中国科学院微生物研究所、中国微生物学会主办
文章信息
- 马湘宁, 张璐佳, 高健玮, 陈芳
- MA Xiangning, ZHANG Lujia, GAO Jianwei, CHEN Fang
- 粪菌移植治疗神经系统疾病的研究进展
- Advances in the application of fecal microbiota transplantation for the treatment of nervous system diseases
- 生物工程学报, 2024, 40(5): 1293-1308
- Chinese Journal of Biotechnology, 2024, 40(5): 1293-1308
- 10.13345/j.cjb.230448
-
文章历史
- Received: June 20, 2023
- Accepted: December 26, 2023
- Published: December 29, 2023
人体的肠道微生物组成复杂,微生物数量超100万亿,在正常生理条件下,肠道菌群相互影响,对维持宿主肠道平衡和健康起着重要的作用[1]。肠道菌群和中枢神经系统之间存在双向交流,即菌群可以通过“肠-脑轴”作用于大脑进而影响神经系统的活动,神经系统受到刺激也会在菌群中有所反馈[2]。研究表明,抑郁症、阿尔茨海默病(Alzheimer’s disease, AD)、帕金森病(Parkinson’s disease, PD)和孤独症谱系障碍(autism spectrum disorder, ASD)等疾病的发生通常伴随着肠道菌群的失调。因此,除临床药物治疗外,研究人员一直致力于通过对肠道菌群的调节来改善神经系统疾病的症状。
粪菌移植(fecal microbiota transplantation, FMT)是临床上一种便捷、高效、副作用少的治疗方法,通过将健康人群粪便中的菌群移植给患者,使患者异常的肠道微生物群恢复,目前在肠易激综合征(irritable bowel syndrome, IBS)的治疗中得到广泛应用[3]。基于神经系统和肠道菌群间的相互作用,近年来科学家开始尝试将FMT作为其新的治疗手段。本文结合“肠道微生物-肠-脑轴”的功能,主要探讨了FMT在神经性疾病治疗中的应用和作用机制,并对未来神经性疾病治疗的研究思路做出了展望。
1 肠道微生物概述肠道微生物由人体肠道内的全部生物群落组成,成年人肠道内微生物约1 000多种,超过99%都是细菌,其余的为真菌和病毒。细菌包括拟杆菌门(Bacteroidetes)、双歧杆菌属(Bifidobacterium)等有益菌,以及金黄色葡萄球菌(Staphylococcus aureus)等有害菌,优势菌门有厚壁菌门(Firmicutes)、放线菌门(Actinobacteria)、变形菌门(Proteobacteria)和拟杆菌门。菌群的丰度与人类健康密切相关,如厚壁菌门和拟杆菌门的比值(F/B)可评估菌群稳态,预测疾病的发生,F/B值在肥胖等代谢疾病中显著增加[4-6]。
肠道菌群在长期的进化过程中,通过个体的适应和自然选择,菌群始终处于动态平衡状态,在人体内发挥生理功能,例如影响体重和消化能力、抵御感染和自体免疫疾病的患病风险[7]。近来研究发现肠道菌群能合成多种人体生长发育所需的维生素,如B族维生素(维生素B1、B2、B6和B12)、维生素K、烟酸和泛酸等。肠道菌群还能利用蛋白质残渣合成人体必需氨基酸,如苯丙氨酸、缬氨酸和苏氨酸等,参与糖类和蛋白质的代谢,同时还能促进铁、镁、锌等矿物元素的吸收,这些营养物质对人类的健康有着重要作用,一旦缺少会引起多种疾病[8-9]。另外肠道菌群还可以通过调节神经营养因子和相关蛋白等影响大脑行为和功能,在神经系统的生长发育过程中起到重要作用。以上证据表明,肠道菌群具有提供营养、影响发育、调节免疫功能和参与宿主代谢等重要作用。
2 肠道微生物对神经系统疾病的影响肠道菌群与脑通过“肠-脑轴”进行双向直接或间接的联系[10] (图 1)。一些肠道微生物可直接作用于肠神经系统并激活其支配的迷走神经,产生的局部信号可通过感觉神经回路传递到参与认知、情绪、恐惧和焦虑等的大脑区域[11]。研究发现,乳酸菌如鼠李糖乳杆菌以区域依赖的方式持续调节γ-氨基丁酸受体mRNA的表达,同时可以降低应激诱导的皮质酮并减轻焦虑、抑郁相关行为。而切除小鼠的迷走神经阻止了鼠李糖乳杆菌的抗抑郁作用,表明迷走神经是暴露于肠道和大脑的细菌之间的主要调节组成通讯途径[12]。
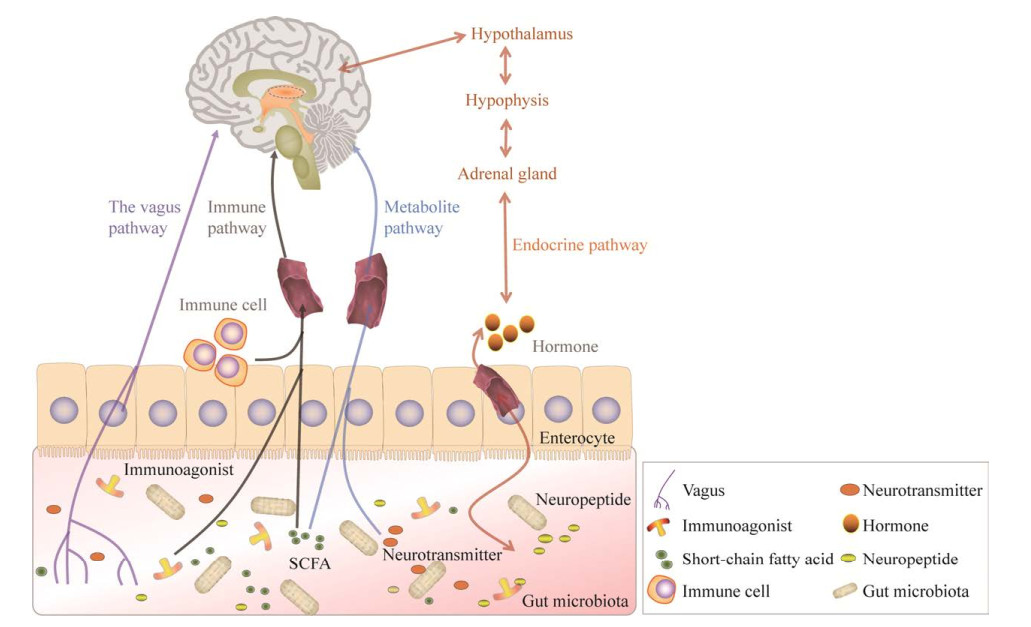
|
| 图 1 肠道菌群与中枢神经系统互作的方式 Fig. 1 The way intestinal flora interacts with the central nervous system. |
| |
