4521. DOI:10.1007/s00253-015-7275-9
|
| [54] |
STEPANKOVA V, BIDMANOVA S, KOUDELAKOVA T, PROKOP Z, CHALOUPKOVA R, DAMBORSKY J. Strategies for stabilization of enzymes in organic solvents. ACS Catalysis, 2013, 3(12): 2823-2836. DOI:10.1021/cs400684x
|
| [55] |
WANG Y, SUBRIZI F, CARTER EM, SHEPPARD TD, WARD JM, HAILES HC. Enzymatic synthesis of benzylisoquinoline alkaloids using a parallel cascade strategy and tyrosinase variants. Nature Communications, 2022, 13(1): 5436. DOI:10.1038/s41467-022-33122-1
|
| [56] |
GAO DK, SONG W, WU J, GUO L, GAO C, LIU J, CHEN XL, LIU LM. Efficient production of L-homophenylalanine by enzymatic-chemical cascade catalysis. Angewandte Chemie International Edition, 2022, 61(36): e202207077.
|
| [57] |
LIU Q, XIE XY, TANG MC, TAO WT, SHI T, ZHANG YZ, HUANG TT, ZHAO YL, DENG ZX, LIN SJ. One-pot asymmetric synthesis of an aminodiol intermediate of florfenicol using engineered transketolase and transaminase. ACS Catalysis, 2021, 11(12): 7477-7488. DOI:10.1021/acscatal.1c01229
|
| [58] |
WANG ZL, LI XR, LI Z. Engineering of cascade reactions and alditol oxidase for high-yielding synthesis of (R)-phenylethanolamine from styrene, L-phenylalanine, glycerol or glucose. ChemCatChem, 2022, 14(17): e202200418.
|
| [59] |
GMELCH TJ, SPERL JM, SIEBER V. Optimization of a reduced enzymatic reaction cascade for the production of L-alanine. Scientific Reports, 2019, 9(1): 11754. DOI:10.1038/s41598-019-48151-y
|
| [60] |
BECKER M, NIKEL P, ANDEXER JN, LÜTZ S, ROSENTHAL K. A multi-enzyme cascade reaction for the production of 2′, 3′-cGAMP. Biomolecules, 2021, 11(4): 590. DOI:10.3390/biom11040590
|
| [61] |
JAKOBLINNERT A, ROTHER D. A two-step biocatalytic cascade in micro-aqueous medium: using whole cells to obtain high concentrations of a vicinal diol. Green Chemistry, 2014, 16(7): 3472-3482. DOI:10.1039/C4GC00010B
|
| [62] |
van SCHIE MMCH, SPÖRING JD, BOCOLA M, DOMÍNGUEZ de MARÍA P, ROTHER D. Applied biocatalysis beyond just buffers-from aqueous to unconventional media. Options and guidelines. Green Chemistry, 2021, 23(9): 3191-3206. DOI:10.1039/D1GC00561H
|
| [63] |
YUN H, KIM J, KINNERA K, KIM BG. Synthesis of enantiomerically puretrans-(1R, 2R)-andcis-(1S, 2R)-1- amino-2-indanol by lipase and ω-transaminase. Biotechnology and Bioengineering, 2006, 93(2): 391-395. DOI:10.1002/bit.20721
|
| [64] |
KIM TH, KANG SH, HAN JE, SEO EJ, JEON EY, CHOI GE, PARK JB, OH DK. Multilayer engineering of enzyme cascade catalysis for one-pot preparation of nylon monomers from renewable fatty acids. ACS Catalysis, 2020, 10(9): 4871-4878. DOI:10.1021/acscatal.9b05426
|
| [65] |
LI JJ, YU SS, WANG YG, YAO PY, WU QQ, ZHU DM. Simultaneous preparation of (S)-2-aminobutane and D-alanine or D-homoalanine via biocatalytic transamination at high substrate concentration. Organic Process Research & Development, 2022, 26(7): 2013-2020.
