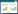中国科学院微生物研究所、中国微生物学会主办
文章信息
- 万赛, 王皓明, 马小清, 谭扬, 刘立成, 李福利
- WAN Sai, WANG Haoming, MA Xiaoqing, TAN Yang, LIU Licheng, LI Fuli
- 碳一气体生物转化中的产乙酸菌改造与发酵工艺优化
- Genetic modification of acetogens and optimization of fermentation process in C1-gas bioconversion
- 生物工程学报, 2023, 39(6): 2410-2429
- Chinese Journal of Biotechnology, 2023, 39(6): 2410-2429
- 10.13345/j.cjb.221004
-
文章历史
- Received: December 14, 2022
- Accepted: March 3, 2023
2. 中国海洋大学化学化工学院, 山东 青岛 266100;
3. 山东能源研究院, 山东 青岛 266101;
4. 青岛新能源山东省实验室, 山东 青岛 266101
2. College of Chemistry and Chemical Engineering, Ocean University of China, Qingdao 266100, Shandong, China;
3. Shandong Energy Institute, Qingdao 266101, Shandong, China;
4. Qingdao New Energy Shandong Laboratory, Qingdao 266101, Shandong, China
现代工业和经济的发展高度依赖化石资源,持续的开采利用和线性的发展模式导致原料短缺,人类排放的大量CO2造成全球变暖和极端天气频发[1]。因此,为了减少对化石资源的依赖,实现碳回收和循环利用,减少温室气体排放和环境污染,人们日益关注和寻找绿色可持续的能源和化学品的生产方式(图 1)。

|
| 图 1 线性经济模型(A)与循环经济模型(B) Fig. 1 Linear economy model (A) and circular economy model (B). |
| |
固定碳一气体(本文仅关注碳氧化物,包括CO和CO2)并转化为化学品和燃料是一种有前景的生产方式。碳一气体来源丰富,炼焦工业和钢铁工业、铁合金生产过程可以产生大量碳一气体,另外,也可通过气化农林废弃物或城市固体废物制得。当前,化学催化方法转化碳一气体需要高温高压,对气体的比例和纯度要求较高,且所用的化学催化剂易被原料气体中微量的污染物损害,相比之下,利用微生物作为催化剂所需的温度和压力条件温和,对污染物的耐受性较好,产物选择性高[2],应用前景广阔。
目前,已知有多种自养微生物可转化利用碳一气体,其中光能自养微生物需要光作为电子来源,发酵系统的复杂度和成本较高;与之相比化能自养生物对发酵系统的要求更简单,与目前的传统工业发酵系统匹配度高,因而受到广泛的关注。部分化能自养微生物可以利用甲烷(CH4),如发孢甲基弯菌(Methylosinus trichosporim)[3];部分好氧型微生物可以固定CO2,如贪铜菌(Cupriavidus necator)[4]。本文聚焦于非甲烷营养专性厌氧的化能自养细菌——产乙酸菌(acetogens)进行综述,这类细菌的主要特点是能够利用CO2和H2自养生长,主要产物为乙酸。
常见的具有碳一气体固定能力的产乙酸菌包括永达尔梭菌(Clostridium ljungdahlii)[5]、自产醇梭菌(Clostridium autoethanogenum)[6]、食一氧化碳梭菌(Clostridium carboxidivorans)[7]、拉格斯代尔梭菌(Clostridium ragsdalei)、克萨氏梭菌(Clostridium coskatii)[8]、伍氏醋酸杆菌(Acetobacterium woodii)[9]等,这类微生物通过伍德-永达尔途径(Wood-Ljungdahl pathway, WLP)将碳一气体转化为乙酰辅酶A及其衍生产物,实现末端电子接受和能量保存的过程。目前,已经完成上述菌株的全基因组测序,且相关分子遗传操作工具日趋成熟[10],可以将外源DNA转化进入宿主细胞并稳定复制,实现了基因表达的操纵和基因组的编辑,从而控制产物的生产[11]。因此,这些菌株是构建碳一气体转化细胞工厂的理想底盘生物。
碳一气体固定效率不仅受到发酵菌株的影响,还与液体培养基组成[12]和发酵反应器的设计及发酵过程中的参数控制密切相关[13]。本文主要综述碳一气体固定产乙酸菌的生理代谢机制、遗传与代谢工程、厌氧发酵工艺、提高碳原子经济性等领域的进展,并展望未来的发展方向。
1 碳一固定产乙酸菌生理代谢机制 1.1 Wood-Ljungdahl途径与电子歧化反应产乙酸菌依赖独特的线性WL途径实现自养生长,该途径由甲基分支和羰基分支组成(图 2),包含一系列以四氢叶酸(tetrahydrofolate, THF)为辅因子的金属酶,在甲基分支中包含6种酶:一氧化碳脱氢酶(CO dehydrogenase, CODH)、甲酸脱氢酶(formate dehydrogenase, FDH)、甲酰四氢叶酸合成酶(formyl-THF synthetase)、甲基四氢叶酸环化酶/脱氢酶(methenyl-THF cyclohydrolase)、亚甲基四氢叶酸还原酶(methylene-THF reductase, MTHFR)和甲基转移酶(methyltransferase),催化产生甲基-THF作为甲基基团供体,经一氧化碳脱氢酶/乙酰辅酶A合成酶(carbon monoxide dehydrogenase/acetyl-CoA synthase, CODH/ACS)介导,与辅酶A和来自羰基分支的一分子CO缩合产生乙酰辅酶A。

|
| 图 2 伍德-永达尔途径与能量合成代谢模式概述(以永达尔梭菌为例) Fig. 2 Summary of Wood-Ljungdahl pathway and energy conservation pattern (using the example of Clostridium ljungdahlii). THF:四氢叶酸;FDH:甲酸脱氢酶;FHS:甲酰四氢叶酸合成酶;FCH:甲酰四氢叶酸环化水解酶;MDH:亚甲基四氢叶酸脱氢酶;MTHFR:亚甲基四氢叶酸还原酶;MT:甲基转移酶;CoFeSP:钴铁硫蛋白;CODH:一氧化碳脱氢酶;ACS:乙酰辅酶A合成酶;PTA:磷酸转乙酰化酶;ACK:乙酸激酶;Fdox:氧化型铁氧还蛋白;Fdred:还原型铁氧还蛋白;Pi:无机磷酸盐 THF: Tetrahydrofolate; FDH: Formate dehydrogenase; FHS: Formyl-tetrahydrofolate synthase; FCH: Formyl-cyclohydrolase; MDH: Methylene-tetrahydrofolate dehydrogenase; MTHFR: Methylene- tetrahydrofolate reductase; MT: Methyltransferase; CoFeSP: Corrinoid iron-sulfur protein; CODH: CO dehydrogenase; ACS: Acetyl-CoA synthase; PTA: Phosphotransacetylase; ACK: Acetate kinase; Fdox: Oxidized ferredoxin; Fdred: Reduced ferredoxin; Pi: Inorganic phosphate. |
| |
WL途径中酶功能的实现依赖于铁氧还蛋白(ferredoxin, Fd)、辅酶Ⅰ (NAD+/NADH)和辅酶Ⅱ (NADP+/NADPH),关键中间产物乙酰辅酶A合成所需的还原力由还原型Fd (reduced Fd, Fdred)、NADH或NADPH提供。在产乙酸菌中存在独特的电子歧化(electron bifurcation)机制,将吸能的氧化还原反应与放能的反应偶联,从而最大限度地减少自由能的浪费[14],在克服热力学能垒的同时满足生命活动的需要。其中Nfn (NADH-dependent Fdred: NADP+ oxidoreductase)复合体能够偶联Fdred还原NADP+的放能过程和NADH还原NADP+的吸能过程,利用放能反应产生的自由能驱动吸能反应,可逆地调节氧化还原平衡[15];红杆菌固氮(Rhodobacter nitrogen fixation, Rnf)复合体使用Fdred还原NAD+驱动质子或钠离子转运至胞外形成跨膜离子梯度,驱动ATP合成,由于WL途径不产生净ATP,因此这种能量合成代谢模式对产乙酸菌的生存至关重要[16];电子歧化氢化酶(electron-bifurcating hydrogenase)偶联H2还原NAD+ (或NADP+)的放能反应和H2还原氧化型Fd (oxidized Fd, Fdox)的吸能反应,产生足够的还原当量,用于在WL途径的羰基分支中还原CO2为CO[17]。
1.2 氧化还原与能量平衡调节机制产乙酸菌的自养代谢在热力学极限(thermodynamic limit)的边缘运行[18],需要维持微妙的氧化还原和能量平衡方可保证正常生长[19]。生命活动必需的电子驱动化学渗透ATP合成机制需要氧化还原辅因子的支持,因此能量平衡与氧化还原平衡存在深层次的联系。研究发现,氧化还原平衡是决定产物合成过程中的碳流动方向和细胞代谢稳健性的关键因素[19],因此氧化还原平衡是产乙酸菌代谢的核心,其中的电子歧化酶在自养条件下保持较高的蛋白丰度,保证NADH/NAD+、NADPH/NADP+、Fdred/Fdox等氧化还原对迅速转换,从而对胞内氧化还原扰动做出反应,保证细胞中还原力的供应[20]。