肠道微生物也可以通过调节免疫功能来影响中枢神经系统[13-14]。菌群失调引起脂多糖(lipopolysaccharide, LPS)和肽聚糖等免疫激动剂增加,并穿过血脑屏障与小胶质细胞Toll样受体4 (Toll-like receptor 4, TLR4)结合,激活小胶质细胞,引起促炎细胞因子如白细胞介素-6 (interleukin-6, IL-6)、γ-干扰素(interferon γ, IFN-γ)等的释放[15],从而参与神经疾病的发生。肠道微生物的代谢产物短链脂肪酸(short chain fatty acid, SCFA)也可以调节肠道黏膜免疫应答,影响中性粒细胞、T细胞等免疫细胞的分化和激活,通过影响外周免疫系统调节大脑功能[16-17]。
同时研究还发现,肠道微生物能够通过内分泌系统与大脑相互调节[18]。下丘脑-垂体-肾上腺(hypothalamic-pituitary-adrenal, HPA)轴能够通过调控神经内分泌系统来影响机体肠道菌群的组成,而改变肠道菌群则会刺激肠内分泌细胞释放多种神经肽等,影响神经系统功能[19]。例如慢性应激诱导的抑郁症模型大鼠会通过HPA轴导致5-羟色胺和去甲肾上腺素水平下降,并引起肠道菌群的紊乱[20];同时,将抑郁症患者的肠道菌群移植到无菌小鼠体内,发现小鼠血清中促肾上腺皮质激素释放激素、肾上腺皮质激素水平显著升高[21],这表明肠道菌群可能通过引起HPA轴的异常而影响分泌系统,进而诱导大鼠的抑郁样行为造成神经功能的损伤。
研究发现,一些神经疾病患者肠道菌群的组成和多样性受到严重干扰,肠道微生物测定结果表明,厚壁菌门、拟杆菌门、放线菌门、变形菌门和疣微菌门(Verrucomicrobia)等都发生显著改变,其中脱硫弧菌属(Desulfovibrio)在抑郁症、AD和ASD中均变化显著,阿克曼菌属(Akkermansia)为AD、PD和ASD中主要变化的属[22],同时这些患者的消化功能严重减弱,并伴随着胃肠道功能紊乱的症状。有趣的是,比起普通小鼠,无菌小鼠的皮层和海马中的脑源性神经营养因子蛋白水平显著降低,且表现出记忆缺陷和神经损伤症状[23]。这意味着肠道菌群也能够影响大脑,参与神经系统疾病的病理和发生,说明稳定的肠道系统对正常中枢功能的维持至关重要,同时表明神经疾病与肠道菌群间存在相互作用关系(表 1)。
| Nervous system disease | Ascend | Descend | References |
| Depression | |||
| Phylum | Firmicutes, Actinobacteria | Bacteroidetes | [24-25] |
| Family | Thermoanaerobacteriaceae | Prevotellaceae | |
| Genus | Desulfovibrio, Oscillospira, Ruminococcus, Eggerthella, Holdemania, Turicibacte, Anaerofilum, Clostridium, Streptococcus | Prevotella, Dialister | |
| AD | |||
| Phylum | Proteobacteria, Verrucomicrobia | Bacteroidetes, Firmicutes | [26-27] |
| Family | Enterobacteriaceae, Veillonellaceae | Clostridiaceae, Lachnospiraceae | |
| Genus | Desulfovibrio, Akkermansia | Alloprevotella, Blautia, Ruminococcus | |
| PD | |||
| Phylum | Verrucomicrobia, Proteobacteria | Firmicutes | [22, 28] |
| Family | Ruminococcaceae, Enterobacteriacea, Odoribacter | Prevotellaceae, Lachnospiraceae, Peptostreptococcaceae | |
| Genus | Proteus, Bilophila, Roseburia, Akkermansia, Veillonella, Enterococcus | Butyricicoccus, Prevotella |
|
| ASD | |||
| Phylum | Bacteroidetes, Proteobacteria | Verrucomicrobia, Actinobacteria, Desulfobacterota, Firmicutes | [29-30] |
| Family | Lachnospiraceae | – | |
| Genus | Turicibacter, Ruminococcus, Streptococcus, Bifidobacterium, Sutterella, Desulfovibrio, Lactobacillus | Faecalibacterium, Akkermansia, Dialister, Clostridium | |
| –: Not mentioned. | |||