|
| [66] |
FAN CW, XU GC, MA BD, BAI YP, ZHANG J, XU JH. A novel D-mandelate dehydrogenase used in three-enzyme cascade reaction for highly efficient synthesis of non-natural chiral amino acids. Journal of Biotechnology, 2015, 195: 67-71. DOI:10.1016/j.jbiotec.2014.10.026
|
| [67] |
SAVILE CK, JANEY JM, MUNDORFF EC, MOORE JC, TAM S, JARVIS WR, COLBECK JC, KREBBER A, FLEITZ FJ, BRANDS J, DEVINE PN, HUISMAN GW, HUGHES GJ. Biocatalytic asymmetric synthesis of chiral amines from ketones applied to sitagliptin manufacture. Science, 2010, 329(5989): 305-309. DOI:10.1126/science.1188934
|
| [68] |
WANG H, ZHENG YC, CHEN FF, XU JH, YU HL. Enantioselective bioamination of aromatic alkanes using ammonia: a multienzymatic cascade approach. ChemCatChem, 2020, 12(7): 2077-2082. DOI:10.1002/cctc.201902253
|
| [69] |
AHSAN MM, JEON H, NADARAJAN SP, CHUNG T, YOO HW, KIM BG, PATIL MD, YUN H. Biosynthesis of the nylon 12 monomer, ω-aminododecanoic acid with novel CYP153A, AlkJ, and ω-TA enzymes. Biotechnology Journal, 2018, 13(4): e1700562. DOI:10.1002/biot.201700562
|
| [70] |
ZHU YL, YUAN JF. A four-step enzymatic cascade for efficient production of L-phenylglycine from biobased L-phenylalanine. ChemBioChem, 2022, 23(8): e202100661.
|
| [71] |
LUO W, HU JG, LU JP, ZHANG HL, WANG XP, LIU YT, DONG LQ, YU XB. One pot cascade synthesis of L-2-aminobutyric acid employing ω-transaminase from Paracoccus pantotrophus. Molecular Catalysis, 2021, 515: 111890. DOI:10.1016/j.mcat.2021.111890
|
| [72] |
BURNS M, BI WY, KIM H, LALL MS, LI C, O'NEILL BT. Ketoreductase/transaminase, one-pot, multikilogram biocatalytic cascade reaction. Organic Process Research & Development, 2021, 25(4): 941-946.
|
| [73] |
LITTLECHILD JA. Enzymes from extreme environments and their industrial applications. Frontiers in Bioengineering and Biotechnology, 2015, 3: 161.
|
| [74] |
ABU R, WOODLEY JM. Application of enzyme coupling reactions to shift thermodynamically limited biocatalytic reactions. ChemCatChem, 2015, 7(19): 3094-3105. DOI:10.1002/cctc.201500603
|
| [75] |
DAWOODAWH, WEIß MS, SCHULZ C, PAVLIDIS IV, IDING H, DEISOUZA ROMA, BORNSCHEUER UT. Isopropylamine as amine donor in transaminase- catalyzed reactions: better acceptance through reaction and enzyme engineering. ChemCatChem, 2018, 10(18): 3943-3949. DOI:10.1002/cctc.201800936
|
| [76] |
SUN ZB, ZHANG ZJ, LI FL, NIE Y, YU HL, XU JH. One pot asymmetric synthesis of (R)-phenylglycinol from racemic styrene oxide via cascade biocatalysis. ChemCatChem, 2019, 11(16): 3802-3807. DOI:10.1002/cctc.201900492
|
| [77] |
TELZEROW A, HOBISCH M, MÜLLER M, SCHÜRMANN M, SCHWAB H, STEINER K. A co-expression system to shift the equilibrium of transamination reactions toward the synthesis of enantiomerically pure amines. Molecular Catalysis, 2019, 471: 38-43. DOI:10.1016/j.mcat.2019.04.006
|
| [78] |
SHIN JS, KIM BG. Asymmetric synthesis of chiral amines with ω‐transaminase. Biotechnology and Bioengineering, 1999, 65(2): 206-211. DOI:10.1002/(SICI)1097-0290(19991020)65:2<206::AID-BIT11>3.0.CO;2-9
|
| [79] |
KOSZELEWSKI D, LAVANDERA I, CLAY D, GUEBITZ G, ROZZELL D, KROUTIL W. Formal asymmetric biocatalytic reductive amination. Angewandte Chemie International Edition, 2008, 47(48): 9337-9340. DOI:10.1002/anie.200803763
|
| [80] |
MUTTI FG, FUCHS CS, PRESSNITZ D, SATTLER JH, KROUTIL W. Stereoselectivity of four (R)-selective transaminases for the asymmetric amination of ketones. Advanced Synthesis & Catalysis, 2011, 353(17): 3227-3233.