产乙酸菌在固定碳一气体的过程中,每形成一分子乙酰辅酶A需要消耗一分子ATP,而后通过底物水平磷酸化产生乙酸并生成一分子ATP,因此WL途径没有净ATP生成,ATP需要由Rnf复合物与ATP合成酶通过电子驱动的化学渗透作用产生[21]。而以永达尔梭菌为代表的产乙酸菌具有乙醛-铁氧还蛋白氧化还原酶(acetaldehyde: Fd oxidoreductase, AOR),可在ATP生成后将乙酸经乙醛转化为乙醇,因此,在高细胞密度的发酵过程中,反应器中具有较高的乙酸浓度,细胞从产乙酸转向产乙醇,以避免细胞外乙酸扩散进入细胞质中干扰ATP的合成,从而维持能量平衡[8, 22]。ATP供应细胞内合成代谢反应、蛋白质与DNA的聚合以及细胞生长代谢所需能量,研究表明维持代谢所需的ATP约占自产醇梭菌气体发酵所产ATP的26%–44%,在热力学上显示出较高的代谢效率[23-24],但若试图调控产乙酸菌代谢以生产高能化学品仍需克服其能量限制[25]。已有研究证明补充营养物质可以为产乙酸菌提供额外的能量,例如在自产醇梭菌自养生长期间补充精氨酸,细胞可以通过精氨酸脱氨酶(arginine deiminase, ADI)途径产生ATP (图 2),从而使生长速率增加一倍,并且抑制乙酸的产生,分解产生的氨可以作为氮源[26];在以H2为能源条件下,补充廉价的硝酸盐作为电子受体,能够促进ATP合成,从而提高永达尔梭菌的生长速率和生物量,但CO2的利用率有所降低[27]。
总之,产乙酸菌为了维持细胞内环境和代谢的稳定,须维持氧化还原和能量的平衡,因此,在不同生长条件下,会导致还原产物和氧化产物的比例变化[19],这种变化更多地受细胞内代谢物浓度和热力学定律的控制,而非从转录或翻译水平上进行调控[28]。例如,在CO供应过剩的发酵条件下,若永达尔梭菌的生长受到营养限制,能量流和碳流将从供应菌体生长转向乙醇生产,从而实现氧化还原平衡[29-30]。对以上生理代谢机制的理解为提高产乙酸菌碳一气体发酵性能和产物选择性提供了理论基础。
2 产乙酸菌的遗传和代谢改造近年来,研究者进一步开发和优化了产乙酸菌的分子遗传操作工具,从细胞密度[31]、DNA浓度、电穿孔转化参数[32]、细胞复苏时间[33]等方面优化了电转化流程,通过接合转移方法[34]实现外源DNA导入;开发了革兰氏阳性和阴性复制子,构建大肠杆菌(Escherichia coli)-梭菌(Clostridium)穿梭质粒[35],结合抗生素抗性筛选标记,实现外源质粒的稳定遗传;利用启动子和核糖体结合位点(ribosome binding sites, RBS)等调控元件或基于RNA的工具[36]操纵宿主细胞基因或外源基因的表达水平;利用基于同源重组的同源臂偶联等位交换(allele-coupled exchange, ACE)技术[37]、基于可移动元件的ClosTron技术[38]以及基于成簇规则间隔短回文重复序列(clustered regularly interspaced short palindromic repeats, CRSIPR)的基因编辑技术,实现产乙酸菌细胞基因组编辑[10]。
分子生物学和合成生物学工具的日趋成熟,促进了产乙酸菌遗传学研究和代谢工程的进展,不仅有利于解析产乙酸菌的关键基因功能和生理代谢机制,更为设计性能优异的碳一气体转化利用细胞工厂奠定了基础(表 1)。
| Species | Substrate | Native products | Modification effects | Modification strategies | References |
| C. ljungdahlii | H2/CO2, CO | Acetate, ethanol, 2, 3-butanediol | Ethanol 4.72 g/L, acetate 4.07 g/L | Knockout of dat1, overexpression of aor2 and adhE1 containing acetylation-mimicking mutations | [39] |
| Butanol* 109 mg/L, hexanol* 393 mg/L | Expression of butanol and hexanol synthesis gene clusters from C. kluyveri and C. acetobutylicum by insertion into the genome | [40] | |||
| Isopropanol* 13.4 g/L, 3-hydroxybutyrate (3-HB)* 3.0 g/L, ethanol 28.4 g/L | Expression of isopropanol synthesis pathway genes from C. acetobutylicum and C. beijerinckii by plasmids | [41] | |||
| Acetate 7.1 g/L | Knocuout of BirA N-terminal structural domain using CRISPR-Cas9 system, and overexpression of acc gene by plasmids | [42] | |||
| Ethanol reduced by 20%−40%, butyrate* 1.2 g/L (20% increase) | Transcriptional repression of adhE1 in a butyrate-producing C. ljungdahlii strain using CRISPR-Cas12a-mediated CRISPRi | [43] | |||
| Butyrate* 1.01 g/L | Insertion of butyrate synthesis system into the genome of C. ljungdahlii through a “dual integrase cassette exchange” strategy | [44] | |||
| Acetone* 0.6 mmol/L, isopropanol* 2.4 mmol/L | Integration of a heterologous acetone synthesis pathway into the C. ljungdahlii genome using a xylose-inducible promoter-regulated Himar1 transposase | [45] | |||
| Poly-3-hydroxybutyrate (PHB)* 1.12%/CDW | Plasmid expression of a novel PHB synthesis pathway in C. ljungdahlii | [46] | |||
| 3-HB* 18.4 mmol/L | Plasmid expression of 3-HB biosynthesis genes, and transcriptional repression of pta or aor2 using CRISPRi | [32] | |||
| Mevalonate* 68 μg/mL | Plasmid expression of the eukaryotic mevalonic acid (MVA) pathway genes | [11] | |||
| C. autoethanogenum | H2/CO2, CO | Acetate, ethanol, 2, 3-butanediol | Ethylene glycol* 0.029 g/g fructose | Expression of an ethylene glycol biosynthesis pathway using a two-plasmid system | [47] |
| Ethanol 3 g/(L·h), isopropanol* 3 g/(L·h) | Mining superior enzymes using a combinatorial metabolic pathway library, and optimizing metabolic flux using omics analysis, kinetic modeling and cell-free prototyping | [48] | |||
| 1, 3-butanediol* 0.5 g/L, 3-HB* 14.63 g/L | Development of a platform for in vitro prototyping and rapid optimization of biosynthetic enzymes (iPROBE), and plasmid expression of a high-performance pathway for 3-HB synthesis | [49] | |||
| PHB* 10% (W/W)/CDW | Plasmid expression of the PHB synthesis pathway from C. necator | [50] | |||
| C. carboxidivorans | H2/CO2, CO | Acetate, ethanol, butyrate, butanol, hexanoate, hexanol | Ethanol 1.79 g/L, butyrate 0.7 g/L, butanol 0.25 g/L | Ethyl methanesulfonate (EMS)-induced mutagenesis | [51] |
| Ethanol 2.44 g/L, butanol 0.35 g/L | Plasmid expression of adhE2 and fnr from C. acetobutylicum ATCC 824 | [52] | |||
| A. woodii | H2/CO2, CO/H2/CO2 | Acetate | Isopropanol* 0.13 g/L | Plasmid expression of a heterologous isopropanol synthesis pathway | [53] |
| Acetone* 3.03 g/L | Plasmid expression of a heterologous acetone synthesis pathway | [8] | |||
| *: Non-native compounds after genetic modification. | |||||