基于肠道菌群通过“肠-脑轴”在神经系统疾病中的重要作用,对肠道菌群干预成为治疗神经疾病的潜在手段,例如施用抗生素、益生菌或粪便微生物群移植等。抗生素治疗一定程度上可以缓解神经病症,但动物实验中发现长期使用广谱抗生素会引发细菌的耐药,损伤肾脏肝脏等器官[31];益生菌对脏器的损害较小,但会破坏免疫缺陷患者本就脆弱的免疫功能[32]。FMT被证实是神经系统疾病的有效治疗手段,是将健康供体的粪便移植到患者胃肠道内,重建具有正常功能的肠道生态系统,实现肠道内外疾病的治疗[33]。FMT补充了抗生素治疗失去微生物多样性的同时,还影响菌群代谢物SCFA、吲哚衍生物、多糖等的生成,进一步改善肠屏障的功能,缓解神经损伤[34-35]。目前在神经疾病上的治疗主要有抑郁症、AD、PD和ASD等。
3.1 抑郁症抑郁症是一种常见且复发率高的神经精神疾病,表现为情绪低落、兴趣下降并且有轻微焦虑的症状[36]。抑郁症的发病机制复杂,其中“单胺缺乏假说”最为常见,但基于此治疗的相关药物并不能完全改善病症[37]。有研究表明,抑郁症患者肠道菌群组成发生了显著变化,尤其是粪杆菌属(Faecalibacterium)和粪球菌属(Coprococcus)的丰度降低[38],小杆菌属(Dialister)在未经治疗的抑郁症患者中也显著减少。研究发现,将抑郁症患者的粪便移植到无菌大鼠一周后表现出抑郁症的行为和生理特征,以及色氨酸代谢异常,由此得出肠道菌群异常可能是引起抑郁症的重要原因之一[24]。
一些动物研究证实了FMT治疗抑郁症的可靠性。NOD样受体热蛋白结构域相关蛋白3 (nod-like receptor thermal protein domain associated protein 3, NLRP3)是固有免疫的重要组成因子,压力或应激等因素激活了静息态细胞中的NLRP3炎性小体,从而进一步裂解出成熟的白细胞介素-1β (interleukin-1β, IL-1β),通过外周免疫系统参与介导了抑郁症的发生发展[39],将NLRP3-KO小鼠的粪便转移到抑郁症小鼠肠道中会显著改善受体小鼠抑郁样行为[40]。Marcondes等[41]发现,普通小鼠接受抑郁样动物的粪便后,小鼠表现出探索和运动活动减少、空间记忆缺陷、体重下降和神经活性物质的变化,如IL-6、肿瘤坏死因子-α (tumour necrosis factor-α, TNF-α)水平和蛋白质氧化水平升高;而后又接受健康供体FMT后,这些因子和蛋白的水平都降低,FMT可以增加小鼠对蔗糖溶液的消耗能力,有效恢复小鼠的抑郁样行为[42]。
临床上,Cai等[43]首次将FMT应用于抑郁症患者治疗,发现对患者病情有显著的缓解作用,表现为便秘症状和精神状况改善;Yang等[44]也在对患者进行FMT治疗中发现了类似的现象,患者的抑郁、焦虑和便秘症状都得到了改善,肠道乳酸菌增多。这说明FMT在抑郁症治疗中起到了重要作用,这为抑郁症的有效治疗提供了新的方向(表 2)。
| Design | Follow-up after FMT | Number of transplants | Administration route | Microbiota effects of FMT | Neurological effects of FMT | Gut effects of FMT | References |
| Animal model (NLRP3-KO mice) | 7 days | 3 | Oral gavage | Bacteroidete relative abundance decreased and Desulfovibrio, Oscillospira, and Ruminococcus increased after FMT | Astrocyte dysfunction decreased; the number of positive cells increased; the number, length, and volume of astrocyte branches increased significantly | Not mentioned | [40] |
| Animal model (depression) | 1 day | 5 | Oral gavage | Not mentioned | The levels of IL-6 and TNF-α in the prefrontal cortex and hippocampus were decreased, and the levels of oxidative damage of proteins were decreased | Not mentioned | [41] |
| Human case series | 6 months | 4 | Duodenum | The number of Firmicutes increased significantly, Bacteroidetes decreased significantly, and the number of Lachnospiraceae increased after FMT | Appetite improved, mental condition improved | Constipation symptoms improved and health questionnaire scores returned to normal | [43] |