|
| [81] |
SCHREWE M, LADKAU N, BÜHLER B, SCHMID A. Direct terminal alkylamino-functionalization via multistep biocatalysis in one recombinant whole-cell catalyst. Advanced Synthesis & Catalysis, 2013, 355(9): 1693-1697.
|
| [82] |
WACHTMEISTER J, ROTHER D. Recent advances in whole cell biocatalysis techniques bridging from investigative to industrial scale. Current Opinion in Biotechnology, 2016, 42: 169-177. DOI:10.1016/j.copbio.2016.05.005
|
| [83] |
FRANCE SP, HUSSAIN S, HILL AM, HEPWORTH LJ, HOWARD RM, MULHOLLAND KR, FLITSCH SL, TURNER NJ. One-pot cascade synthesis of mono- and disubstituted piperidines and pyrrolidines using carboxylic acid reductase (CAR), ω-transaminase (ω-TA), and imine reductase (IRED) biocatalysts. ACS Catalysis, 2016, 6(6): 3753-3759. DOI:10.1021/acscatal.6b00855
|
| [84] |
HEPWORTH LJ, HUSSAIN S, BOTH P, TURNER N, FLITSCH S. Enzyme cascades in whole cells for the synthesis of chiral cyclic amines. ACS Catalysis, 2017, 7(4): 2920-2925. DOI:10.1021/acscatal.7b00513
|
| [85] |
SONG H, DING MZ, JIA XQ, MA Q, YUAN YJ. Synthetic microbial consortia: from systematic analysis to construction and applications. Chemical Society Reviews, 2014, 43(20): 6954-6981. DOI:10.1039/C4CS00114A
|
| [86] |
JIA Q, ZHENG YC, LI HP, QIAN XL, ZHANG ZJ, XU JH. Engineering isopropanol dehydrogenase for efficient regeneration of nicotinamide cofactors. Applied and Environmental Microbiology, 2022, 88(9): e0034122. DOI:10.1128/aem.00341-22
|
| [87] |
CHENG F, ZHOU SY, CHEN LX, ZHANG W, LI SF, WENG CY, WANG YJ, ZHENG YG. Reaction-kinetic model-guided biocatalyst engineering for dual-enzyme catalyzed bioreaction system. Chemical Engineering Journal, 2023, 452: 138997. DOI:10.1016/j.cej.2022.138997
|
| [88] |
KATAOKA M, ROHANI LPS, WADA M, KITA K, YANASE H, URABE I, SHIMIZU S. Escherichia coli transformant expressing the glucose dehydrogenase gene from Bacillus megaterium as a cofactor regenerator in a chiral alcohol production system. Bioscience, Biotechnology, and Biochemistry, 1998, 62(1): 167-169. DOI:10.1271/bbb.62.167
|
| [89] |
CHENG F, LI QH, ZHANG HY, WEI L, ZHANG JM, LI JM, XUE YP, ZHENG YG. Simultaneous directed evolution of coupled enzymes for efficient asymmetric synthesis of L-phosphinothricin. Applied and Environmental Microbiology, 2021, 87(5): e0256320. DOI:10.1128/AEM.02563-20
|
| [90] |
JIANG HW, CHEN Q, PAN J, ZHENG GW, XU JH. Rational engineering of formate dehydrogenase substrate/cofactor affinity for better performance in NADPH regeneration. Applied Biochemistry and Biotechnology, 2020, 192(2): 530-543. DOI:10.1007/s12010-020-03317-7
|
| [91] |
HATRONGJIT R, PACKDIBAMRUNG K. A novel NADP+-dependent formate dehydrogenase from Burkholderia stabilis 15516: screening, purification and characterization. Enzyme and Microbial Technology, 2010, 46(7): 557-561. DOI:10.1016/j.enzmictec.2010.03.002
|
| [92] |
SHOU C, ZHENG YC, ZHAN JR, LI CX, XU JH. Removing the obstacle to (–)-menthol biosynthesis by building a microbial cell factory of (+)- cis-isopulegone from (–)-limonene. ChemSusChem, 2022, 15(9): e202101741.