在发酵过程中,通过增加气体分压等手段可以提高碳一气体的固定效率(将在“3发酵工艺优化策略”中详细讨论),但具有物理上的局限性[54],因此科研人员通过代谢改造提高产乙酸菌的生产性能,克服其低生长速率、低产量和低细胞密度的缺点,使其更加适用于工业生产(图 3)。改造产乙酸菌中的WL途径(甲基分支和羰基分支)或引入外源固碳途径,可以提高其利用碳一气体效率。
|
| 图 3 产乙酸菌碳一气体转化效率提升策略概述 Fig. 3 Summary of strategies to improve the efficiency of C1-gas bioconversion in acetogens. A:遗传工程. B:发酵工艺. C:二氧化碳电还原 A: Genetic engineering. B: Fermentation process. C: CO2 electroreduction. |
| |
有研究推测,甲基分支途径中,CO2还原生成甲酸后,下游THF依赖的酶对甲酸的处理过程可能是CO2和H2转化的限速过程[55]。基于此推测,Straub等[56]将来自永达尔梭菌的甲基分支中的4种酶(FHS, FCH, MDH, MTHFR)在伍氏醋酸杆菌(Acetacillus woodii)中过表达,在控制pH的分批发酵条件下,重组菌株相比于原始菌株,乙酸浓度提高了14%。在WL途径中,CODH/ ACS复合物在乙酰辅酶A的合成过程中发挥重要作用,当CODH编码基因被敲除时,自产醇梭菌无法在自养条件下生长[57],当将CODH/ ACS基因(CAETHG_1620–1621)通过质粒进行过表达时,延滞期减少了4.2 d,乙醇和乳酸的产量分别提高1.2倍和2.7倍[58]。此外,在德雷克氏梭菌(Clostridium drakei)中,甲基分支途径与甘氨酸合成酶-还原酶途径(glycine synthase- reductase pathway, GSRP)相连,GSRP可介导甲基分支中间产物亚甲基-THF转化为甘氨酸,而后进一步还原为乙酰磷酸,最终转化为乙酸。GSRP的异源表达使粘液真杆菌(Eubacterium limosum)的二氧化碳消耗速率提高了40%,乙酸盐生产速率增加了1.1倍,证明了在WL途径中接入外源固碳途径的可行性[59]。
尽管产乙酸菌可以利用CO为碳源和能源自养生长,但在高浓度CO条件下,自养生长通常会受到抑制。Kang等[60]通过实验室适应性进化(adaptive laboratory evolution),经过150代的培养后,使得粘液真杆菌在44% CO浓度下,细胞密度提高至出发菌株的2.14倍,生长速率提高至1.44倍。随后的单克隆筛选及基因组重测序证明,引起表型改变的关键突变位点出现在CODH/ACS复合体的编码基因上,表明实验室适应性进化策略可以有效提高菌株发酵性能[61]。
2.2 增强天然产物合成的遗传改造所有的产乙酸菌都能够通过WL途径产生乙酰辅酶A并产生乙酸。乙酰辅酶A是许多生化物质的重要前体,产乙酸菌除了主要生成乙酸外,还可以生成多种有机酸和醇类。但在气体发酵条件下,仅有少数产乙酸菌能够作为生产化学品的细胞工厂,产物一般为乙醇、丁醇和2, 3-丁二醇[62]等。例如,伍氏醋酸杆菌在H2/CO2条件下以乙酸为唯一终产物,采用分批发酵可使乙酸滴度达到44 g/L[63],乙酸可作为各种化学衍生物的前体,具有较高的商业价值,2020年全球需求量高达1 800万t/年[64];自产醇梭菌、永达尔梭菌和拉格斯代尔梭菌能够生产乙醇和2, 3-丁二醇[21, 65];较特殊的是,食一氧化碳梭菌能够利用合成气生产丁酸、丁醇和己醇,这些高级醇有较高的能量密度,是高效的生物燃料[66]。
为增强产乙酸菌的气体发酵性能,提高天然产物的产量和选择性,除改变发酵底物、发酵条件以及对WL途径进行遗传改造外,研究者们通过解析产乙酸菌在碳一气体发酵过程中的产物合成机制、转录调节机制[67]以及翻译后调节机制,采取相应的遗传操作策略改善发酵性能(图 3)。例如,抑制自产醇梭菌的旁路途径基因(乙酰乳酸脱羧酶、乳酸脱氢酶和2, 3-丁二醇脱氢酶)可提高乙醇产量[68-70]。永达尔梭菌中,在由乙酰辅酶A向乙醇转化过程中,存在AOR和AdhE两条途径,其基因组编码2个醛铁氧还蛋白氧化还原酶AOR1和AOR2以及2个双功能醛醇脱氢酶AdhE1和AdhE2。在自养条件下,AOR途径是乙醇合成的关键途径,但敲除aor2可使乙醇合成提高70%,而敲除adhE1或adhE2可使自养条件下的乙醇合成最高提高80%[71]。Liu等[72]研究发现,AOR和AdhE途径不仅可以还原乙酸产生乙醇,还可以氧化乙醇生成乙酸。乙醇合成主要发生于指数期,而在稳定生长期,AOR2和AdhE1也会参与乙醇氧化,生成乙酸,这就解释了为什么这两个基因的敲除会增强乙醇合成。
转录因子BirA一方面是一个多效调控因子,可以调节多基因的表达;另一方面具有生物素蛋白连接酶(biotin protein ligase, BPL)活性,能够调节下游酶的活性[73]。在永达尔梭菌中敲除BirA N端DNA结合域阻断其调控功能,并过表达缺失DNA结合功能的突变体强化其BPL活性,同时过表达由其生物素化的下游蛋白乙酰辅酶A羧化酶(acetyl-CoA carboxylase, ACC),可显著提高重组菌株的生长速率、细胞密度和乙酸的最终滴度[73]。此外,研究者证明永达尔梭菌中主要的乙酰化/脱乙酰化系统(acetylation/deacetylation system)与转录因子Rex (redox-sensing protein)形成交叉调节机制调节碳代谢流分布,在敲除去乙酰化酶基因(deacetylase, dat1)的基础上,过表达乙醛铁氧还原蛋白酶(aldehyde ferredoxin oxidoreductase, aor2),并且采取关键赖氨酸位点突变模拟去乙酰化的策略过表达醇醛脱氢酶(acetaldehyde/ alcohol dehydrogenase, adhE1),显著提高了乙醇的产量和分子比例[42]。
值得注意的是,关键基因的敲除往往导致细胞死亡,CRISPR基因表达抑制(CRISPR interference, CRISPRi)系统可调节性地降低靶基因的表达并防止基因功能的完全丧失,可用于调节产乙酸菌气体发酵过程中的碳代谢流分配,研究者已成功利用基于Cas9和Cas12a系统的CRISPRi技术,提高目标产物(乙醇、丁酸等)的产量和比例,降低碳向乙酸的流动[32, 39]。
2.3 引入非天然产物的遗传改造自从首次完成产乙酸菌的遗传改造以来,日趋成熟的遗传操作工具使异源途径在产乙酸菌底盘中的表达更加便利,产乙酸菌已经能够利用碳一气体产生多种重要的工业化学品,包括丁醇、丁二醇、丙二醇、异丙醇、乳酸、琥珀酸、丙酮、丁烯和异戊二烯等[43, 48],例如引入来自丙酮丁醇梭菌(Clostridium acetobutylicum)的丙酮合成途径在永达尔梭菌[47]和伍氏醋酸杆菌[74]中实现丙酮生产。
充分开发乙酸梭菌转化利用碳一气体,需要进一步扩大产物谱及相应的生产性能(图 3)。在产乙酸菌中,非天然产物的合成需要引入异源途径并且稳定表达,而已报道的绝大多数成功案例基于质粒表达系统,容易受到质粒拷贝数及遗传稳定性的限制,同时需要抗生素维持从而增加细胞的代谢负担[29]。应该强调的是,向产乙酸菌中引入更复杂的代谢途径需要将这些代谢途径整合入其基因组中,例如,通过噬菌体丝氨酸整合酶实现大片段基因簇染色体整合表达以实现永达尔梭菌生产丁酸[75];利用Himar1转座酶将异源丙酮生物合成途径的整合到永达尔梭菌基因组整合实现丙酮生产[44]。此外,基于RNA引导的CRISPR系统也可用于基因组整合[45],仍有待在产乙酸菌中进一步研究利用。最近,研究者利用无细胞基因表达系统综合分析数百种酶组合优化代谢途径,结合基因组敲除修饰、代谢通量优化以及工艺优化,使自产醇梭菌在中试规模利用合成气连续发酵生产丙酮和异丙醇,生产效率达到3 g/(L·h)以上,选择性达到90%[48],为产乙酸菌在商业规模转化碳一气体生产非天然化学品提供了很好的范式。
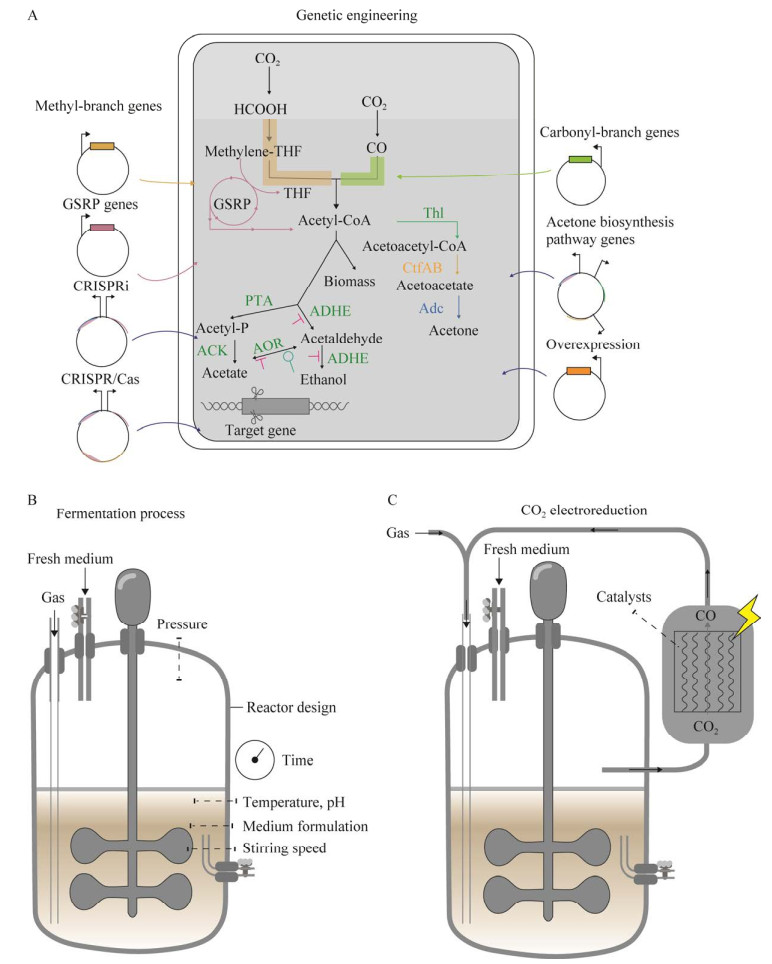
3 发酵工艺优化策略 3.1 反应器设计与工艺优化策略由于CO和H2在水中的溶解度极低(37 ℃, 100 kPa, CO和H2在水中的饱和浓度分别为8.2×10−4 mol/L和7.1×10−4 mol/L),气液界面的高液膜阻力导致气体底物传质受限。因此,如何提高生物反应器的气液传质速率成为合成气发酵工艺中的主要问题(图 3)。根据传质速率方程(1)[76],提高体积传质系数kLa值和传质驱动力(
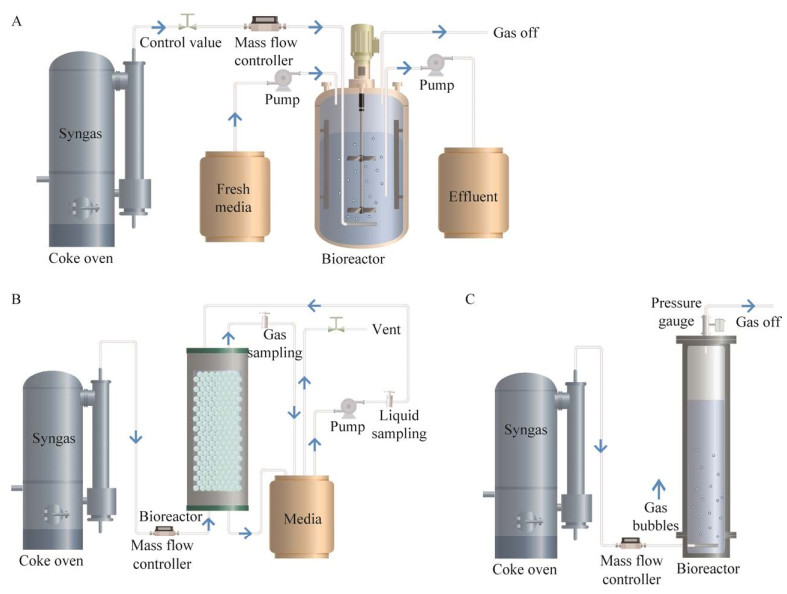
|
| 图 4 典型的厌氧发酵反应器构型 Fig. 4 Typical reactor configuration for anaerobic fermentation. A:搅拌釜反应器. B:滴流床反应器. C:鼓泡塔反应器 A: Continuous stirred tank bioreactor. B: Trickle bed reactor. C: Bubble column reactor. |
| |
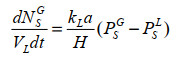
|
(1) |
式中,NSG为气相中气体底物摩尔数,mol;VL为液相体积,L;kLa为体积传质系数,h–1;H为亨利定律常数,L·atm/mol;PSG为气体底物在气相中的分压,atm;PSL为气体底物在液相中的分压,atm。
目前,搅拌釜式反应器(stirred tank reactor, STR)是应用最广泛的气体发酵生物反应器,可以在间歇或连续模式下单独运行或与不同反应器耦合联用[77-78]。通过提高STR叶轮的搅拌转速将气泡破碎,能够增加气液传质界面积,从而提高气液传质效率。Klasson等[79]将搅拌转速由300 r/min提高至700 r/min,kLa (CO)提高了138%。尽管提高搅拌转速可增强传质,但在工业化生产中,转速提高导致的高能耗会提高运行成本[80]。因此有研究者使用微气泡扩散器以提升传质效率[81-82],气体经过微孔扩散到发酵液中,可有效地提高气液接触面积。Lee等[83]将STR与外置中空纤维膜扩散器相结合使得kLa (CO)可达到385/h。此外,还可通过增加挡板、优化叶轮选择和提高气速等方式来改善传质效果[84-86]。
