中国科学院微生物研究所、中国微生物学会主办
文章信息
- 李可心, 王颖, 姚明东, 肖文海
- LI Kexin, WANG Ying, YAO Mingdong, XIAO Wenhai
- 脱落酸生物合成研究进展
- Advances in abscisic acid biosynthesis
- 生物工程学报, 2023, 39(6): 2190-2203
- Chinese Journal of Biotechnology, 2023, 39(6): 2190-2203
- 10.13345/j.cjb.220574
-
文章历史
- Received: July 25, 2022
- Accepted: November 10, 2022
- Published: November 14, 2022
2. 天津大学合成生物学前沿科学中心 系统生物工程教育部重点实验室, 天津 300072;
3. 天津大学前沿技术研究院, 天津 301700
2. Key Laboratory of Systems Bioengineering (Ministry of Education), Frontier Science Center for Synthetic Biology, Tianjin University, Tianjin 300072, China;
3. Frontier Technology Research Institute, Tianjin University, Tianjin 301700, China
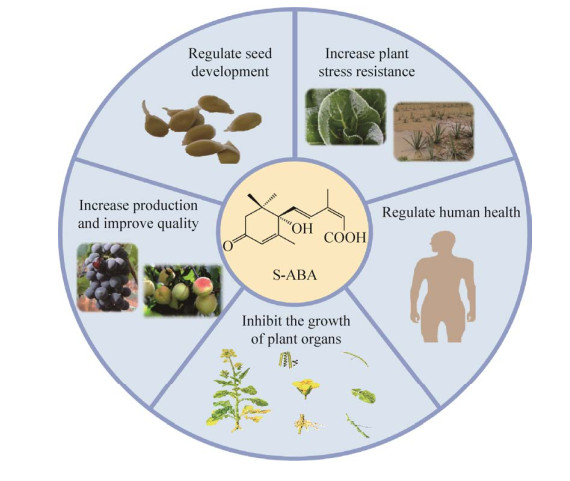
脱落酸(abscisic acid, ABA),又称休眠素,是一种具有倍半萜结构的植物激素[1],因其能促使叶子脱落而得名,是植物5大天然生长调节剂之一[2]。天然的ABA存在对映异构体,起主要活性作用的是右旋异构体S-ABA。ABA是平衡植物内源激素和调节生长代谢的关键因子,具有增强作物抗旱[3-4]、耐盐[5]以及减少果实褐变[6]等作用,可用于提高农作物的品质和产量[7];除在农业上的应用外,ABA还可应用于人体,对免疫系统、心血管细胞、干细胞和糖尿病[8-10]等有广泛的调节作用(图 1)。ABA实用制剂应用市场的打开,将会带来巨大的经济效益和社会效益。
|
| 图 1 脱落酸的应用 Fig. 1 Application of abscisic acid. |
| |
ABA广泛存在于高等植物中,也存在于一些植物病原真菌、细菌[11-13]中。ABA的生物合成途径分为间接途径和直接途径[14-15]:在高等植物中,主要通过间接途径合成ABA,该途径最显著的特点是通过氧化裂解C40类胡萝卜素来形成C15骨架,随后通过多步酶催化反应合成ABA;在真菌中,ABA则直接由法尼基焦磷酸(farnesyl pyrophosphate, FPP)经多步氧化形成,此途径称为C15直接途径,不同真菌形成ABA的代谢途径略有不同。目前ABA主要通过化学合成方法获得,但化学合成的ABA是S-ABA和R-ABA的混合物,效价较低。随着合成生物学技术的发展,利用微生物合成天然产物已经成为研究热点。目前,利用天然微生物合成ABA的研究已取得诸多进展,而ABA的异源微生物合成研究相对较少。相较于细胞内部代谢情况尚不明晰、可用遗传工具数量有限的天然合成ABA的真菌,异源微生物如酿酒酵母、解脂耶氏酵母、大肠杆菌等的遗传背景清晰,代谢已被详细研究,可应用高效的遗传操作工具,具有较好的安全性,是合成ABA的有希望的候选者。利用这些工程菌株进行ABA的异源合成将是一种更具潜力的生产方式。本文在介绍ABA生物合成途径和关键酶的基础上,将重点对微生物异源合成ABA的研究进展及相关工程化策略进行综述,并对其前景进行分析与展望。
1 脱落酸的生物合成途径和关键酶 1.1 脱落酸的生物合成途径在植物中,ABA生物合成主要发生在质体中,根冠和叶片是合成ABA的主要部位[1]。ABA在植物体内的合成以2-C-甲基-d-赤藻糖醇-4-磷酸(methylerythritol-4-phosphate pathway, MEP)途径为起始,随后经牻牛儿基焦磷酸(geranyl pyrophosphate, GPP)、FPP、牻牛儿基牻牛儿基焦磷酸(geranylgeranyl pyrophosphate, GGPP)等物质合成关键中间体β-胡萝卜素[16] (图 2)。β-胡萝卜素经β-胡萝卜素羟化酶(β-carotene hydroxylase, BCH/CrtZ)连续氧化依次生成隐黄质和玉米黄质;之后玉米黄质在玉米黄质环氧化酶(zeaxanthin epoxidase, ZEP)作用下依次生成花药黄质和全反式紫黄质,同时全反式紫黄质可以在光照作用下经紫黄质脱环氧化酶(violaxanthin de-epoxidase, VDE)脱环氧生成玉米黄质[17-19]。研究表明,从全反式紫黄质到ABA的合成途径有两种:全反式紫黄质可以经新黄质合酶(neoxanthin synthase, NSY)催化生成全反式新黄质,随后经异构化变为9-顺-新黄质[20];同时全反式紫黄质也可以异构化为9-顺-紫黄质。在9-顺-环氧类胡萝卜素双加氧酶(9-cis-epoxycarotenoid dioxygenase, NCED)作用下,9-顺-新黄质和9-顺-紫黄质的C11、C12键断裂生成黄质醛。最后,黄质醛经黄素脱氢酶(xanthoxin dehydrogenase, ABA2)、脱落醛氧化酶(abscisic-aldehyde oxidase, AAO3)两步氧化生成ABA[21-23]。
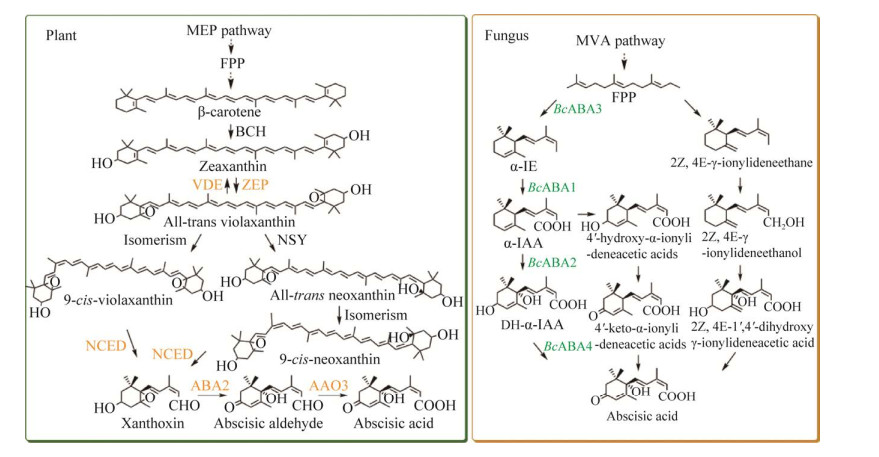
|
| 图 2 脱落酸在植物(左)和真菌(右)中的生物合成途径 Fig. 2 Biosynthetic pathway of abscisic acid in plants (left) and fungi (right). α-IE: α-ionylideneethane; α-IAA: α-ionylideneacetic acid; DH-α-IAA: 1′, 4′-trans-dihydroxy-α-ionylideneacetic acid. α-IE:α-芷香乙烷;α-IAA:α-芷香乙酸;DH-α-IAA:1ʹ, 4ʹ-ABA二醇 |
| |
除植物外,许多真菌也能合成ABA,如葡萄孢霉属、尾孢霉属等[24]。尽管所有的真菌普遍采用C15途径直接合成ABA,但ABA的生物合成途径根据物种不同存在一定差异[24-25] (图 2)。研究较多的真菌有灰葡萄孢霉菌[26-27]、豆类煤污尾孢菌[24, 28]、蔷薇色尾孢菌[24],这些真菌通过不同的氧化步骤,分别以1′, 4′-ABA二醇、1′, 4′-二羟基-亚硫代乙酸或4-酮-芷香乙酸为中间体,直接从FPP合成ABA[29]。在灰葡萄尾孢菌(Botrytis cinerea)中,倍半萜合成酶ABA3 (alpha-ionylideneethane synthase aba3, ABA3)将FPP环化为α-芷香乙烷,之后α-芷香乙烷经细胞色素P450单加氧酶ABA1 (cytochrome P450 monooxygenase aba1, ABA1)、细胞色素P450单加氧酶ABA2 (cytochrome P450 monooxygenase aba2, ABA2)氧化为1′, 4′-ABA二醇,最后1′, 4′-ABA二醇经短链脱氢酶ABA4 (short-chain dehydrogenase/reductase aba4, ABA4)氧化生成ABA[27];在豆类煤污尾孢菌中,FPP经环化形成γ-芷香乙烷后进一步被氧化形成1′, 4′-二羟基-亚硫代乙酸,最终合成ABA[29];蔷薇色尾孢菌则是氧化顺序的差别,先氧化α-芷香乙烷生成4-酮-芷香乙酸,最终氧化生成ABA[24]。