|
| [93] |
JOHANNES TW, WOODYER RD, ZHAO HM. Directed evolution of a thermostable phosphite dehydrogenase for NAD(P)H regeneration. Applied and Environmental Microbiology, 2005, 71(10): 5728-5734. DOI:10.1128/AEM.71.10.5728-5734.2005
|
| [94] |
ZHANG LY, KING E, BLACK WB, HECKMANN CM, WOLDER A, CUI YT, NICKLEN F, SIEGEL JB, LUO R, PAUL CE, LI H. Directed evolution of phosphite dehydrogenase to cycle noncanonical redox cofactors via universal growth selection platform. Nature Communications, 2022, 13(1): 5021. DOI:10.1038/s41467-022-32727-w
|
| [95] |
VRTIS JM, WHITE AK, METCALF WW, van der DONK WA. Phosphite dehydrogenase: a versatile cofactor-regeneration enzyme. Angewandte Chemie International Edition, 2002, 114(17): 3391-3393.
|
| [96] |
ZHENG MM, WANG RF, LI CX, XU JH. Two-step enzymatic synthesis of ursodeoxycholic acid with a new 7β-hydroxysteroid dehydrogenase from Ruminococcus torques. Process Biochemistry, 2015, 50(4): 598-604. DOI:10.1016/j.procbio.2014.12.026
|
| [97] |
LI HP, YOU ZN, LIU YY, ZHENG GW, GONG H, MO YM, ZHU N, BAI YP, XU JH. Continuous-flow microreactor-enhanced clean NAD+ regeneration for biosynthesis of 7-oxo-lithocholic acid. ACS Sustainable Chemistry & Engineering, 2021, 10(1): 456-463.
|
| [98] |
GEUEKE B, RIEBEL B, HUMMEL W. NADH oxidase from Lactobacillus brevis: a new catalyst for the regeneration of NAD. Enzyme and Microbial Technology, 2003, 32(2): 205-211. DOI:10.1016/S0141-0229(02)00290-9
|
| [99] |
LEE LG, WHITESIDES GM. Enzyme-catalyzed organic synthesis: a comparison of strategies for in situ regeneration of NAD from NADH. Journal of the American Chemical Society, 1985, 107(24): 6999-7008. DOI:10.1021/ja00310a043
|
| [100] |
MICHAEL B, HANNES L, LUO L, SCHMID ROLF D, DIRK WB. Biocatalytic process optimization based on mechanistic modeling of cholic acid oxidation with cofactor regeneration. Biotechnology and Bioengineering, 2011, 108(6): 1307-1317. DOI:10.1002/bit.23047
|
| [101] |
SATTLER JH, FUCHS M, MUTTI FG, GRISCHEK B, ENGEL P, PFEFFER J, WOODLEY J, KROUTIL W. Introducing an in situ capping strategy in systems biocatalysis to access 6-aminohexanoic acid. Angewandte Chemie International Edition, 2014, 53(51): 14153-14157. DOI:10.1002/anie.201409227
|
| [102] |
SHIMANE M, SUGAI Y, KAINUMA R, NATSUME M, KAWAIDE H. Mevalonate-dependent enzymatic synthesis of amorphadiene driven by an ATP- regeneration system using polyphosphate kinase. Bioscience, Biotechnology, and Biochemistry, 2012, 76(8): 1558-1560. DOI:10.1271/bbb.120177
|
| [103] |
MENG QL, ZHANG YF, JU XZ, MA CL, MA HW, CHEN JZ, ZHENG P, SUN JB, ZHU J, MA YH, ZHAO XM, CHEN T. Production of 5-aminolevulinic acid by cell free multi-enzyme catalysis. Journal of Biotechnology, 2016, 226: 8-13. DOI:10.1016/j.jbiotec.2016.03.024
|
| [104] |