鼓泡塔反应器(bubble column reactor, BCR)因其结构简单,运行和维护成本低、具有较高的传质效率等优势,在工业规模得到推广和应用。在气体发酵过程中,塔内充满液体,依靠底部气体喷射带动液体流动。气泡在上升过程中与液体充分接触并起到搅拌作用[87]。由于气液界面面积大,且气体在液体中的停留时间长,使BCR具有更好的传质效果。Chang等[88]报道在BCR中kLa (CO)可达到72/h。Munasinghe等[89]将BCR与不同类型气体扩散器相结合比较发现,柱状扩散器(孔径0.5−1.0 mm)和环形扩散器(孔径0.5 mm)的kLa (CO)约为45/h,其传质能力明显低于孔径为20 μm的球形微气泡扩散器[kLa (CO)=79/h]。为避免在BCR中出现轴向混合和气泡聚并等不利于传质的现象,可在BCR的基础上加入导流筒改进为气升式反应器(gas lift reactor, GLR)。Munasinghe等[90]通过在GLR底部加装20 μm孔径的球形气体扩散器,使该反应器的kLa (CO)最高可达到129.6/h。但由于水力剪切力过大,细胞生长受到一定限制。
在滴流床式反应器(trickle bed reactor, TBR)中,将微生物细胞固定在反应器内部的惰性填料上,气液以逆流或并流方式进行连续流动。由于液体以薄膜的形式与气体接触,从而减小液膜阻力提高传质效率[91]。Devarapalli等[92]报道在1 L的TBR中,kLa (CO)可高达664/h。此外,其他具有细胞固定化能力的膜生物反应器也逐渐应用于气体发酵工艺,如中空纤维膜反应器(hollow fiber membrane bioreactor, HFMBR)[93]、单片生物膜反应器(monolithic biofilm reactor, MBR)[94]和水平旋转填料床膜生物反应器(horizontal rotating packed bed biofilm reactor)[95]。在膜生物反应器中,提高液体流速可以有效提高传质效率,是保持高细胞密度发酵的理想反应器。但在长期运行之后,会产生积碳、生物淤积使得反应器堵塞,影响使用寿命[96]。
3.2 发酵反应参数控制优化策略pH是调控合成气发酵过程的重要因素,影响微生物的生长速率和产物生成(图 3)。大部分产乙酸菌的最适pH为5.0–8.3之间[97],研究发现,相对较高的pH 5.0–6.5有利于菌体生长和有机酸的生成,当pH降至4.5–5.0时,则会有助于醇的生成。永达尔梭菌在生长过程中,由于乙酸的产生会导致pH下降。当胞内未解离的乙酸浓度达到热力学阈值时,会使细胞由产酸阶段转变为产醇阶段,生成乙醇和其他还原性的物质[28]。根据这一特性,Richter等[77]以永达尔梭菌为平台建立了两级连续发酵工艺:第一个反应器为连续搅拌釜式反应器(continuous stirred- tank reactor, CSTR)并设置pH为5.7,负责生长阶段;第二反应器为BCR并设置pH为4.8,负责产乙醇阶段,最终乙醇产量为20.7 g/L。这种两级连续发酵模式在食一氧化碳梭菌进行了验证应用[98-99]。
发酵温度对微生物的生长和代谢有着直接影响。大多数产乙酸菌的最适生长温度在30−37 ℃范围内,这有利于有机酸的产生和积累,而对醇的生成则有所限制。在37 ℃,食一氧化碳梭菌P7仅能生成1.56 mmol/L的乙醇,但当温度降至25 ℃,能够促进乙醇和丁醇的生成,浓度分别为32.1和14.5 mmol/L[67]。此外,Shen等[13]研究发现,虽然37 ℃条件有利于食一氧化碳梭菌P7的快速生长,但会导致细胞絮结。通过两步温度培养方法(先37 ℃后25 ℃)不仅能够减少细胞絮结,还可以有效提高醇的产量。此外,Kundiyana等[100]发现拉格斯代尔梭菌在32 ℃条件下,可以获得较高的乙醇产量并且能够缩短由产酸期到产醇期的转换时间。
由于CO、H2的溶解度低,使得气液传质受限成为合成气发酵的主要瓶颈。为了改善传质效率,提高气体流速成为一种有效的方法。Devarapalli等[92]研究表明,当气体流速由2.3 mL/min增加至4.6 mL/min,拉格斯代尔梭菌对CO和H2的利用提高80%以上,乙酸的产量也明显提高。永达尔梭菌以CO为气体底物时,当气体流速由5 mL/min提高至10 mL/min,乙酸和乙醇的累积有所增加。但随着气体流速进一步提高至15 mL/min时,CO转化率由75%下降至55%,并且出现乙醇回用的现象[101]。Shen等[94]研究了不同气体流速下食一氧化碳梭菌P7以CO和H2作为气体底物时的发酵性能,发现当气体流速为50−300 mL/min时,乙醇和乙酸的产率会随气速增加而增加,但继续提高气速至500 mL/min,两者的产量则会轻微下降。
合成气主要是由CO、H2和CO2三种气体组成,但合成气来源不同导致其三者的比例也有所不同[102]。产乙酸菌可将CO作为发酵过程所需的碳源和能源,当利用CO2为碳源时,则需要额外的H2或者CO为其提供能量。有研究表明,永达尔梭菌利用CO和H2进行发酵时,H2/CO比例影响发酵产物比例,较高的H2浓度有利于乙酸的形成,而较高的CO浓度,则有利于乙醇和2, 3-丁二醇的产生[103]。在一定范围内增加CO的分压能够提高永达尔梭菌和醋酸梭菌(Clostridium aceticum)的生物量和乙醇产量[104-105]。尽管提高CO浓度有利于醇的产生,但有报道称当顶空中CO的体积分数为10%时,则会对拉格斯代尔梭菌中氢化酶活性产生严重的抑制[106]。
4 碳负生产与原子经济性提升策略目前,大多数燃料和化学品的生产完全依赖化石资源,如石油、天然气和煤炭,这些传统的生产过程排放温室气体且污染环境,因此需要开发环境友好的能源和化学品生产制造方式,降低生产过程中的温室气体(greenhouse gas, GHG)排放总量,即降低“碳足迹”(carbon footprint)。我国《“十四五”生态环境领域科技创新专项规划》强调开发低碳零碳负碳技术,而气体发酵技术能够在废碳流进入大气层环境之前,对其进行捕获和回收利用,是实现负碳制造和循环经济的有效途径之一[48]。厌氧产乙酸菌具有优秀的气体发酵潜力,且已经实现工业规模气体发酵生产乙醇[38]、丙酮和异丙醇[48]。美国朗泽科技公司(LanzaTech)在最新的研究中,通过全生命周期分析(life-cycle analysis, LCA)[107],在划定系统边界的基础上对生产过程中所需的能源和材料进行统计,分析相应的温室气体排放和生产过程对环境的影响,证明该生产过程的碳足迹为负。
减少碳排放的另一方式是提高碳的“原子经济性” (atom economy)[108],即提高生产过程中原料中的碳原子进入产品中的比例,这样可以充分利用资源,防止污染[109]。基于此理念,科研人员将微生物气体发酵系统与电化学催化系统耦合组成微生物电合成系统(microbial electrosynthesis system, MES)[110],在这个过程中微生物细胞通过从电极获得电子将CO2转化为含碳有机物[111],可显著提高CO2还原的能量效率,但MES系统的效率易受到培养基成分、pH值、反应器设计、气体浓度、电极材料、电压等参数的影响[112]。此外,在产乙酸菌利用CO生产乙醇的过程中,CO担负着碳源和能源的双重角色,6 mol CO合成1 mol乙醇,同时伴随着4 mol CO2的生成,只有三分之一的碳被固定,碳原子经济性差,生物发酵本身这一代谢特点,不能提高碳的固定率,需要耦合其他转化方式[71, 113]。电化学反应可将CO2还原为CO且反应条件温和,不需要复杂的换热和热管理,维持反应条件的能耗低,且容易与微生物气体发酵工艺衔接匹配,产生的CO通过循环再次进入产乙酸菌的代谢体系,从而提高转化过程中的碳利用率(图 3)。目前,研究者已经开发出能够还原CO2的电还原催化剂,例如,Zn-N共掺杂石墨烯(Zn-N-G)单原子催化剂,具有单分散Zn-N4活性位,可以催化电还原CO2,产物CO法拉第效率最高达到91%,过电位仅有0.39 V;负载Pd2双原子位点催化剂相比Pd1单原子催化剂具有更优异的活性,产CO法拉第效率最高达到98.2%;利用氨基化修饰单原子催化剂,增强CO2吸附、调控单原子局部电荷分布,显著提高产CO反应电流密度,并调控产物选择性[114-115],可应用于产乙酸菌的气体发酵系统。尽管如此,电化学催化还原CO2反应过程开发、设备放大等仍面临诸多挑战,例如气体扩散电极、离子交换膜等关键材料和部件的稳定性问题,以及电解池的设计和电解堆技术的开发,是目前研究的重点[116]。
5 总结与展望碳一气体生物发酵是一项前景广阔的技术,能够有效减少温室气体排放,降低对环境的污染和对化石燃料的依赖,绿色可持续地生产生物燃料和化学品。产乙酸菌具有高效的CO2固定途径、天然的产醇能力、广泛的代谢产物谱、成熟的分子生物学工具、温和易控的发酵条件以及与工业发酵工艺较高的匹配度,因此成为碳一气体生物转化生产高价值化学品和燃料的理想底盘生物,并已在工业规模上应用。
近年来,研究者利用多组学分析、代谢建模、遗传学分析等多种手段,在揭示产乙酸菌的生理代谢机制、转录调控机制以及翻译后调控机制等方面取得诸多进展,并大大促进了相关的合成生物学工具和元件的开发。为了通过菌株的理性设计开发更加适用于工业规模发酵的底盘菌株,提高气体转化效率、生产率,扩大代谢产物谱,还需要进一步开发功能更为强大的遗传操作工具和基因调控元件,以能够快速高效地进行工程化改造。
除菌株遗传和代谢工程改造外,发酵参数和过程控制的优化、反应器构型的改进,结合相应的提升气液传质效率的手段,将在产乙酸菌碳一气体发酵的工业化放大方面发挥重要作用。将气体发酵与电还原CO2相结合,利用来自风能和太阳能的可再生电力,发展混合营养的发酵策略,控制碳流向,减少副产物生成,有利于提高碳原子经济性,实现以负碳方式生产生物燃料和化学品。
| [1] |
IPCC. Contribution of working group Ⅰ to the sixth assessment report of the intergovernmental panel on climate change[R]. Climate Change 2021: the Physical Science Basis, 2021.