1.2 脱落酸生物合成途径的关键酶近年来,随着有关ABA合成物种基因组和转录组分析的报道越来越多,多种物种中参与ABA合成的酶已成功被表征,其中ABA生物合成途径的关键酶汇总如表 1所示。在高等植物中,对ABA生物合成起关键作用的反应存在于β-胡萝卜素之后的步骤,其中ZEP、NCED被认为是途径关键酶。
| Synthetic pathway | Key enzyme | Name | Species | Research progress | References |
| C40 pathway | ZEP | CzZEP | Chlorella zofingiensis | Heterologous gene complementation proves the function of the enzyme | [30] |
| PtZEP2, PtZEP3 | Phaeodactylum tricornutum | Pathway complementation proves the function and catalytic activity of the enzyme | [31] | ||
| NCED | PaNCED1, PaNCED3 | Persea americana | Vitro experiments showed the function of the enzyme | [32] | |
| AhNCED1 | Arachis hypogaea | The results showed that NCED located in chloroplasts, roots and leaves were the main parts of ABA synthesis in response to water stress | [33] | ||
| AcNCED1 | Actinidia chinensis | It is proved that AcNCED1 is a key enzyme involved in the synthesis of ABA in Actinidia chinensis | [34] | ||
| C15 pathway | BcABA1 | BcABA1 | Botrytis cinerea | It is proved that BcABA1 participates in ABA biosynthesis and identifies the functions and properties of enzymes | [26-27, 29] |
| BcABA2 | BcABA2 | Botrytis cinerea | It is proved that BcABA2 participates in ABA biosynthesis and identifies the functions and properties of enzymes | [26-27, 35] | |
| BcABA3 | BcABA3 | Botrytis cinerea | Identify the properties and reaction mechanism of the enzyme | [26-27, 35] | |
| BcABA4 | BcABA4 | Botrytis cinerea | Determine the nature and function of the enzyme | [26-27] |
ZEP,一个定位于类囊体膜基质侧的双功能单加氧酶,被认为是ABA生物合成的关键酶之一[36-37]。编码ZEP的cDNA序列最先是从烟草中分离获得[38],随后也陆续在拟南芥、番茄等植物中解析。Couso等[30]将绿色微藻小球藻(Chlorella zofingiensis)来源的CzZEP导入缺乏ZEP活性的衣藻突变体,阳性转化子能够有效地将玉米黄质转化为紫黄质,侧面验证了CzZEP的功能。ABA生物合成的另一关键是通过NCED裂解类胡萝卜素以产生黄质醛。NCED与其他4个类胡萝卜素裂解双加氧酶(carotenoid cleavage dixoygenases, CCDs)亚家族(CCD1、CCD4、CCD7、CCD8)裂解位点有所不同[39-40],它在C11、C12双键位置上切割9-顺-紫黄质或9-顺-新黄质产生15碳的黄质醛。NCED最初在玉米viviparous 14 (vp14)突变体中发现[41],并在多种植物中被证明是ABA合成的限速酶[39, 42-44]。Hu等[33]利用蛋白印迹及荧光显色确定了花生(Arachis hypogaea)来源的AhNCED1的细胞器定位。Chernys等[32]证明了鳄梨(Persea americana)来源的PaNCED1和PaNCED3能够在体外切割9-顺式叶黄素为黄质醛。
ABA在真菌中的合成途径与植物不同,驱动真菌中ABA生物合成的分子机制研究有限,仅在灰葡萄尾孢菌中发现了一个包含4个基因的基因簇。该基因簇由倍半萜环化酶(BcABA3)、P450单加氧酶(BcABA1和BcABA2)和短链脱氢酶/还原酶(BcABA4)组成[27, 29]。该BcABA基因簇的存在是罕见的,研究进展见表 1。2004年,Siewers等[29]通过靶向失活编码细胞色素P450氧化还原酶的基因,证明了BcABA1对ABA的生物合成是必不可少的。2006年,Siewers等[35]继续通过邻域分析及基因的靶向失活,证明了BcABA2和BcABA3参与ABA的生物合成,还表明了BcABA4的贡献。近年来,BcABA基因簇的功能逐渐被确定。Takino等[26]鉴定了新型倍半萜合酶BcABA3以及ABA合成的3步反应机制,之后Takino等[27]通过生物转化实验和体外酶反应,进一步阐明了BcABA基因簇的功能。
2 异源合成脱落酸底盘细胞的比较和选择异源生物合成目标产物的产量与底盘细胞的选择和代谢途径密切相关,异源合成ABA底盘细胞的比较如表 2所示。酿酒酵母、解脂耶氏酵母和大肠杆菌作为最常用的微生物底盘,它们的遗传背景清晰、操作简便,是工业化生产的理想菌株。有研究者曾尝试在大肠杆菌中生产紫黄质未成功[38, 45],大多所表达的单个基因不能产生目标化合物,有时需要添加前体和优化培养条件获得目标产物。因此,将大肠杆菌用于体外酶的表达或者功能验证是不错的选择。
| Chassis cell | Advantage | Disadvantages |
| Escherichia coli | Fermentation cycle is short (2–3 d), and the precursors are not shunted Culture and metabolism are easy to control Accumulate precursors and cofactors |