AD是一种常见的神经退行性疾病,患病症状表现为记忆丧失、行为障碍[45]。AD的发病机制与遗传和环境因素均有关,目前“β-淀粉样蛋白积聚假说”最为经典[46],研究发现,AD患者的大脑中β-淀粉样蛋白发生聚积,且白细胞介素(IL-5、IL-6、IL-8、IL-1β)和TNF-α等神经炎症表达显著上调[47],并伴随着肠道菌群的紊乱。由于肠道菌群与神经炎症的发生密切相关,通过调节肠道微生物降低这些促炎因子的表达,这可能是预防或降低AD风险的有效策略(表 3)。
| Design | Follow-up after FMT | Number of transplants | Administration route | Microbiota effects of FMT | Neurological effects of FMT | Gut effects of FMT | References |
| Animal model (transgenosis AD mice) | 4 weeks | 28 | Oral gavage | Desulfovibrio decreased and Bacteroidete increased after FMT | The mice stayed in the target quadrant much more frequently and much longer than the model | Not mentioned | [48] |
| Animal model (transgenosis AD mice) | 16 days | 16 | Oral gavage | Not mentioned | The total plaque area of frontal cortex and hippocampus decreased significantly, and the levels of soluble and insoluble Aβ40 in cerebral cortex decreased significantly. Glial cell formation decreased | Intestinal permeability and intestinal barrier integrity were enhanced | [49] |
| Human case series | 6 months | 1 | Not mentioned | Not mentioned | Cognitive function score increased from 20 points (cognitive impairment) to 26 points (normal function); after 6 months, mood improved and the score rose to 29 | Not mentioned | [50] |
| Animal model (APPPS1-21 mice) | 24 days | 24 | Oral gavage | There was no difference in alpha-diversity between donor and FMT, and the difference was significant in the control group without FMT | The cortical plaques and microglia body area increased, the total number of microglia cells did not change, the dendritic branch length of microglia shortened, and the dendritic branch points decreased | The cecum weight increased after FMT | [51] |
研究发现,将健康小鼠的粪便移植给AD小鼠,可以改善其肠道菌群及代谢物组成,上调粪便中SCFA的含量[48]。与此类似,Kim等[49]研究发现,在小鼠中FMT治疗可以改善AD小鼠β-淀粉样蛋白斑块沉积、tau蛋白病理学特征和小胶质细胞激活等,并且逆转了AD小鼠的认知障碍表型。
临床上FMT治疗AD的报道迄今为止仅有一例,该病例显示一名82岁患者接受FMT后,记忆、认知、情绪等AD症状均得到了显著改善[50]。尽管目前FMT在AD的临床应用较少,但动物实验中的结果明确提出了FMT能够恢复肠道稳态的证据,表明FMT在AD治疗上具有很好的前景。
3.3 PDPD是一种多因素的神经退行性疾病,患者表现为步态障碍、运动受限及四肢震颤等,其主要特征是多巴胺能神经元的丧失,黑质和纹状体中的“α-突触核蛋白”表达积聚[52-53]。研究发现PD患者肠道菌群失调,从而引起肠上皮细胞损伤,激活TNF-α/NF-κB等促炎信号通路,通过肠-脑轴影响α-突触核蛋白的表达[54],因此,通过FMT调节肠道菌群可能对PD的治疗具有重要作用(表 4)。