|
| [2] |
MOLITOR B, RICHTER H, MICHAEL EM, RASMUS OJ, ALEX J, CHRISTOPHE M, LARGUS TA. Carbon recovery by fermentation of CO-rich off gases-turning steel mills into biorefineries. Bioresource Technology, 2016, 215: 386-396. DOI:10.1016/j.biortech.2016.03.094
|
| [3] |
NGUYEN DTN, LEE OK, LIM C, LEE J, NA JG, LEE EY. Metabolic engineering of type Ⅱ methanotroph, Methylosinus trichosporium OB3b, for production of 3-hydroxypropionic acid from methane via a malonyl-CoA reductase-dependent pathway. Metabolic Engineering, 2020, 59: 142-150. DOI:10.1016/j.ymben.2020.02.002
|
| [4] |
NYBO SE, KHAN NE, WOOLSTON BM, CURTIS WR. Metabolic engineering in chemolithoautotrophic hosts for the production of fuels and chemicals. Metabolic Engineering, 2015, 30: 105-120. DOI:10.1016/j.ymben.2015.04.008
|
| [5] |
KÖPKE M, HELD C, HUJER S, LIESEGANG H, WIEZER A, WOLLHERR A, EHRENREICH A, LIEBL W, GOTTSCHALK G, DÜRRE P. Clostridium ljungdahlii represents a microbial production platform based on syngas. Proceedings of the National Academy of Sciences of the United States of America, 2010, 107(29): 13087-13092. DOI:10.1073/pnas.1004716107
|
| [6] |
HUMPHREYS CM, MCLEAN S, SCHATSCHNEIDER S, MILLAT T, HENSTRA AM, ANNAN FJ, BREITKOPF R, PANDER B, PIATEK P, ROWE P, WICHLACZ AT, WOODS C, NORMAN R, BLOM J, GOESMAN A, HODGMAN C, BARRETT D, THOMAS NR, WINZER K, MINTON NP. Whole genome sequence and manual annotation of Clostridium autoethanogenum, an industrially relevant bacterium. BMC Genomics, 2015, 16(1): 1-10. DOI:10.1186/1471-2164-16-1
|
| [7] |
LI N, YANG JJ, CHAI CS, YANG S, JIANG WH, GU Y. Complete genome sequence of Clostridium carboxidivorans P7T, a syngas-fermenting bacterium capable of producing long-chain alcohols. Journal of Biotechnology, 2015, 211: 44-45. DOI:10.1016/j.jbiotec.2015.06.430
|
| [8] |
BENGELSDORF FR, POEHLEIN A, LINDER S, ERZ C, HUMMEL T, HOFFMEISTER S, DANIEL R, DÜRRE P. Industrial acetogenic biocatalysts: a comparative metabolic and genomic analysis. Frontiers in Microbiology, 2016, 7: 1036.
|
| [9] |
MOON J, DÖNIG J, KRAMER S, POEHLEIN A, DANIEL R, MÜLLER V. Formate metabolism in the acetogenic bacterium Acetobacterium woodii. Environmental Microbiology, 2021, 23(8): 4214-4227. DOI:10.1111/1462-2920.15598
|
| [10] |
BOURGADE B, MINTON NP, ISLAM MA. Genetic and metabolic engineering challenges of C1-gas fermenting acetogenic chassis organisms. FEMS Microbiology Reviews, 2021, 45(2): fuab008. DOI:10.1093/femsre/fuab008
|
| [11] |
DINER BA, FAN J, SCOTCHER MC, WELLS DH, WHITED GM. Synthesis of heterologous mevalonic acid pathway enzymes in Clostridium ljungdahlii for the conversion of fructose and of syngas to mevalonate and isoprene. Applied and Environmental Microbiology, 2018, 84(1): e01723-e01717.
|
| [12] |
SINGH V, HAQUE S, NIWAS R, SRIVASTAVA A, PASUPULETI M, TRIPATHI CKM. Strategies for fermentation medium optimization: an in-depth review. Frontiers in Microbiology, 2017, 7: 2087.
|
| [13] |
SHEN SH, WANG G, ZHANG M, TANG Y, GU Y, JIANG WH, WANG YH, ZHUANG YP. Effect of temperature and surfactant on biomass growth and higher-alcohol production during syngas fermentation by Clostridium carboxidivorans P7. Bioresources and Bioprocessing, 2020, 7(1): 1-13. DOI:10.1186/s40643-019-0289-x
|
| [14] |
LI FL, HINDERBERGER J, SEEDORF H, ZHANG J, BUCKEL W, THAUER RK. Coupled ferredoxin and crotonyl coenzyme A (CoA) reduction with NADH catalyzed by the butyryl-CoA dehydrogenase/etf complex from Clostridium kluyveri. Journal of Bacteriology, 2008, 190(3): 843-850. DOI:10.1128/JB.01417-07
|
| [15] |
WANG SN, HUANG HY, MOLL J, THAUER RK. NADP+ reduction with reduced ferredoxin and NADP+ reduction with NADH are coupled via an electron-bifurcating enzyme complex in Clostridium kluyveri. Journal of Bacteriology, 2010, 192(19): 5115-5123. DOI:10.1128/JB.00612-10
|
| [16] |
HESS V, GALLEGOS R, JONES JA, BARQUERA B, MALAMY MH, MÜLLER V. Occurrence of ferredoxin: NAD+ oxidoreductase activity and its ion specificity in several Gram-positive and Gram-negative bacteria. PeerJ, 2016, 4: e1515. DOI:10.7717/peerj.1515
|
| [17] |
MÜLLER V, CHOWDHURY NP, BASEN M. Electron bifurcation: a long-hidden energy-coupling mechanism. Annual Review of Microbiology, 2018, 72: 331-353. DOI:10.1146/annurev-micro-090816-093440
|
| [18] |
SCHUCHMANN K, MÜLLER V. Autotrophy at the thermodynamic limit of life: a model for energy conservation in acetogenic bacteria. Nature Reviews Microbiology, 2014, 12(12): 809-821. DOI:10.1038/nrmicro3365
|
| [19] |
MAHAMKALI V, VALGEPEA K, de SOUZA PINTO LEMGRUBER R, PLAN M, TAPPEL R, KÖPKE M, SIMPSON SD, NIELSEN LK, MARCELLIN E. Redox controls metabolic robustness in the gas-fermenting acetogen Clostridium autoethanogenum. Proceedings of the National Academy of Sciences of the United States of America, 2020, 117(23): 13168-13175. DOI:10.1073/pnas.1919531117
|
| [20] |
VALGEPEA K, TALBO G, TAKEMORI N, TAKEMORI A, LUDWIG C, MAHAMKALI V, MUELLER AP, TAPPEL R, KÖPKE M, DENNIS SIMPSON S, NIELSEN LK, MARCELLIN E. Absolute proteome quantification in the gas-fermenting acetogen Clostridium autoethanogenum. mSystems, 2022, 7(2): e00026-22.
|
| [21] |
LIEW F, MARTIN ME, TAPPEL RC, HEIJSTRA BD, MIHALCEA C, KÖPKE M. Gas fermentation—a flexible platform for commercial scale production of low-carbon-fuels and chemicals from waste and renewable feedstocks. Frontiers in Microbiology, 2016, 7: 694.
|
| [22] |
VALGEPEA K, de SOUZA PINTO LEMGRUBER R, MEAGHAN K, PALFREYMAN RW, ABDALLA T, DANIEL HEIJSTRA B, BEHRENDORFF JB, TAPPEL R, KÖPKE M, DENNIS SIMPSON S, NIELSEN LK, MARCELLIN E. Maintenance of ATP homeostasis triggers metabolic shifts in gas-fermenting acetogens. Cell Systems, 2017, 4(5): 505-515.e5. DOI:10.1016/j.cels.2017.04.008
|
| [23] |
HEFFERNAN JK, VALGEPEA K, de SOUZA PINTO LEMGRUBER R, CASINI I, PLAN M, TAPPEL R, SIMPSON SD, KÖPKE M, NIELSEN LK, MARCELLIN E. Enhancing CO2-valorization using Clostridium autoethanogenum for sustainable fuel and chemicals production. Frontiers in Bioengineering and Biotechnology, 2020, 8: 204. DOI:10.3389/fbioe.2020.00204
|
| [24] |
LAHTVEE PJ, SEIMAN A, ARIKE L, ADAMBERG K, VILU R. Protein turnover forms one of the highest maintenance costs in Lactococcus lactis. Microbiology, 2014, 160(7): 1501-1512. DOI:10.1099/mic.0.078089-0
|
| [25] |
KATSYV A, MÜLLER V. Overcoming energetic barriers in acetogenic C1 conversion. Frontiers in Bioengineering and Biotechnology, 2020, 8: 621166. DOI:10.3389/fbioe.2020.621166
|
| [26] |
VALGEPEA K, LOI KQ, BEHRENDORFF JB, de SP LEMGRUBER R, PLAN M, HODSON MP, KÖPKE M, NIELSEN LK, MARCELLIN E. Arginine deiminase pathway provides ATP and boosts growth of the gas- fermenting acetogen Clostridium autoethanogenum. Metabolic Engineering, 2017, 41: 202-211. DOI:10.1016/j.ymben.2017.04.007
|
| [27] |
EMERSON DF, WOOLSTON BM, LIU N, DONNELLY M, CURRIE DH, STEPHANOPOULOS G. Enhancing hydrogen-dependent growth of and carbon dioxide fixation by Clostridium ljungdahlii through nitrate supplementation. Biotechnology and Bioengineering, 2019, 116(2): 294-306. DOI:10.1002/bit.26847
|
| [28] |
RICHTER H, MOLITOR B, WEI H, CHEN W, ARISTILDE L, ANGENENT LT. Ethanol production in syngas-fermenting Clostridium ljungdahlii is controlled by thermodynamics rather than by enzyme expression. Energy & Environmental Science, 2016, 9(7): 2392-2399.