Rapid gene expression system may adversely affect the balance of metabolic pathways Lacking post-translational modification, it is difficult to express more complex enzymes |
| Saccharomyces cerevisiae | Provide a large number of precursors for terpenoid synthesis Intracellular environment is suitable for terpene synthesis Suitable for CYPs expression |
Fermentation cycle is long (5–7 d), and the endogenous terpenoids are shunted Incomplete processing of signal peptide When secreted and expressed, the secretion efficiency is relatively low |
| Yarrowia lipolytica | The accumulation of acyl coenzyme A and acetyl coenzyme A in cells is high, which is suitable for the synthesis of terpenoids The rich subcellular structure of lipid droplets can provide a storage place for carotenoid hydrophobic products and other substances It has the ability to secrete protein efficiently |
Strictly aerobic non fermenting yeast Intracellular non homologous recombination is highly efficient and difficult to operate Difficult to regulate the intrinsic metabolic pathway, and adapt the heterologous and endogenous pathways |
与大肠杆菌相比,具有真核表达系统的酿酒酵母、解脂耶氏酵母更适合作为ABA的合成平台。以紫黄质的合成为例,无论是大肠杆菌还是酿酒酵母虽然都能合成紫黄质的前体玉米黄质,但由于重要的单加氧酶ZEP表达效率低下,最终产物紫黄质在大肠杆菌中的产量远低于工程化酿酒酵母。解脂耶氏酵母是一种非常规酵母,该酵母与酿酒酵母相比具有明显不同的代谢特点[46-47]。利用解脂耶氏酵母作为异源表达宿主,成功生产了有价值的萜类化合物ABA (263.5 mg/L),在所有的测试底盘细胞中产量最高[48]。
在这些底盘生物中,不同微生物生产萜类化合物的优势各不相同,需要根据天然产物的特性选择最适宿主细胞,实现高效价生产。ABA合成途径依靠多种酶的参与,是一个多因素调节的复杂的动态过程,不同的胞内环境影响ABA合成关键酶的高效异源表达。Arnesen等[48]通过工程化改造解脂耶氏酵母实现了异源微生物生产ABA的最高产量,表明解脂耶氏酵母有希望通过C15直接途径实现ABA高效生产。Cataldo等[49]表明酿酒酵母是表达铁氧还蛋白NADPH氧化还原酶(ferredoxin-NADPH oxidoreductase, FNR)、铁氧还蛋白(ferredoxin, FD)、ZEP全功能系统的合适宿主,适合植物来源的ABA生物合成酶的表达,有望实现ABA间接途径的全合成。在异源ABA生产成为可行的工业选择之前,需要对酵母底盘和培养条件进行进一步的工程设计。
3 微生物异源合成脱落酸的研究进展 3.1 微生物异源合成脱落酸的研究现状在发现部分真菌可以天然合成ABA后,研究者们已采取多种诱变策略来提高ABA的产量[50-51]。从目前报道来看,ABA大规模产量约为2‒4 g/L。目前天然ABA生产菌的研究达到一定瓶颈,缺乏高效的通量系统来筛选改良的高性能菌株;以及缺少方便的遗传工具,使得合理设计ABA高产菌株变得困难。研究者们开始探究微生物异源合成ABA,表 3总结了微生物异源合成ABA的研究进展。Takino等[15]将含灰葡萄尾孢菌来源的BcABA1、BcABA2、BcABA3、BcABA4基因的质粒导入米曲霉中,研究是否有ABA的产生,发现在MPY培养基中ABA产量达8 mg/L。Otto等[52]将灰葡萄尾孢菌来源的BcABA1、BcABA2、BcABA3、BcABA4、BcABA5导入酿酒酵母,通过敲除对比发现BcABA1、BcABA2、BcABA3和BcABA4的表达足以使异源宿主产生ABA;之后引入异源细胞色素P450还原酶(Cytochrome P450 reductase, CPR)、增加BcABA1、BcABA2基因拷贝数,使ABA的产量提高到11 mg/L。Arnesen等[48]将BcABA1、BcABA2、BcABA3、BcABA4、Bccpr1导入解脂耶氏酵母,通过整合额外拷贝的甲羟戊酸途径(mevalonate pathway, MVA)基因、ABA生物合成编码基因,表达异源ABA转运蛋白,获得了一株ABA产量为263.5 mg/L (9.1 mg/g DCW)的工程菌。
| Chassis cell | Engineering means | Yield (mg/L) | Fermentation time (h) | References |
| Yarrowia lipolytica | ·Introduction of heterologous (Botrytis cinerea) ABA synthesis pathway ·Add BcABA1/ABA3/ABA4 copy ·Add ERG20, POS5 copy ·Expression of exogenous ABA transporter AtDTX50 |
263.5 | 72 | [48] |
| Saccharomyces cerevisiae | ·Introduction of heterologous (Botrytis cinerea) ABA synthesis pathway ·Knock out the competitive pathways LPP1/DPP1/ERG9 ·Overexpression of tHMG1, ERG20 ·Add BcABA1/BcABA2 copy ·Introduction of heterologous cytochrome P450 reductase |
11.0 | 48 | [52] |
| Aspergillus oryzae | ·Introduction of heterologous (Botrytis cinerea) ABA synthesis pathway |
8.0 | ‒ | [15] |
| ‒ indicates that relevant data are not disclosed in the literature. | ||||