| Design | Follow-up after FMT | Number of transplants | Administration route | Microbiota effects of FMT | Neurological effects of FMT | Gut effects of FMT | References |
| Animal model (MPTP induce PD mice) | 8 days | 7 | Oral gavage | Firmicutes increased, Proteobacteria decreased; in the order level, Clostridiales decreased, Turicibacterales and Enterobacterales increased | Activated astrocytes and microglia decreased; reduce the expression of TLR4/TBK1/ NF-κB/TNF-α signaling pathway | Not mentioned | [55] |
| Animal model (rotenone induce PD mice) | 6 weeks | 14 | Oral gavage | Increased alpha-diversity; there was no difference between FMT and control group | Restoring neuron loss, decreasing α-synuclein aggregation and decreasing astrocyte proliferation; inhibition of TLR4/MyD88/ NF-κB signaling pathway | Increased frequency of bowel movements; the blood-brain barrier connection and tissue structure were restored, the damage of endothelial cells was reduced | [56] |
| Human case series 1 | 12 weeks | 27 | Capsule | FMT alleviate the clinical symptoms of patients with PD by strengthening the correlation between the microbial genera | Not mentioned | Those participants had a significantly better quality of life regarding abdominal pain, flatulence, nausea, etc. | [57] |
| Human case series 2 | 12 months | 1 | Fibercolonoscopy, injected into the end of the ileum | Ruminococcus, Blautia, Prevotella and Faecalibacterium increased, Bacteroidete decresed | Constipation, mood and sleep quality were improved, and tremor and bradykinesia were significantly improved after 4 weeks | Not mentioned | [58] |
FMT处理对PD小鼠的病症具有明显的改善作用,一方面,FMT减少PD小鼠肠道微生物失调,缓解小鼠的体重减轻、运动及胃肠道功能障碍,恢复肠道菌群多样性,并增加厚壁菌门丰度等;另一方面,FMT增加纹状体中神经递质含量,抑制黑质中小胶质细胞和星形胶质细胞的激活,降低炎症因子和LPS水平,并通过阻断TLR4/TNF-α/NF-κB/MyD88信号通路激活及下游促炎蛋白的产生来改善PD小鼠的症状[55-56]。
FMT治疗PD在临床上也得到了一定的应用,科学家们对27名轻度至中度PD患者进行FMT治疗,随访期间未观察到严重不良反应,且患者的胃肠道疾病得到了改善,肠道微生态系统的复杂性显著增加[57]。另外,南京医科大学附属医院对一名PD患者进行FMT治疗后发现,在4周时患者情绪、睡眠质量均有所好转,但在12周时稍有恶化的趋势[58]。尽管FMT能够在一定程度上改善PD患者的症状,但结果也存在争议,还需更多的临床试验来支撑。
3.4 ASDASD是一种在儿童中常见的神经发育障碍,特征是社会交流和互动的改变以及重复的刻板行为,孤独症是其中的一种典型疾病[59-60]。免疫功能障碍是ASD发病的主要机制,在ASD模型中观察到与免疫相关的蛋白如接触蛋白相关样蛋白4 (contactin associated protein-like 4, CNTNAP4) 和脆性X智力低下蛋白(familial mental retardation protein, FMRP)的显著下调[61-62],研究中常将CNTNAP4和FMRP基因敲除小鼠作为ASD模型。