|
| [29] |
PAVAN M, REINMETS K, GARG S, MUELLER AP, MARCELLIN E, KÖPKE M, VALGEPEA K. Advances in systems metabolic engineering of autotrophic carbon oxide-fixing biocatalysts towards a circular economy. Metabolic Engineering, 2022, 71: 117-141. DOI:10.1016/j.ymben.2022.01.015
|
| [30] |
MARTIN ME, RICHTER H, SAHA S, ANGENENT LT. Traits of selected Clostridium strains for syngas fermentation to ethanol. Biotechnology and Bioengineering, 2016, 113(3): 531-539. DOI:10.1002/bit.25827
|
| [31] |
KITA A, IWASAKI Y, SAKAI S, OKUTO S, TAKAOKA K, SUZUKI T, YANO S, SAWAYAMA S, TAJIMA T, KATO J, NISHIO N, MURAKAMI K, NAKASHIMADA Y. Development of genetic transformation and heterologous expression system in carboxydotrophic thermophilic acetogen Moorella thermoacetica. Journal of Bioscience and Bioengineering, 2013, 115(4): 347-352. DOI:10.1016/j.jbiosc.2012.10.013
|
| [32] |
WOOLSTON BM, EMERSON DF, CURRIE DH, STEPHANOPOULOS G. Rediverting carbon flux in Clostridium ljungdahlii using CRISPR interference (CRISPRi). Metabolic Engineering, 2018, 48: 243-253. DOI:10.1016/j.ymben.2018.06.006
|
| [33] |
SHIN J, KANG S, SONG Y, JIN S, LEE JS, LEE JK, KIM DR, KIM SC, CHO S, CHO BK. Genome engineering of Eubacterium limosum using expanded genetic tools and the CRISPR-Cas9 system. ACS Synthetic Biology, 2019, 8(9): 2059-2068. DOI:10.1021/acssynbio.9b00150
|
| [34] |
MOCK J, ZHENG YN, MUELLER AP, LY S, TRAN L, SEGOVIA S, NAGARAJU S, KÖPKE M, DÜRRE P, THAUER RK. Energy conservation associated with ethanol formation from H2 and CO2 in Clostridium autoethanogenum involving electron bifurcation. Journal of Bacteriology, 2015, 197(18): 2965-2980. DOI:10.1128/JB.00399-15
|
| [35] |
HEAP JT, PENNINGTON OJ, CARTMAN ST, MINTON NP. A modular system for Clostridium shuttle plasmids. Journal of Microbiological Methods, 2009, 78(1): 79-85. DOI:10.1016/j.mimet.2009.05.004
|
| [36] |
CHOI KR, JANG WD, YANG D, CHO JS, PARK D, LEE SY. Systems metabolic engineering strategies: integrating systems and synthetic biology with metabolic engineering. Trends in Biotechnology, 2019, 37(8): 817-837. DOI:10.1016/j.tibtech.2019.01.003
|
| [37] |
ANNAN FJ, AL-SINAWI B, HUMPHREYS CM, NORMAN R, WINZER K, KÖPKE M, SIMPSON SD, MINTON NP, HENSTRA AM. Engineering of vitamin prototrophy in Clostridium ljungdahlii and Clostridium autoethanogenum. Applied Microbiology and Biotechnology, 2019, 103(11): 4633-4648. DOI:10.1007/s00253-019-09763-6
|
| [38] |
MARCELLIN E, BEHRENDORFF JB, NAGARAJU S, DETISSERA S, SEGOVIA S, PALFREYMAN RW, DANIELL J, LICONA-CASSANI C, QUEK LE, SPEIGHT R, HODSON MP, SIMPSON SD, MITCHELL WP, KÖPKE M, NIELSEN LK. Low carbon fuels and commodity chemicals from waste gases-systematic approach to understand energy metabolism in a model acetogen. Green Chemistry, 2016, 18(10): 3020-3028. DOI:10.1039/C5GC02708J
|
| [39] |
LIU YQ, ZHANG ZW, JIANG WH, GU Y. Protein acetylation-mediated cross regulation of acetic acid and ethanol synthesis in the gas-fermenting Clostridium ljungdahlii. Journal of Biological Chemistry, 2022, 298(2): 101538. DOI:10.1016/j.jbc.2021.101538
|
| [40] |
LAUER I, PHILIPPS G, JENNEWEIN S. Metabolic engineering of Clostridium ljungdahlii for the production of hexanol and butanol from CO2 and H2. Microbial Cell Factories, 2022, 21(1): 1-18. DOI:10.1186/s12934-021-01718-9
|
| [41] |
JIA DC, HE MY, TIAN Y, SHEN SH, ZHU XF, WANG YH, ZHUANG YP, JIANG WH, GU Y. Metabolic engineering of gas-fermenting Clostridium ljungdahlii for efficient co-production of isopropanol, 3-hydroxybutyrate, and ethanol. ACS Synthetic Biology, 2021, 10(10): 2628-2638. DOI:10.1021/acssynbio.1c00235
|
| [42] |
ZHANG C, NIE XQ, ZHANG H, WU YW, HE HQ, YANG C, JIANG WH, GU Y. Functional dissection and modulation of the BirA protein for improved autotrophic growth of gas-fermenting Clostridium ljungdahlii. Microbial Biotechnology, 2021, 14(5): 2072-2089. DOI:10.1111/1751-7915.13884
|
| [43] |
ZHAO R, LIU YQ, ZHANG H, CHAI CS, WANG J, JIANG WH, GU Y. CRISPR-Cas12a-mediated gene deletion and regulation in Clostridium ljungdahlii and its application in carbon flux redirection in synthesis gas fermentation. ACS Synthetic Biology, 2019, 8(10): 2270-2279. DOI:10.1021/acssynbio.9b00033
|
| [44] |
HUANG H, CHAI CS, YANG S, JIANG WH, GU Y. Phage serine integrase-mediated genome engineering for efficient expression of chemical biosynthetic pathway in gas-fermenting Clostridium ljungdahlii. Metabolic Engineering, 2019, 52: 293-302. DOI:10.1016/j.ymben.2019.01.005
|
| [45] |
PHILIPPS G, de VRIES S, JENNEWEIN S. Development of a metabolic pathway transfer and genomic integration system for the syngas-fermenting bacterium Clostridium ljungdahlii. Biotechnology for Biofuels, 2019, 12(1): 1-14. DOI:10.1186/s13068-018-1346-y
|
| [46] |
FLÜCHTER S, FOLLONIER S, SCHIEL-BENGELSDORF B, BENGELSDORF FR, ZINN M, DÜRRE P. Anaerobic production of poly(3-hydroxybutyrate) and its precursor 3-hydroxybutyrate from synthesis gas by autotrophic clostridia. Biomacromolecules, 2019, 20(9): 3271-3282. DOI:10.1021/acs.biomac.9b00342
|
| [47] |
BOURGADE B, HUMPHREYS CM, MILLARD J, MINTON NP, ISLAM MA. Design, analysis, and implementation of a novel biochemical pathway for ethylene glycol production in Clostridium autoethanogenum. ACS Synthetic Biology, 2022, 11(5): 1790-1800. DOI:10.1021/acssynbio.1c00624
|
| [48] |
LIEW FE, NOGLE R, ABDALLA T, RASOR BJ, CANTER C, JENSEN RO, WANG L, STRUTZ J, CHIRANIA P, de TISSERA S, MUELLER AP, RUAN ZH, GAO A, TRAN L, ENGLE NL, BROMLEY JC, DANIELL J, CONRADO R, TSCHAPLINSKI TJ, GIANNONE RJ, et al. Carbon-negative production of acetone and isopropanol by gas fermentation at industrial pilot scale. Nature Biotechnology, 2022, 40(3): 335-344. DOI:10.1038/s41587-021-01195-w
|
| [49] |
KARIM AS, DUDLEY QM, JUMINAGA A, YUAN YB, CROWE SA, HEGGESTAD JT, GARG S, ABDALLA T, GRUBBE WS, RASOR BJ, COAR DN, TORCULAS M, KREIN M, LIEW F, QUATTLEBAUM A, JENSEN RO, STUART JA, SIMPSON SD, KÖPKE M, JEWETT MC. In vitro prototyping and rapid optimization of biosynthetic enzymes for cell design. Nature Chemical Biology, 2020, 16(8): 912-919. DOI:10.1038/s41589-020-0559-0
|
| [50] |
de SOUZA PINTO LEMGRUBER R, VALGEPEA K, TAPPEL R, BEHRENDORFF JB, PALFREYMAN RW, PLAN M, HODSON MP, DENNIS SIMPSON S, NIELSEN LK, KÖPKE M, MARCELLIN E. Systems-level engineering and characterisation of Clostridium autoethanogenum through heterologous production of poly-3-hydroxybutyrate (PHB). Metabolic Engineering, 2019, 53: 14-23. DOI:10.1016/j.ymben.2019.01.003
|
| [51] |
LAKHSSASSI N, BAHARLOUEI A, MEKSEM J, HAMILTON-BREHM SD, LIGHTFOOT DA, MEKSEM K, LIANG YN. EMS-induced mutagenesis of Clostridium carboxidivorans for increased atmospheric CO2 reduction efficiency and solvent production. Microorganisms, 2020, 8(8): 1239. DOI:10.3390/microorganisms8081239
|
| [52] |
CHENG C, LI WM, LIN M, YANG ST. Metabolic engineering of Clostridium carboxidivorans for enhanced ethanol and butanol production from syngas and glucose. Bioresource Technology, 2019, 284: 415-423. DOI:10.1016/j.biortech.2019.03.145
|
| [53] |
WEITZ S, HERMANN M, LINDER S, BENGELSDORF FR, TAKORS R, DÜRRE P. Isobutanol production by autotrophic acetogenic bacteria. Frontiers in Bioengineering and Biotechnology, 2021, 9: 657253. DOI:10.3389/fbioe.2021.657253
|
| [54] |
SATHISH A, SHARMA A, GABLE P, SKIADAS I, BROWN R, WEN ZY. A novel bulk-gas-to- atomized-liquid reactor for enhanced mass transfer efficiency and its application to syngas fermentation. Chemical Engineering Journal, 2019, 370: 60-70. DOI:10.1016/j.cej.2019.03.183
|
| [55] |
PETERS V, JANSSEN PH, CONRAD R. Transient production of formate during chemolithotrophic growth of anaerobic microorganisms on hydrogen. Current Microbiology, 1999, 38(5): 285-289. DOI:10.1007/PL00006803
|
| [56] |
STRAUB M, DEMLER M, WEUSTER-BOTZ D, DÜRRE P. Selective enhancement of autotrophic acetate production with genetically modified Acetobacterium woodii. Journal of Biotechnology, 2014, 178: 67-72. DOI:10.1016/j.jbiotec.2014.03.005
|
| [57] |
LIEW F, HENSTRA AM, WINZER K, KÖPKE M, SIMPSON SD, MINTON NP. Insights into CO2 fixation pathway of Clostridium autoethanogenum by targeted mutagenesis. mBio, 2016, 7(3): e00427-16.