在ABA合成的间接途径中,目前已在异源底盘细胞实现中间体-紫黄质的生产。Cataldo等[49]利用一株产β-胡萝卜素的酿酒酵母菌株,通过适配CrtZ和ZEP,并截短ZEP、增加β-胡萝卜素合成基因的基因拷贝数,使紫黄质分批补料发酵产量达7.3 mg/g DCW。Takemura等[53]在大肠杆菌中合成紫黄质时探究了不同来源ZEP对紫黄质产量的影响,发现辣椒(Capsicum annuum)来源的CaZEP有最好的转化效率,后经宿主、表达载体的适配和核糖体结合位点(ribosome binding site, RBS)序列优化使紫黄质的产量达231 μg/g DCW。ABA间接途径的异源合成未能实现紫黄质下游产物合成的瓶颈可能是:异源代谢途径长度的增加将会产生更多的碳分流,需要不断地累积增加前体供应,维持长途径碳流的供给;C40间接途径发掘于高等植物,大部分酶位于叶绿体中表达,而微生物宿主缺乏此种细胞器,酶在异源表达过程中可能存在一定限制,需进一步优化。
3.2 异源合成脱落酸的工程化策略在发现ABA生物合成途径的基因后,研究者们已采取多种方法构建、优化异源合成ABA的微生物菌株以提高ABA的产量,相关工程化策略如图 3所示。这些研究除提高ABA的产量外,还揭示了在微生物中异源合成ABA的限制性因素。

|
| 图 3 异源合成脱落酸的工程化策略 Fig. 3 Engineering strategy for heterologous synthesis of abscisic acid. A: Screening and expression enhancement strategy of key enzymes. B: Cofactor regulation strategy. C: Enhanced precursor supply strategy. D: Strategies to promote abscisic acid efflux. A:关键酶的筛选与表达强化策略. B:辅因子调节策略. C:增强前体供应策略. D:促进脱落酸外排策略 |
| |
近年来,灰葡萄尾孢菌中的ABA生物合成途径被挖掘,ABA合成基因簇的功能逐渐被确定,BcABA1和BcABA2已被Siewers等[29, 35]证明是ABA生物合成必不可少的P450单加氧酶。Otto等[52]通过过表达ABA合成基因簇基因来测试灰葡萄尾孢菌的ABA基因簇中是否有至少一种酶的活性限制ABA的产生,结果表明过表达BcABA1、BcABA2、BcABA3均不同程度提高了ABA的产量,尤其是BcABA1的过表达;通过进一步比较中间副产物的积累,发现ABA的产生主要受BcABA1活性的限制,同时较高的BcABA2活性可以避免BcABA1下游副产物的积累,这可能会增加ABA途径的代谢通量。基于这些结果,Otto等[52]同时过表达BcABA1和BcABA2使ABA产量增加了4.1倍。Arnesen等[48]研究在解脂耶氏酵母中过表达ABA生物合成途径基因BcABA1、BcABA2、BcABA3、BcABA4对ABA产量的影响,发现只有过表达BcABA1的菌株ABA的产量增加了2.8倍。这些结果证明了BcABA1和BcABA2的活性是菌株异源合成ABA的瓶颈,额外的基因拷贝可以增加ABA的合成通量。
在ABA的间接合成途径中,催化玉米黄质合成紫黄质的ZEP是途径关键酶之一。Cataldo等[49]在合成β-胡萝卜素的酿酒酵母产生菌SM14中共表达泛菌(Pantoea ananatis)来源的PaCrtZ和湖泊红球藻(Haematoccocus lacustris)来源的HlZEP实现了紫黄质的合成;为继续提高紫黄质的产量,作者预测了全长ZEP酶的结构和转运肽位置,HlZEP在N端截短30个或59个氨基酸时,紫黄质产量提高了4倍,该结果揭示了植物源的酶在微生物表达可能需要截掉信号肽和定位序列促使蛋白异源表达的正确性。Takemura等[53]在大肠杆菌中异源合成紫黄质时,对大肠杆菌底盘细胞、表达载体和核糖体结合位点(ribosome binding site, RBS)序列进行了适配优化。结果表明,在菌株JM101(DE3)中,用pUC18载体表达CaZEP且所使用的RBS为RBS5000时,紫黄质产量最高(231 μg/g DCW)。
3.2.2 辅因子的调节在ABA生物合成的直接途径中,BcABA1和BcABA2作为P450单加氧酶(cytochrome P450, CYP450),需要CPR介导NADPH向CYP450传递电子,电子传递给CYP450之后,CYP450才能与底物发生氧化还原反应。研究者们尝试调节相关辅因子促进途径代谢,Arnesen等[48]尝试过表达酵母内源的NADH激酶(NADH kinase POS5, POS5)以改善NADPH氧化还原辅因子的供应;Otto等[52]通过敲除NADPH依赖的氨同化谷氨酸脱氢酶(NADP-specific glutamate dehydrogenase 1, GDH1)和过表达NADH依赖的谷氨酸脱氢酶(NAD-specific glutamate dehydrogenase, GDH2)增加NADPH的供应,并导入异源Bccpr1和过表达酿酒酵母内源CPR (NCP1)测试对ABA产量的影响。结果发现,只有异源Bccpr1的表达以及酵母内源NCP1的过表达使ABA的产量增加了3.5倍,体现了CPR过表达的重要性,增加通向CYP450的电子。但NCP1的过表达导致菌株的生长受限,OD600下降30%,可能对细胞产生一定毒性。
在ABA生物合成的间接途径中,ZEP是一个黄素腺嘌呤二核苷酸(flavin adenine dinucleotide, FAD)、NAD(P)H、O2依赖性双功能单加氧酶[54]。异源表达的ZEP从酵母内源代谢中获得所需的还原当量较少,电子转移到ZEP是有限的。因此,为了提高紫黄质的产量,Cataldo等[49]在表达tr59-HlZEP的紫黄质合成菌株中共表达FNR和FD。引入来自拟南芥(Arabidopsis thaliana)来源的氧化还原伙伴铁氧还蛋白-NADP还原酶(ferredoxin-NADP reductase, RFNR1)和铁氧还蛋白(ferredoxin-3, FD3)使紫黄质的含量增加了47%;进一步截短铁氧还蛋白(RFNR1/tr-FD3)或同时截断铁氧还蛋白和铁氧还蛋白-NADP还原酶(tr-RFNR1/tr-FD3),紫黄质的积累增加了2.2倍。可见,酿酒酵母适合FNR/FD/ZEP系统的表达。
3.2.3 增强前体供应随着生物技术的不断发展,利用基因工程手段改造菌株减少副产物的积累、提高前体物质的供给从而提高目标产物的产量成为了研究热点。萜类化合物发酵过程中副产物法尼醇、角鲨烯等相关合成基因的敲除、MVA途径基因的过表达成为了研究者们普遍选择的改造策略。目前,紫黄质的合成已在酿酒酵母中实现,而β-胡萝卜素是紫黄质合成的关键中间体。Cataldo等[49]尝试过表达香叶基香叶基二磷酸合酶(geranylgeranyl diphosphate synthase, CrtE)、八氢番茄红素去饱和酶(phytoene desaturase, CrtI)和双功能番茄红素环化酶/八氢番茄红素合酶(bifunctional lycopene cyclase/phytoene synthase, CrtYB)来增加β-胡萝卜素合成途径通量,发现只有增加CrtYB的拷贝数使类胡萝卜素总量显著增加(从9.1 mg/g DCW增加至12.3 mg/g DCW),为紫黄质的产生提供了更多前体物质,同时也为微生物生产C40途径中其他有价值的环氧类胡萝卜素以及ABA提供了一定的参考价值。