SATO M, MASUDA Y, KIRIMURA K, KINO K. Thermostable ATP regeneration system using polyphosphate kinase from Thermosynechococcus elongatus BP-1 for D-amino acid dipeptide synthesis. Journal of Bioscience and Bioengineering, 2007, 103(2): 179-184. DOI:10.1263/jbb.103.179
|
| [105] |
ZHANG X, WU H, HUANG B, LI ZM, YE Q. One-pot synthesis of glutathione by a two-enzyme cascade using a thermophilic ATP regeneration system. Journal of Biotechnology, 2017, 241: 163-169. DOI:10.1016/j.jbiotec.2016.11.034
|
| [106] |
LIU S, LI Y, ZHU J. Enzymatic production of L-theanine by γ-glutamylmethylamide synthetase coupling with an ATP regeneration system based on polyphosphate kinase. Process Biochemistry, 2016, 51(10): 1458-1463. DOI:10.1016/j.procbio.2016.06.006
|
| [107] |
RESNICK SM, ZEHNDER AJB. In vitro ATP regeneration from polyphosphate and AMP by polyphosphate: AMP phosphotransferase and adenylate kinase from Acinetobacter johnsonii 210A. Applied and Environmental Microbiology, 2000, 66(5): 2045-2051. DOI:10.1128/AEM.66.5.2045-2051.2000
|
| [108] |
STROHMEIER GA, EITELJÖRG IC, SCHWARZ A, WINKLER M. Enzymatic one-step reduction of carboxylates to aldehydes with cell-free regeneration of ATP and NADPH. Chemistry-A European Journal, 2019, 25(24): 6119-6123. DOI:10.1002/chem.201901147
|
| [109] |
MORDHORST S, MAURER A, POPADIĆ D, BRECH J, ANDEXER JN. A flexible polyphosphate-driven regeneration system for coenzyme A dependent catalysis. ChemCatChem, 2017, 9(22): 4164-4168. DOI:10.1002/cctc.201700848
|
| [110] |
SUZUKI S, HARA R, KINO K. Production of aminoacyl prolines using the adenylation domain of nonribosomal peptide synthetase with class Ⅲ polyphosphate kinase 2-mediated ATP regeneration. Journal of Bioscience and Bioengineering, 2018, 125(6): 644-648. DOI:10.1016/j.jbiosc.2017.12.023
|
| [111] |
NOGUCHI T, SHIBA T. Use of Escherichia coli polyphosphate kinase for oligosaccharide synthesis. Bioscience, Biotechnology, and Biochemistry, 1998, 62(8): 1594-1596. DOI:10.1271/bbb.62.1594
|
| [112] |
MORDHORST S, SIEGRIST J, MÜLLER M, RICHTER M, ANDEXER JN. Catalytic alkylation using a cyclic S-adenosylmethionine regeneration system. Angewandte Chemie International Edition, 2017, 56(14): 4037-4041. DOI:10.1002/anie.201611038
|
| [113] |
李元, 刘珊, 祝俊. PPK和GMAS共表达重组菌株的构建及其在L-茶氨酸合成中的应用. 生物工程学报, 2016, 32(12): 1745-1749. LI Y, LIU S, ZHU J. Construction of recombinant strains co-expressing PPK and GMAS for the synthesis of L-theanine. Chinese Journal of Biotechnology, 2016, 32(12): 1745-1749 (in Chinese). DOI:10.13345/j.cjb.160218 |
| [114] |
KULMER ST, GUTMANN A, LEMMERER M, NIDETZKY B. Biocatalytic cascade of polyphosphate kinase and sucrose synthase for synthesis of nucleotide- activated derivatives of glucose. Advanced Synthesis & Catalysis, 2017, 359(2): 292-301.