|
| [58] |
KÖPKE M, LIEW F. Genetically engineered bacterium with altered carbon monoxide dehydrogenase (codh) activity[P]. 2016, U. S. Patent 20160040193 A1.
|
| [59] |
SONG Y, LEE JS, SHIN J, LEE GM, JIN S, KANG S, LEE JK, KIM DR, LEE EY, KIM SC, CHO S, KIM D, CHO BK. Functional cooperation of the glycine synthase-reductase and Wood-Ljungdahl pathways for autotrophic growth of Clostridium drakei. Proceedings of the National Academy of Sciences of the United States of America, 2020, 117(13): 7516-7523. DOI:10.1073/pnas.1912289117
|
| [60] |
KANG S, SONG Y, JIN S, SHIN J, BAE JY, KIM DR, LEE JK, KIM SC, CHO S, CHO BK. Adaptive laboratory evolution of Eubacterium limosum ATCC 8486 on carbon monoxide. Frontiers in Microbiology, 2020, 11: 402. DOI:10.3389/fmicb.2020.00402
|
| [61] |
刘正, 侍永江, 周泰然, 高磊, 郭羽芬, 唐士杰, 罗洪镇. 木质纤维素预处理衍生抑制物对产溶剂梭菌的胁迫机制及解抑制策略研究. 南京工业大学学报(自然科学版), 2022, 44(5): 566-576. LIU Z, SHI YJ, ZHOU TR, GAO L, GUO YF, TANG SJ, LUO HZ. Study on response mechanism of solventogenic Clostridia against lignocellulose pretreatment⁃derived inhibitors stress and recent strategies for eliminating the inhibitory effects. Journal of Nanjing Tech University (Natural Science Edition), 2022, 44(5): 566-576 (in Chinese). DOI:10.3969/j.issn.1671-7627.2022.05.009 |
| [62] |
MÜLLER V, FRERICHS J.. Acetogenic Bacteria. eLS. Chichester: John Wiley & Sons Ltd, 2020.
|
| [63] |
DEMLER M, WEUSTER-BOTZ D. Reaction engineering analysis of hydrogenotrophic production of acetic acid by Acetobacterium woodii. Biotechnology and Bioengineering, 2011, 108(2): 470-474. DOI:10.1002/bit.22935
|
| [64] |
KAREKAR S, SRINIVAS K, AHRING B. Kinetic study on heterotrophic growth of Acetobacterium woodii on lignocellulosic substrates for acetic acid production. Fermentation, 2019, 5(1): 17. DOI:10.3390/fermentation5010017
|
| [65] |
KÖPKE M, MIHALCEA C, LIEW F, TIZARD JH, ALI MS, CONOLLY JJ, AL-SINAWI B, SIMPSON SD. 2, 3-butanediol production by acetogenic bacteria, an alternative route to chemical synthesis, using industrial waste gas. Applied and Environmental Microbiology, 2011, 77(15): 5467-5475. DOI:10.1128/AEM.00355-11
|
| [66] |
RAMIÓ-PUJOL S, GANIGUÉ R, BAÑERAS L, COLPRIM J. Incubation at 25 ℃ prevents acid crash and enhances alcohol production in Clostridium carboxidivorans P7. Bioresource Technology, 2015, 192: 296-303. DOI:10.1016/j.biortech.2015.05.077
|
| [67] |
张俊哲, 张全, 刘自勇, 马小清, 刘立成, 李福利. 细菌微室及其在合成生物学中的应用进展. 南京工业大学学报(自然科学版), 2022, 44(5): 490-499. ZHANG JZ, ZHANG Q, LIU ZY, MA XQ, LIU LC, LI FL. Bacterial microcompartments and application progress in synthetic biology. Journal of Nanjing Tech University (Natural Science Edition), 2022, 44(5): 490-499 (in Chinese). DOI:10.3969/j.issn.1671-7627.2022.05.002 |
| [68] |
KÖPKE M, LIEW F. Recombinant microorganism and methods of production thereof[P]. 2011, U. S. Patent 20110236941 A1.
|
| [69] |
NAGARAJU S, DAVIES NK, WALKER DJF, KÖPKE M, DENNIS SIMPSON S. Genome editing of Clostridium autoethanogenum using CRISPR/Cas9. Biotechnology for Biofuels, 2016, 9(1): 1-8. DOI:10.1186/s13068-015-0423-8
|
| [70] |
李福利, 顾阳, 刘自勇, 等. 一种提高合成气发酵产乙醇含量的突变株和利用突变株的应用: CN2021104358862[P]. 2021-04-22. LI FL, GU Y, LIU ZY, et al. A mutant strain for improving ethanol production from syngas fermentation and its application: CN2021104358862[P]. 2021-04-22 (in Chinese). |
| [71] |
LIEW F, HENSTRA AM, KӦPKE M, WINZER K, SIMPSON SD, MINTON NP. Metabolic engineering of Clostridium autoethanogenum for selective alcohol production. Metabolic Engineering, 2017, 40: 104-114. DOI:10.1016/j.ymben.2017.01.007
|
| [72] |
LIU ZY, JIA DC, ZHANG KD, ZHU HF, ZHANG Q, JIANG WH, GU Y, LI FL. Ethanol metabolism dynamics in Clostridium ljungdahlii grown on carbon monoxide. Applied and Environmental Microbiology, 2020, 86(14): e00730-20.
|
| [73] |
PETERS-WENDISCH P, STANSEN KC, GÖTKER S, WENDISCH VF. Biotin protein ligase from Corynebacterium glutamicum: role for growth and l-lysine production. Applied Microbiology and Biotechnology, 2012, 93(6): 2493-2502. DOI:10.1007/s00253-011-3771-8
|
| [74] |
BANERJEE A, LEANG C, UEKI T, NEVIN KP, LOVLEY DR. Lactose-inducible system for metabolic engineering of Clostridium ljungdahlii. Applied and Environmental Microbiology, 2014, 80(8): 2410-2416. DOI:10.1128/AEM.03666-13
|
| [75] |
HOFFMEISTER S, GERDOM M, BENGELSDORF FR, LINDER S, FLÜCHTER S, ÖZTÜRK H, BLÜMKE W, MAY A, FISCHER RJ, BAHL H, DÜRRE P. Acetone production with metabolically engineered strains of Acetobacterium woodii. Metabolic Engineering, 2016, 36: 37-47. DOI:10.1016/j.ymben.2016.03.001
|
| [76] |
KLASSON K, ACKERSON C, CLAUSEN E, GADDY J. Biological conversion of synthesis gas into fuels. International Journal of Hydrogen Energy, 1992, 17(4): 281-288. DOI:10.1016/0360-3199(92)90003-F
|
| [77] |
RICHTER H, MARTIN M, ANGENENT L. A two-stage continuous fermentation system for conversion of syngas into ethanol. Energies, 2013, 6(8): 3987-4000. DOI:10.3390/en6083987
|
| [78] |
LIU K, PHILLIPS JR, SUN X, MOHAMMAD S, HUHNKE RL, ATIYEH HK. Investigation and modeling of gas-liquid mass transfer in a sparged and non-sparged continuous stirred tank reactor with potential application in syngas fermentation. Fermentation, 2019, 5(3): 75. DOI:10.3390/fermentation5030075
|
| [79] |
KLASSON KT, ACKERSON MD, CLAUSEN EC, GADDY JL. Bioconversion of synthesis gas into liquid or gaseous fuels. Enzyme and Microbial Technology, 1992, 14(8): 602-608. DOI:10.1016/0141-0229(92)90033-K
|
| [80] |
RIEGLER P, CHRUSCIEL T, MAYER A, DOLL K, WEUSTER-BOTZ D. Reversible retrofitting of a stirred-tank bioreactor for gas-lift operation to perform synthesis gas fermentation studies. Biochemical Engineering Journal, 2019, 141: 89-101. DOI:10.1016/j.bej.2018.09.021
|
| [81] |
LI XY, DUAN YG, WANG HL, CHENG JC, YANG C. Internal optimization for enhancing the microbubble dispersion characteristics of a stirred tank. Industrial & Engineering Chemistry Research, 2022, 61(45): 16815-16822.