3.2.4 促进脱落酸外排拟南芥ABC转运蛋白G亚家族(ABC transporter G family, ABCG)是拟南芥中最大的转运蛋白亚家族[55]。Kuromori等[56]通过融合荧光蛋白方式发现AtABCG25在植物细胞中定位于细胞质膜,后经囊泡转运实验及过表达AtABCG25证明了AtABCG25是ABA的输出蛋白,并参与形成细胞间ABA信号通路。ABA转运蛋白的第二大家族是神经肽F (neuropeptide F, NPF) (NRT1/PTR)家族。Kanno等[57]利用改良的酵母双杂交系统以及异源转运分析,表明了AIT1/NRT1.2是参与ABA输入的蛋白。2014年,Zhang等[58]在大肠杆菌和非洲爪蟾卵母细胞中异源表达AtDTX50 (detoxification 50, DTX50),证明了拟南芥中DTX/Mate (multidrug and toxic extrusion transporter, Mate)家族成员AtDTX50作为ABA输出蛋白的功能。
在异源合成ABA的菌株中,Otto等[52]发现采用上清萃取法(乙酸乙酯-甲酸)处理ABA发酵样品可以回收93%的ABA,表明ABA可以通过细胞膜运输转运至胞外。于是,Arnesen等[48]为工程菌提供额外的ABA植物转运蛋白测试是否可以进一步强化ABA的分泌,从而缓解细胞压力。作者表达了来自拟南芥的两个转运蛋白AtDTX50p和AtABCG25p。然而,与原始菌株相比,异源转运蛋白的表达并没有对菌株产生正向影响。这表明在目前的产量水平下,天然酵母转运蛋白足以输出大部分的ABA。
4 总结与展望ABA应用广泛且市场需求逐渐增大,促使关于ABA合成的研究也越来越深入。由于植物提取中ABA含量较低、资源消耗过大,微生物发酵生产ABA起步较晚、生产成本很高等问题,在较长时间里化学合成是获得ABA的主要工业方法。近年来,随着ABA合成物种(主要是植物和真菌)基因组和转录组数据的挖掘[26, 59-60],完整的ABA生物合成途径逐渐被阐明,一些ABA生物合成基因也已通过体内或体外测定被鉴定出来。利用合成生物学在微生物中人工构建高价值天然产物的生物合成途径将会是一种更便捷、更经济的生产方式。在ABA的异源合成方面,已有研究者在解脂耶氏酵母[48]和酿酒酵母[52]中构建了异源代谢通路实现了ABA的合成。与此同时,如何提高工业平台菌株的生产力,成为ABA实现产业化首先要解决的问题。
一些新技术和方法可用于改造微生物菌株以提高ABA产量,例如:(1) 利用高通量筛选技术选育出优良菌株[61];(2) 利用细菌微区室(bacterial microcompartments, BMCs)技术在不影响细胞代谢的基础上高效合成ABA[62];(3) 在充分了解ABA生物合成和副产物积累的基础上,利用全局转录调控以及辅因子调控,进一步提高ABA产量[63];(4) 对限制性酶进行理性设计或非理性设计,提高酶活;(5) 利用基因组再造以及诱导重排,提高底盘细胞的稳定性和可操作性等。
综上所述,合成生物学技术的不断进步,为ABA生物合成和代谢工程的进一步研究提供了新思路。ABA工业化的生产不仅将带来巨大的经济利益,还会对农业和医药行业产生有益影响。
| [1] |
KARL D. The discovery of abscisic acid: a retrospect. Journal of Plant Growth Regulation, 2015, 34(4): 795-808. DOI:10.1007/s00344-015-9525-6
|
| [2] |
SAH SK, REDDY KR, LI JX. Abscisic acid and abiotic stress tolerance in crop plants. Frontiers in Plant Science, 2016, 7: 571.
|
| [3] |
项洪涛, 李琬, 何宁, 王雪扬, 曹大为, 曹良子, 唐晓东, 李一丹. 外源脱落酸(ABA)调节植物抗旱机制的研究进展[J/OL]. 东北农业科学, 2022, 47(5): 37-41. XIANG HT, LI W, HE N, WANG XY, CAO DW, CAO LZ, TANG XD, LI YD. Research progress on exogenous abscisic acid (ABA) regulating plant drought resistance[J/OL]. Journal of Northeast Agricultural Sciences, 2022, 47(5): 37-41 (in Chinese). |
| [4] |
AHANGER MA, SIDDIQUE KHM, AHMAD P. Understanding drought tolerance in plants. Physiologia Plantarum, 2021, 172(2): 286-288. DOI:10.1111/ppl.13442
|
| [5] |
GÓMEZ-CADENAS A, ARBONA V, JACAS J, PRIMO-MILLO E, TALON M. Abscisic acid reduces leaf abscission and increases salt tolerance in Citrus plants. Journal of Plant Growth Regulation, 2002, 21(3): 234-240. DOI:10.1007/s00344-002-0013-4
|
| [6] |
ZHANG Q, LIU YL, HE CC, ZHU SJ. Postharvest exogenous application of abscisic acid reduces internal browning in pineapple. Journal of Agricultural and Food Chemistry, 2015, 63(22): 5313-5320. DOI:10.1021/jf506279x
|
| [7] |
CHEN TT, LI GY, ISLAM MR, FU WM, FENG BH, TAO LX, FU GF. Abscisic acid synergizes with sucrose to enhance grain yield and quality of rice by improving the source-sink relationship. BMC Plant Biology, 2019, 19(1): 525. DOI:10.1186/s12870-019-2126-y
|
| [8] |
BOOZ V, CHRISTIANSEN CB, KUHRE RE, SALTIEL MY, SOCIALI G, SCHALTENBERG N, FISCHER AW, HEEREN J, ZOCCHI E, HOLST JJ, BRUZZONE S. Abscisic acid stimulates the release of insulin and of GLP-1 in the rat perfused pancreas and intestine. Diabetes/Metabolism Research and Reviews, 2019, 35(2): e3102.