|
| [115] |
TAVANTI M, HOSFORD J, LLOYD RC, BROWN MJB. ATP regeneration by a single polyphosphate kinase powers multigram-scale aldehyde synthesis in vitro. Green Chemistry, 2021, 23(2): 828-837. DOI:10.1039/D0GC03830J
|
| [116] |
LEE H, SUGIHARTO YEC, LEE H, JEON W, AHN J, LEE H. Biotransformation of dicarboxylic acids from vegetable oil-derived sources: current methods and suggestions for improvement. Applied Microbiology and Biotechnology, 2019, 103(4): 1545-1555. DOI:10.1007/s00253-018-9571-7
|
| [117] |
DASGUPTA S, HAMMOND WB, GODDARD WA. Crystal structures and properties of nylon polymers from theory. Journal of the American Chemical Society, 1996, 118(49): 12291-12301. DOI:10.1021/ja944125d
|
| [118] |
NOACK H, GEORGIEV V, BLOMBERG MRA, SIEGBAHN PE, JOHANSSON AJ. Theoretical insights into heme-catalyzed oxidation of cyclohexane to adipic acid. Inorganic Chemistry, 2011, 50(4): 1194-1202. DOI:10.1021/ic101405u
|
| [119] |
RAJ K, PARTOW S, CORREIA K, KHUSNUTDINOVA A, YAKUNIN A, MAHADEVAN R. Biocatalytic production of adipic acid from glucose using engineered Saccharomyces cerevisiae. Metabolic Engineering Communications, 2018, 6: 28-32. DOI:10.1016/j.meteno.2018.02.001
|
| [120] |
BOWEN CH, BONIN J, KOGLER A, BARBA- OSTRIA C, ZHANG FZ. Engineering Escherichia coli for conversion of glucose to medium-chain ω-hydroxy fatty acids and α, ω-dicarboxylic acids. ACS Synthetic Biology, 2016, 5(3): 200-206. DOI:10.1021/acssynbio.5b00201
|
| [121] |
ZHAO M, HUANG DX, ZHANG XJ, KOFFAS MAG, ZHOU JW, DENG Y. Metabolic engineering of Escherichia coli for producing adipic acid through the reverse adipate-degradation pathway. Metabolic Engineering, 2018, 47: 254-262. DOI:10.1016/j.ymben.2018.04.002
|
| [122] |
YU JL, XIA XX, ZHONG JJ, QIAN ZG. Direct biosynthesis of adipic acid from a synthetic pathway in recombinant Escherichia coli. Biotechnology and Bioengineering, 2014, 111(12): 2580-2586. DOI:10.1002/bit.25293
|
| [123] |
CHAE TU, AHN JH, KO YS, KIM JW, LEE JA, LEE EH, LEE SY. Metabolic engineering for the production of dicarboxylic acids and diamines. Metabolic Engineering, 2020, 58: 2-16. DOI:10.1016/j.ymben.2019.03.005
|
| [124] |
DENG Y, MAO Y. Production of adipic acid by the native-occurring pathway in Thermobifida fusca B6. Journal of Applied Microbiology, 2015, 119(4): 1057-1063. DOI:10.1111/jam.12905
|
| [125] |
SUN J, RAZA M, SUN XX, YUAN QP. Biosynthesis of adipic acid via microaerobic hydrogenation of cis, cis-muconic acid by oxygen-sensitive enoate reductase. Journal of Biotechnology, 2018, 280: 49-54. DOI:10.1016/j.jbiotec.2018.06.304
|
| [126] |
WANG F, ZHAO J, LI Q, YANG J, LI RJ, MIN J, YU XJ, ZHENG GW, YU HL, ZHAI C, ACEVEDO- ROCHA CG, MA LX, LI AT. One-pot biocatalytic route from cycloalkanes to α, ω-dicarboxylic acids by designed Escherichia coli consortia. Nature Communications, 2020, 11(1): 5035. DOI:10.1038/s41467-020-18833-7
|
| [127] |
ADKINS J, JORDAN J, NIELSEN DR. Engineering Escherichia coli for renewable production of the 5-carbon polyamide building-blocks 5-aminovalerate and glutarate. Biotechnology and Bioengineering, 2013, 110(6): 1726-1734. DOI:10.1002/bit.24828
|
| [128] |
CLOMBURG JM, BLANKSCHIEN MD, VICK JE, CHOU A, KIM S, GONZALEZ R. Integrated engineering of β-oxidation reversal and ω-oxidation pathways for the synthesis of medium chain ω-functionalized carboxylic acids. Metabolic Engineering, 2015, 28: 202-212. DOI:10.1016/j.ymben.2015.01.007
|
| [129] |
SONG JW, LEE JH, BORNSCHEUER UT, PARK JB. Microbial synthesis of medium-chain α, ω-dicarboxylic acids and ω-aminocarboxylic acids from renewable long-chain fatty acids. Advanced Synthesis & Catalysis, 2014, 356(8): 1782-1788.