|
| [82] |
SUN X, ATIYEH HK, ZHANG HL, TANNER RS, HUHNKE RL. Enhanced ethanol production from syngas by Clostridium ragsdalei in continuous stirred tank reactor using medium with poultry litter biochar. Applied Energy, 2019, 236: 1269-1279. DOI:10.1016/j.apenergy.2018.12.010
|
| [83] |
LEE PH, NI SQ, CHANG SY, SUNG S, KIM SH. Enhancement of carbon monoxide mass transfer using an innovative external hollow fiber membrane (HFM) diffuser for syngas fermentation: experimental studies and model development. Chemical Engineering Journal, 2012, 184: 268-277. DOI:10.1016/j.cej.2011.11.103
|
| [84] |
LI GL, LI H, WEI GG, HE X, XU S, CHEN KQ, OUYANG P, JI XJ. Hydrodynamics, mass transfer and cell growth characteristics in a novel microbubble stirred bioreactor employing sintered porous metal plate impeller as gas sparger. Chemical Engineering Science, 2018, 192: 665-677. DOI:10.1016/j.ces.2018.08.025
|
| [85] |
BENEVENUTI C, BRANCO M, DO NASCIMENTO- CORREA M, BOTELHO A, FERREIRA T, AMARAL P. Residual gas for ethanol production by Clostridium carboxidivorans in a dual impeller stirred tank bioreactor (STBR). Fermentation, 2021, 7(3): 199. DOI:10.3390/fermentation7030199
|
| [86] |
SHARMA AM, KUMAR A, Madihally S, Whiteley JR, Huhnke RL. Prediction of biomass-generated syngas using extents of major reactions in a continuous stirred-tank reactor. Energy, 2014, 72: 222-232. DOI:10.1016/j.energy.2014.05.027
|
| [87] |
LI XG, GRIFFIN D, LI XL, HENSON MA. Incorporating hydrodynamics into spatiotemporal metabolic models of bubble column gas fermentation. Biotechnology and Bioengineering, 2019, 116(1): 28-40. DOI:10.1002/bit.26848
|
| [88] |
CHANG IS, KIM BH, LOVITT RW, BANG JS. Effect of CO partial pressure on cell-recycled continuous CO fermentation by Eubacterium limosum KIST612. Process Biochemistry, 2001, 37(4): 411-421. DOI:10.1016/S0032-9592(01)00227-8
|
| [89] |
MUNASINGHE PC, KHANAL SK. Syngas fermentation to biofuel: evaluation of carbon monoxide mass transfer coefficient (kLa) in different reactor configurations. Biotechnology Progress, 2010, 26(6): 1616-1621. DOI:10.1002/btpr.473
|
| [90] |
MUNASINGHE PC, KHANAL SK. Evaluation of hydrogen and carbon monoxide mass transfer and a correlation between the myoglobin-protein bioassay and gas chromatography method for carbon monoxide determination. RSC Adv, 2014, 4(71): 37575-37581. DOI:10.1039/C4RA04696J
|
| [91] |
ORGILL JJ, ATIYEH HK, DEVARAPALLI M, PHILLIPS JR, LEWIS RS, HUHNKE RL. A comparison of mass transfer coefficients between trickle-bed, hollow fiber membrane and stirred tank reactors. Bioresource Technology, 2013, 133: 340-346. DOI:10.1016/j.biortech.2013.01.124
|
| [92] |
DEVARAPALLI M, ATIYEH HK, PHILLIPS JR, LEWIS RS, HUHNKE RL. Ethanol production during semi-continuous syngas fermentation in a trickle bed reactor using Clostridium ragsdalei. Bioresource Technology, 2016, 209: 56-65. DOI:10.1016/j.biortech.2016.02.086
|
| [93] |
SHEN YW, BROWN R, WEN ZY. Syngas fermentation of Clostridium carboxidivoran P7 in a hollow fiber membrane biofilm reactor: evaluating the mass transfer coefficient and ethanol production performance. Biochemical Engineering Journal, 2014, 85: 21-29. DOI:10.1016/j.bej.2014.01.010
|
| [94] |
SHEN YW, BROWN R, WEN ZY. Enhancing mass transfer and ethanol production in syngas fermentation of Clostridium carboxidivorans P7 through a monolithic biofilm reactor. Applied Energy, 2014, 136: 68-76. DOI:10.1016/j.apenergy.2014.08.117
|
| [95] |
SHEN YW, BROWN R, WEN ZY. Syngas fermentation by Clostridium carboxidivorans P7 in a horizontal rotating packed bed biofilm reactor with enhanced ethanol production. Applied Energy, 2017, 187: 585-594. DOI:10.1016/j.apenergy.2016.11.084
|
| [96] |
GUNES B. A critical review on biofilm-based reactor systems for enhanced syngas fermentation processes. Renewable and Sustainable Energy Reviews, 2021, 143: 110950. DOI:10.1016/j.rser.2021.110950
|
| [97] |
BAE JY, SONG Y, LEE H, SHIN J, JIN S, KANG S, CHO BK. Valorization of C1 gases to value-added chemicals using acetogenic biocatalysts. Chemical Engineering Journal, 2022, 428: 131325. DOI:10.1016/j.cej.2021.131325
|
| [98] |
ABUBACKAR HN, VEIGA MC, KENNES C. Production of acids and alcohols from syngas in a two-stage continuous fermentation process. Bioresource Technology, 2018, 253: 227-234. DOI:10.1016/j.biortech.2018.01.026
|
| [99] |
DOLL K, RÜCKEL A, KÄMPF P, WENDE M, WEUSTER-BOTZ D. Two stirred-tank bioreactors in series enable continuous production of alcohols from carbon monoxide with Clostridium carboxidivorans. Bioprocess and Biosystems Engineering, 2018, 41(10): 1403-1416. DOI:10.1007/s00449-018-1969-1
|
| [100] |
KUNDIYANA DK, WILKINS MR, MADDIPATI P, HUHNKE RL. Effect of temperature, pH and buffer presence on ethanol production from synthesis gas by "Clostridium ragsdalei". Bioresource Technology, 2011, 102(10): 5794-5799. DOI:10.1016/j.biortech.2011.02.032
|
| [101] |
ACHARYA B, DUTTA A, BASU P. Ethanol production by syngas fermentation in a continuous stirred tank bioreactor using Clostridium ljungdahlii. Biofuels, 2019, 10(2): 221-237. DOI:10.1080/17597269.2017.1316143
|
| [102] |
王悦琳, 晁伟, 蓝晓程, 莫志朋, 佟淑环, 王铁峰. 合成气生物发酵法制乙醇的研究进展. 化工学报, 2022, 73(8): 3448-3460. WANG YL, CHAO W, LAN XC, MO ZP, TONG SH, WANG TF. Review of ethanol production via biological syngas fermentation. CIESC Journal, 2022, 73(8): 3448-3460 (in Chinese). |
| [103] |
JACK J, LO J, MANESS PC, REN ZJ. Directing Clostridium ljungdahlii fermentation products via hydrogen to carbon monoxide ratio in syngas. Biomass and Bioenergy, 2019, 124: 95-101. DOI:10.1016/j.biombioe.2019.03.011
|
| [104] |
YOUNESI H, NAJAFPOUR G, MOHAMED AR. Ethanol and acetate production from synthesis gas via fermentation processes using anaerobic bacterium, Clostridium ljungdahlii. Biochemical Engineering Journal, 2005, 27(2): 110-119. DOI:10.1016/j.bej.2005.08.015
|
| [105] |
MAYER A, SCHÄDLER T, TRUNZ S, STELZER T, WEUSTER-BOTZ D. Carbon monoxide conversion with Clostridium aceticum. Biotechnology and Bioengineering, 2018, 115(11): 2740-2750. DOI:10.1002/bit.26808
|
| [106] |
SKIDMORE BE. Syngas fermentation: quantification of assay techniques, reaction kinetics, and pressure dependencies of the clostridial P11 hydrogenase[D]. Provo, UT, USA: Brigham Young University, 2010.
|
| [107] |
FACKLER N, HEIJSTRA BD, RASOR BJ, BROWN H, MARTIN J, NI ZF, SHEBEK KM, ROSIN RR, SIMPSON SD, TYO KE, GIANNONE RJ, HETTICH RL, TSCHAPLINSKI TJ, LEANG C, BROWN SD, JEWETT MC, KÖPKE M. Stepping on the gas to a circular economy: accelerating development of carbon-negative chemical production from gas fermentation. Annual Review of Chemical and Biomolecular Engineering, 2021, 12: 439-470. DOI:10.1146/annurev-chembioeng-120120-021122
|
| [108] |
TROST BM. The atom economy—a search for synthetic efficiency. Science, 1991, 254(5037): 1471-1477. DOI:10.1126/science.1962206
|
| [109] |
LI CJ, TROST BM. Green chemistry for chemical synthesis. Proceedings of the National Academy of Sciences of the United States of America, 2008, 105(36): 13197-13202. DOI:10.1073/pnas.0804348105
|
| [110] |
THARAK A, MOHAN SV. Syngas fermentation to acetate and ethanol with adaptative electroactive carboxydotrophs in single chambered microbial electrochemical system. Micromachines, 2022, 13(7): 980. DOI:10.3390/mi13070980
|
| [111] |
NEVIN KP, HENSLEY SA, FRANKS AE, SUMMERS ZM, OU JH, WOODARD TL, SNOEYENBOS-WEST OL, LOVLEY DR. Electrosynthesis of organic compounds from carbon dioxide is catalyzed by a diversity of acetogenic microorganisms. Applied and Environmental Microbiology, 2011, 77(9): 2882-2886. DOI:10.1128/AEM.02642-10
|
| [112] |
LIU C, COLÓN BC, ZIESACK M, SILVER PA, NOCERA DG. Water splitting-biosynthetic system with CO2 reduction efficiencies exceeding photosynthesis. Science, 2016, 352(6290): 1210-1213. DOI:10.1126/science.aaf5039
|
| [113] |
ZHU HF, LIU ZY, ZHOU X, YI JH, LUN ZM, WANG SN, TANG WZ, LI FL. Energy conservation and carbon flux distribution during fermentation of CO or H2/CO2 by Clostridium ljungdahlii. Frontiers in Microbiology, 2020, 11: 416. DOI:10.3389/fmicb.2020.00416
|
| [114] |
ZHANG NQ, ZHANG XX, KANG YK, YE CL, JIN R, YAN H, LIN R, YANG JR, XU Q, WANG Y, ZHANG QH, GU L, LIU LC, SONG WY, LIU J, WANG DS, LI YD. A supported Pd2 dual-atom site catalyst for efficient electrochemical CO2 reduction. Angewandte Chemie International Edition, 2021, 60(24): 13388-13393. DOI:10.1002/anie.202101559
|
| [115] |
ZHANG XX, JIAO MY, CHEN ZP, MA X, WANG ZH, WANG NL, ZHANG XP, LIU LC. An integrated gradually thinning and dual-ion co-substitution strategy modulated in-O-ultrathin-SnS2 nanosheets to achieve efficient electrochemical reduction of CO2. Chemical Engineering Journal, 2022, 429: 132145. DOI:10.1016/j.cej.2021.132145
|
| [116] |
CHENG YY, HOU PF, WANG XP, KANG P. CO2 electrolysis system under industrially relevant conditions. Accounts of Chemical Research, 2022, 55(3): 231-240. DOI:10.1021/acs.accounts.1c00614
|
 2023, Vol. 39
2023, Vol. 39