|
| [9] |
GURI AJ, HONTECILLAS R, BASSAGANYA- RIERA J. Abscisic acid ameliorates experimental IBD by downregulating cellular adhesion molecule expression and suppressing immune cell infiltration. Clinical Nutrition, 2010, 29(6): 824-831. DOI:10.1016/j.clnu.2010.02.009
|
| [10] |
GLENNON EKK, ADAMS LG, HICKS DR, DEHESH K, LUCKHART S. Supplementation with abscisic acid reduces malaria disease severity and parasite transmission. The American Journal of Tropical Medicine and Hygiene, 2016, 94(6): 1266-1275. DOI:10.4269/ajtmh.15-0904
|
| [11] |
DARMA R, LUTZ A, ELLIOTT CE, IDNURM A. Identification of a gene cluster for the synthesis of the plant hormone abscisic acid in the plant pathogen Leptosphaeria maculans. Fungal Genetics and Biology, 2019, 130: 62-71. DOI:10.1016/j.fgb.2019.04.015
|
| [12] |
PAN W, LU Q, XU QR, ZHANG RR, LI HY, YANG YH, LIU HJ, DU ST. Abscisic acid-generating bacteria can reduce Cd concentration in pakchoi grown in Cd-contaminated soil. Ecotoxicology and Environmental Safety, 2019, 177: 100-107. DOI:10.1016/j.ecoenv.2019.04.010
|
| [13] |
NAGAMUNE K, HICKS LM, FUX B, BROSSIER F, CHINI EN, SIBLEY LD. Abscisic acid controls calcium-dependent egress and development in Toxoplasma gondii. Nature, 2008, 451(7175): 207-210. DOI:10.1038/nature06478
|
| [14] |
万小荣, 李玲. 高等植物脱落酸生物合成途径及其酶调控. 植物学通报, 2004, 21(3): 352-359. WAN XR, LI L. Pathways and related enzymes of ABA biosynthesis in higher plants. Chinese Bulletin of Botany, 2004, 21(3): 352-359 (in Chinese). DOI:10.3969/j.issn.1674-3466.2004.03.015 |
| [15] |
INOMATA M, HIRAI N, YOSHIDA R, OHIGASHI H. The biosynthetic pathway to abscisic acid via ionylideneethane in the fungus Botrytis cinerea. Phytochemistry, 2004, 65(19): 2667-2678. DOI:10.1016/j.phytochem.2004.08.025
|
| [16] |
FINKELSTEIN R. Abscisic acid synthesis and response. The Arabidopsis Book, 2013, 11: e0166. DOI:10.1199/tab.0166
|
| [17] |
杨秋玲, 季静, 王罡, 关春峰. 类胡萝卜素合成途径终产物脱落酸的合成调控与生物学效应. 天津农业科学, 2011, 17(5): 24-27. YANG QL, JI J, WANG G, GUAN CF. Regulation and biological effects of end-product abscisic acid of carotenoid biosynthetic pathway. Tianjin Agricultural Sciences, 2011, 17(5): 24-27 (in Chinese). DOI:10.3969/j.issn.1006-6500.2011.05.007 |
| [18] |
HIEBER AD, BUGOS RC, YAMAMOTO HY. Plant lipocalins: violaxanthin de-epoxidase and zeaxanthin epoxidase. Biochimica et Biophysica Acta: BBA-Protein Structure and Molecular Enzymology, 2000, 1482(1/2): 84-91.
|
| [19] |
SIMIONATO D, BASSO S, ZAFFAGNINI M, LANA T, MARZOTTO F, TROST P, MOROSINOTTO T. Protein redox regulation in the thylakoid lumen: the importance of disulfide bonds for violaxanthin de-epoxidase. FEBS Lett, 2015, 589(8): 919-923. DOI:10.1016/j.febslet.2015.02.033
|
| [20] |
NEUMAN H, GALPAZ N, CUNNINGHAM FX Jr, ZAMIR D, HIRSCHBERG J. The tomato mutation nxd1 reveals a gene necessary for neoxanthin biosynthesis and demonstrates that violaxanthin is a sufficient precursor for abscisic acid biosynthesis. The Plant Journal: for Cell and Molecular Biology, 2014, 78(1): 80-93. DOI:10.1111/tpj.12451
|
| [21] |
NURBEKOVA Z, SRIVASTAVA S, STANDING D, KURMANBAYEVA A, BEKTUROVA A, SOLTABAYEVA A, OSHANOVA D, TURECKOVA V, STRAND M, BISWAS MS, MANO J, SAGI M. Arabidopsis aldehyde oxidase 3, known to oxidize abscisic aldehyde to abscisic acid, protects leaves from aldehyde toxicity. The Plant Journal: for Cell and Molecular Biology, 2021, 108(5): 1439-1455. DOI:10.1111/tpj.15521
|
| [22] |
GONZÁLEZ-GUZMÁN M, APOSTOLOVA N, BELLÉS JM, BARRERO JM, PIQUERAS P, PONCE MR, MICOL JL, SERRANO R, RODRÍGUEZ PL. The short-chain alcohol dehydrogenase ABA2 catalyzes the conversion of xanthoxin to abscisic aldehyde. The Plant Cell, 2002, 14(8): 1833-1846. DOI:10.1105/tpc.002477
|
| [23] |
WU J, KAMANGA BM, ZHANG WY, XU YH, XU L. Research progress of aldehyde oxidases in plants. PeerJ, 2022, 10: e13119. DOI:10.7717/peerj.13119
|
| [24] |
YAMAMOTO H, INOMATA M, TSUCHIYA S, NAKAMURA M, ORITANI T. Metabolism of chiral ionylideneacetic acids on the abscisic acid biosynthetic pathway in Cercospora. Bioscience, Biotechnology, and Biochemistry, 2000, 64(12): 2644-2650. DOI:10.1271/bbb.64.2644
|
| [25] |
莫才清, 周俊初. 真菌中脱落酸的代谢及定量分析技术. 氨基酸和生物资源, 1996, 18(1): 44-48. MO CQ, ZHOU JC. Metabolism of abscisic acid in fungi and its quantitative analysis methods. Amino Acids & Biotic Resources, 1996, 18(1): 44-48 (in Chinese). DOI:10.14188/j.ajsh.1996.01.015 |
| [26] |
TAKINO J, KOZAKI T, SATO Y, LIU CW, OZAKI T, MINAMI A, OIKAWA H. Unveiling biosynthesis of the phytohormone abscisic acid in fungi: unprecedented mechanism of core scaffold formation catalyzed by an unusual sesquiterpene synthase. Journal of the American Chemical Society, 2018, 140(39): 12392-12395. DOI:10.1021/jacs.8b08925
|
| [27] |
TAKINO J, KOZAKI T, OZAKI T, LIU CW, MINAMI A, OIKAWA H. Elucidation of biosynthetic pathway of a plant hormone abscisic acid in phytopathogenic fungi. Bioscience, Biotechnology, and Biochemistry, 2019, 83(9): 1642-1649. DOI:10.1080/09168451.2019.1618700
|
| [28] |
INOMATA M, HIRAI N, YOSHIDA R, OHIGASHI H. Biosynthesis of abscisic acid by the direct pathway via ionylideneethane in a fungus, Cercospora cruenta. Bioscience, Biotechnology, and Biochemistry, 2004, 68(12): 2571-2580. DOI:10.1271/bbb.68.2571
|
| [29] |
SIEWERS V, SMEDSGAARD J, TUDZYNSKI P. The P450 monooxygenase BcABA1 is essential for abscisic acid biosynthesis in Botrytis cinerea. Applied and Environmental Microbiology, 2004, 70(7): 3868-3876. DOI:10.1128/AEM.70.7.3868-3876.2004
|
| [30] |
COUSO I, CORDERO BF, VARGAS MÁ, RODRÍGUEZ H. Efficient heterologous transformation of Chlamydomonas reinhardtii npq2 mutant with the zeaxanthin epoxidase gene isolated and characterized from Chlorella zofingiensis. Marine Drugs, 2012, 10(9): 1955-1976.