|
| [130] |
LADKAU N, ASSMANN M, SCHREWE M, JULSING MK, SCHMID A, BÜHLER B. Efficient production of the nylon 12 monomer ω-aminododecanoic acid methyl ester from renewable dodecanoic acid methyl ester with engineered Escherichia coli. Metabolic Engineering, 2016, 36: 1-9. DOI:10.1016/j.ymben.2016.02.011
|
| [131] |
AHSAN M, PATIL M, JEON H, SUNG S, CHUNG T, YUN H. Biosynthesis of nylon 12 monomer, ω-aminododecanoic acid using artificial self-sufficient P450, AlkJ and ω-TA. Catalysts, 2018, 8(9): 400. DOI:10.3390/catal8090400
|
| [132] |
GE JW, YANG XH, YU HW, YE LD. High-yield whole cell biosynthesis of nylon 12 monomer with self-sufficient supply of multiple cofactors. Metabolic Engineering, 2020, 62: 172-185. DOI:10.1016/j.ymben.2020.09.006
|
| [133] |
MARIA R, HECKMANN CHRISTIAN M, FRANCESCA P. Biocatalytic production of a nylon 6 precursor from caprolactone in continuous flow. ChemSusChem, 2022, 15(16): e202200811.
|
| [134] |
SARAK S, SUNG S, JEON H, PATIL MD, KHOBRAGADE TP, PAGAR AD, DAWSON PE, YUN H. An integrated cofactor/co-product recycling cascade for the biosynthesis of nylon monomers from cycloalkylamines. Angewandte Chemie International Edition, 2021, 60(7): 3481-3486. DOI:10.1002/anie.202012658
|
| [135] |
FEDORCHUK TP, KHUSNUTDINOVA AN, EVDOKIMOVA E, FLICK R, Di LEO R, STOGIOS P, SAVCHENKO A, YAKUNIN AF. One-pot biocatalytic transformation of adipic acid to 6-aminocaproic acid and 1, 6-hexamethylenediamine using carboxylic acid reductases and transaminases. Journal of the American Chemical Society, 2020, 142(2): 1038-1048. DOI:10.1021/jacs.9b11761
|
| [136] |
BELLUSSI G, PEREGO C. Industrial catalytic aspects of the synthesis of monomers for nylon production. Cattech, 2000, 4(1): 4-16. DOI:10.1023/A:1011905009608
|
| [137] |
ZHANG ZW, LI Q, WANG F, LI RJ, YU XJ, KANG LX, ZHAO J, LI AT. One-pot biosynthesis of 1, 6-hexanediol from cyclohexane by de novo designed cascade biocatalysis. Green Chemistry, 2020, 22(21): 7476-7483. DOI:10.1039/D0GC02600J
|
| [138] |
LIU YF, WANG W, ZENG AP. Biosynthesizing structurally diverse diols via a general route combining oxidative and reductive formations of OH-groups. Nature Communications, 2022, 13(1): 1595. DOI:10.1038/s41467-022-29216-5
|
| [139] |
AHSAN MM, SUNG S, JEON H, PATIL MD, CHUNG T, YUN H. Biosynthesis of medium- to long-chain α, ω-diols from free fatty acids using CYP153A monooxygenase, carboxylic acid reductase, and E. coli endogenous aldehyde reductases. Catalysts, 2017, 8(1): 4. DOI:10.3390/catal8010004
|
| [140] |
McDONALD AD, BRUFFY SK, KASAT AT, BULLER AR. Engineering enzyme substrate scope complementarity for promiscuous cascade synthesis of 1, 2-amino alcohols. Angewandte Chemie International Edition, 2022, 61(46): e202212637.
|
| [141] |
LIU S, ZHANG X, LIU F, XU MJ, YANG TW, LONG MF, ZHOU JP, OSIRE T, YANG ST, RAO ZM. Designing of a cofactor self-sufficient whole-cell biocatalyst system for production of 1, 2-amino alcohols from epoxides. ACS Synthetic Biology, 2019, 8(4): 734-743. DOI:10.1021/acssynbio.8b00364
|
| [142] |
CORRADO MARIA L, TANJA K, ULRICH S, MUTTI FRANCESCO G. High-yield synthesis of enantiopure 1, 2-amino alcohols from L-phenylalanine via linear and divergent enzymatic cascades. Organic Process Research & Development, 2022, 26(7): 2085-2095.
|
| [143] |
AHARONI SM. N-nylons: Their Synthesis, Structure, and Properties[M]. John Wiley & Sons Incorporated, 1997.
|
| [144] |
SATTLER JH, FUCHS M, TAUBER K, MUTTI FG, FABER K, PFEFFER J, HAAS T, KROUTIL W. Redox self-sufficient biocatalyst network for the amination of primary alcohols. Angewandte Chemie International Edition, 2012, 124(36): 9290-9293.
|
 2023, Vol. 39
2023, Vol. 39