|
| [31] |
EILERS U, DIETZEL L, BREITENBACH J, BÜCHEL C, SANDMANN G. Identification of genes coding for functional zeaxanthin epoxidases in the diatom Phaeodactylum tricornutum. Journal of Plant Physiology, 2016, 192: 64-70. DOI:10.1016/j.jplph.2016.01.006
|
| [32] |
CHERNYS JT, ZEEVAART JAD. Characterization of the 9-cis-epoxycarotenoid dioxygenase gene family and the regulation of abscisic acid biosynthesis in avocado. Plant Physiology, 2000, 124(1): 343-354. DOI:10.1104/pp.124.1.343
|
| [33] |
HU B, HONG L, LIU X, LI L, LUO GY. Comparative study of the tissue-specific distribution of ABA from Arachis hypogaea L. and expression of the 9-cis epoxycarotenoid dioxygenase 1 (AhNCED1) during plant development. Biotechnology & Biotechnological Equipment, 2012, 26(5): 3201-3205.
|
| [34] |
GAN ZY, SHAN N, FEI LY, WAN CP, CHEN JY. Isolation of the 9-cis-epoxycarotenoid dioxygenase (NCED) gene from kiwifruit and its effects on postharvest softening and ripening. Scientia Horticulturae, 2020, 261: 109020. DOI:10.1016/j.scienta.2019.109020
|
| [35] |
SIEWERS V, KOKKELINK L, SMEDSGAARD J, TUDZYNSKI P. Identification of an abscisic acid gene cluster in the grey mold Botrytis cinerea. Applied and Environmental Microbiology, 2006, 72(7): 4619-4626. DOI:10.1128/AEM.02919-05
|
| [36] |
PASTENES C, PIMENTEL P, LILLO J. Leaf movements and photoinhibition in relation to water stress in field-grown beans. Journal of Experimental Botany, 2005, 56(411): 425-433.
|
| [37] |
YAMAMOTO HY, NAKAYAMA TOM, CHICHESTER CO. Studies on the light and dark interconversions of leaf xanthophylls. Archives of Biochemistry and Biophysics, 1962, 97(1): 168-173. DOI:10.1016/0003-9861(62)90060-7
|
| [38] |
MARIN E, NUSSAUME L, QUESADA A, GONNEAU M, SOTTA B, HUGUENEY P, FREY A, MARION-POLL A. Molecular identification of zeaxanthin epoxidase of Nicotiana plumbaginifolia, a gene involved in abscisic acid biosynthesis and corresponding to the ABA locus of Arabidopsis thaliana. The EMBO Journal, 1996, 15(10): 2331-2342. DOI:10.1002/j.1460-2075.1996.tb00589.x
|
| [39] |
YAO YX, JIA L, CHENG Y, RUAN MY, YE QJ, WANG RQ, YAO ZP, ZHOU GZ, LIU J, YU JH, ZHANG P, YIN YH, DIAO WP, WAN HJ. Evolutionary origin of the carotenoid cleavage oxygenase family in plants and expression of pepper genes in response to abiotic stresses. Frontiers in Plant Science, 2022, 12: 792832. DOI:10.3389/fpls.2021.792832
|
| [40] |
DARUWALLA A, KISER PD. Structural and mechanistic aspects of carotenoid cleavage dioxygenases (CCDs). Biochimica et Biophysica Acta: BBA- Molecular and Cell Biology of Lipids, 2020, 1865(11): 158590.
|
| [41] |
TAN BC, SCHWARTZ SH, ZEEVAART JA, MCCARTY DR. Genetic control of abscisic acid biosynthesis in maize. Proceedings of the National Academy of Sciences of the United States of America, 1997, 94(22): 12235-12240. DOI:10.1073/pnas.94.22.12235
|
| [42] |
QI KJ, WU X, XIE ZH, SUN XJ, GU C, TAO ST, ZHANG SL. Seed coat removal in pear accelerates embryo germination by down- regulating key genes in ABA biosynthesis. The Journal of Horticultural Science and Biotechnology, 2019, 94(6): 718-725. DOI:10.1080/14620316.2019.1602001
|
| [43] |
LIANG JH, YANG LX, CHEN X, LI L, GUO DL, LI HH, ZHANG BY. Cloning and characterization of the promoter of the 9-cis-epoxycarotenoid dioxygenase gene in Arachis hypogaea L.. Bioscience, Biotechnology, and Biochemistry, 2009, 73(9): 2103-2106. DOI:10.1271/bbb.90133
|
| [44] |
HUANG Y, GUO YM, LIU YT, ZHANG F, WANG ZK, WANG HY, WANG F, LI DP, MAO DD, LUAN S, LIANG MZ, CHEN LB. 9-cis-epoxycarotenoid dioxygenase 3 regulates plant growth and enhances multi-abiotic stress tolerance in rice. Frontiers in Plant Science, 2018, 9: 162. DOI:10.3389/fpls.2018.00162
|
| [45] |
DAMBEK M, EILERS U, BREITENBACH J, STEIGER S, BUCHEL C, SANDMANN G. Biosynthesis of fucoxanthin and diadinoxanthin and function of initial pathway genes in Phaeodactylum tricornutum. Journal of Experimental Botany, 2012, 63(15): 5607-5612. DOI:10.1093/jxb/ers211
|
| [46] |
MAMAEV D, ZVYAGILSKAYA R. Yarrowia lipolytica: a multitalented yeast species of ecological significance. FEMS Yeast Research, 2021, 21(2): foab008. DOI:10.1093/femsyr/foab008
|
| [47] |
张金宏, 崔志勇, 祁庆生, 侯进. 解脂耶氏酵母表达调控工具的开发及天然产物合成的研究进展. 生物工程学报, 2022, 38(2): 478-505. ZHANG JH, CUI ZY, QI QS, HOU J. The recent advances in developing gene editing and expression tools and the synthesis of natural products in Yarrowia lipolytica. Chinese Journal of Biotechnology, 2022, 38(2): 478-505 (in Chinese). DOI:10.13345/j.cjb.210327 |
| [48] |
ARNESEN JA, JACOBSEN IH, DYEKJÆR JD, RAGO D, KRISTENSEN M, KLITGAARD AK, RANDELOVIC M, MARTINEZ JL, BORODINA I. Production of abscisic acid in the oleaginous yeast Yarrowia lipolytica. FEMS Yeast Research, 2022, 22(1): foac015. DOI:10.1093/femsyr/foac015
|
| [49] |
CATALDO VF, ARENAS N, SALGADO V, CAMILO C, LBANEZ F, AGOSIN E. Heterologous production of the epoxycarotenoid violaxanthin in Saccharomyces cerevisiae. Metabolic Engineering, 2020, 59: 53-63. DOI:10.1016/j.ymben.2020.01.006
|
| [50] |
谭红, 周金燕, 钟娟, 杨杰, 肖亮. 一种高效制备天然脱落酸的方法: CN102399827B[P]. 2013-06-05. TAN H, ZHOU JY, ZHONG J, YANG J, XIAO L. An efficient method for preparing natural abscisic acid: CN102399827B[P]. 2013-06-05 (in Chinese). |
| [51] |
DING ZT, ZHANG Z, ZHONG J, LUO D, ZHOU JY, YANG J, XIAO L, SHU D, TAN H. Comparative transcriptome analysis between an evolved abscisic acid-overproducing mutant Botrytis cinerea TBC-A and its ancestral strain Botrytis cinerea TBC-6. Scientific Reports, 2016, 6: 37487. DOI:10.1038/srep37487
|
| [52] |
OTTO M, TEIXEIRA PG, VIZCAINO MI, DAVID F, SIEWERS V. Integration of a multi-step heterologous pathway in Saccharomyces cerevisiae for the production of abscisic acid. Microbial Cell Factories, 2019, 18(1): 205. DOI:10.1186/s12934-019-1257-z
|
| [53] |
TAKEMURA M, KUBO A, HIGUCHI Y, MAOKA T, SAHARA T, YAOI K, OHDAN K, UMENO D, MISAWA N. Pathway engineering for efficient biosynthesis of violaxanthin in Escherichia coli. Applied Microbiology and Biotechnology, 2019, 103(23): 9393-9399.
|
| [54] |
BÜCH K, STRANSKY H, HAGER A. FAD is a further essential cofactor of the NAD(P)H and O2-dependent zeaxanthin-epoxidase. FEBS Letters, 1995, 376(1/2): 45-48.
|
| [55] |
VERRIER PJ, BIRD D, BURLA B, DASSA E, FORESTIER C, GEISLER M, KLEIN M, KOLUKISAOGLU U, LEE Y, MARTINOIA E, MURPHY A, REA PA, SAMUELS L, SCHULZ B, SPALDING EJ, YAZAKI K, THEODOULOU FL. Plant ABC proteins-a unified nomenclature and updated inventory. Trends in Plant Science, 2008, 13(4): 151-159. DOI:10.1016/j.tplants.2008.02.001
|
| [56] |
KUROMORI T, MIYAJI T, YABUUCHI H, SHIMIZU H, SUGIMOTO E, KAMIYA A, MORIYAMA Y, SHINOZAKI K. ABC transporter AtABCG25 is involved in abscisic acid transport and responses. Proceedings of the National Academy of Sciences of the United States of America, 2010, 107(5): 2361-2366. DOI:10.1073/pnas.0912516107
|
| [57] |
KANNO Y, HANADA A, CHIBA Y, ICHIKAWA T, NAKAZAWA M, MATSUI M, KOSHIBA T, KAMIYA Y, SEO M. Identification of an abscisic acid transporter by functional screening using the receptor complex as a sensor. Proceedings of the National Academy of Sciences of the United States of America, 2012, 109(24): 9653-9658. DOI:10.1073/pnas.1203567109
|
| [58] |
ZHANG HW, ZHU HF, PAN YJ, YU YX, LUAN S, LI LG. A DTX/MATE-type transporter facilitates abscisic acid efflux and modulates ABA sensitivity and drought tolerance in Arabidopsis. Molecular Plant, 2014, 7(10): 1522-1532. DOI:10.1093/mp/ssu063
|
| [59] |
YANG WL, LI N, FAN YX, DONG BY, SONG ZH, CAO HY, DU TT, LIU TY, QI M, NIU LL, MENG D, YANG Q, FU YJ. Transcriptome analysis reveals abscisic acid enhancing drought resistance by regulating genes related to flavonoid metabolism in pigeon pea. Environmental and Experimental Botany, 2021, 191: 104627. DOI:10.1016/j.envexpbot.2021.104627
|
| [60] |
GOGOLEVA NE, NIKOLAICHIK YA, ISMAILOV TT, GORSHKOV VY, SAFRONOVA VI, BELIMOV AA, GOGOLEV Y. Complete genome sequence of the abscisic acid-utilizing strain Novosphingobium sp. P6W. 3 Biotech, 2019, 9(3): 94. DOI:10.1007/s13205-019-1625-8
|
| [61] |
孙怡, 张腾, 吕波, 李春. 胞内生物传感器提高微生物细胞工厂的精细调控. 化工学报, 2022, 73(2): 521-534. SUN Y, ZHANG T, LV B, LI C. Improvement for fine regulation of microbial cell factory by intracellular biosensors. CIESC Journal, 2022, 73(2): 521-534 (in Chinese). |
| [62] |
KIRST H, KERFELD CA. Bacterial microcompartments: catalysis-enhancing metabolic modules for next generation metabolic and biomedical engineering. BMC Biology, 2019, 17(1): 79. DOI:10.1186/s12915-019-0691-z
|
| [63] |
PATEL ZM, HUGHES TR. Global properties of regulatory sequences are predicted by transcription factor recognition mechanisms. Genome Biology, 2021, 22(1): 285. DOI:10.1186/s13059-021-02503-y
|
 2023, Vol. 39
2023, Vol. 39