肠道菌群可以通过调节免疫应答来影响神经系统的功能,近年来ASD的治疗多数集中在菌群改善方面(表 5)。Zhang等[62]发现CNTNAP4基因敲除小鼠表现出孤独症样行为,肠道菌群结构发生变化,乳杆菌属的丰度显著降低,而进行了正常小鼠的粪便移植后可缓解其恐惧和孤独症样行为。FMRP基因敲除小鼠中也会观察到同样的表型,而喂养鱼油(fish oil, FO)提供多不饱和脂肪酸可以缓解小鼠出现的肠道炎症、菌群紊乱和孤独症行为。同时,在FMRP基因敲除小鼠中,将FO喂养组的粪便移植入无FO喂养组可以显著恢复小鼠肠道稳态,降低结肠中TNF-α和ZO-3的表达,从而改善小鼠的孤独症行为[61]。
| Design | Follow-up after FMT | Number of transplants | Administration route | Microbiota effects of FMT | Neurological effects of FMT | Gut effects of FMT | References |
| Animal model (FMRP-KO mice) | Not mentioned | 7 | Oral gavage | Akkermansia and Gordonibacter increased | FMT significantly reduced the expression of TNF-α mRNA in the colon of recipient mice | Intestinal homeostasis improved after FMT, significantly increasing the expression level of ZO-3 in the colon of recipient mice | [61] |
| Animal model (CNTNAP4-KO mice) |
15 days | 7 | Oral gavage | Lactobacillus increased colonisation in the colon | The time residence time in the open regional center area increased; the rate of social interaction in the interaction zone increased | Not mentioned | [62] |
| Human case series 1 | 8 weeks | 10 | Oral or rectal (upper digestive mixed drink, lower digestive enema) | Bifidobacterium increasing 4 times significantly, Prevotella and Desulfovibrio also increased | There was no difference between oral and rectal ingestion, and behavioral symptoms improved significantly | The gastrointestinal symptom score scale decreased, and the number of days with abnormal or no stool decreased significantly | [63] |
| Human case series 2 | 10 weeks | 18 | Not mentioned | Iignificant increases in levels of Bifidobacteria, Prevotella, and Desulfovibrio | Not mentioned | Levels of sulfuric acid against cresol were similarly reduced in healthy children | [64] |
FMT临床试验发现,ASD儿童的胃肠道症状得到了改善,腹痛、腹泻和便秘等症状显著减少,社交技能等行为症状也显著缓解,并且测序分析表明,细菌多样性得以提升[63],同时还发现FMT推动了血浆中各种代谢特征的变化,包括烟酸/烟酰胺和嘌呤代谢[64]。综上所述,实验证明了FMT治疗ASD的可行性,为其提供临床治疗的新方法,后续需要进一步地探究ASD的发病机制,为寻找FMT的靶点以便于更高效更快速的解决病症。
4 FMT治疗神经性疾病的潜在机制在神经系统疾病中,肠道微生态紊乱诱导了肠道炎症与氧化应激反应,减少了短链脂肪酸的产生,引起疾病标志性蛋白(如β-淀粉样蛋白、tau蛋白[46]、α-突触核蛋白[54])变化,并通过肠-脑轴的传递和转移,最终造成神经系统损伤。从该角度考虑,推测FMT治疗神经疾病可能参与减轻或抑制肠道炎症与氧化应激,进而保护神经系统[65] (图 2)。
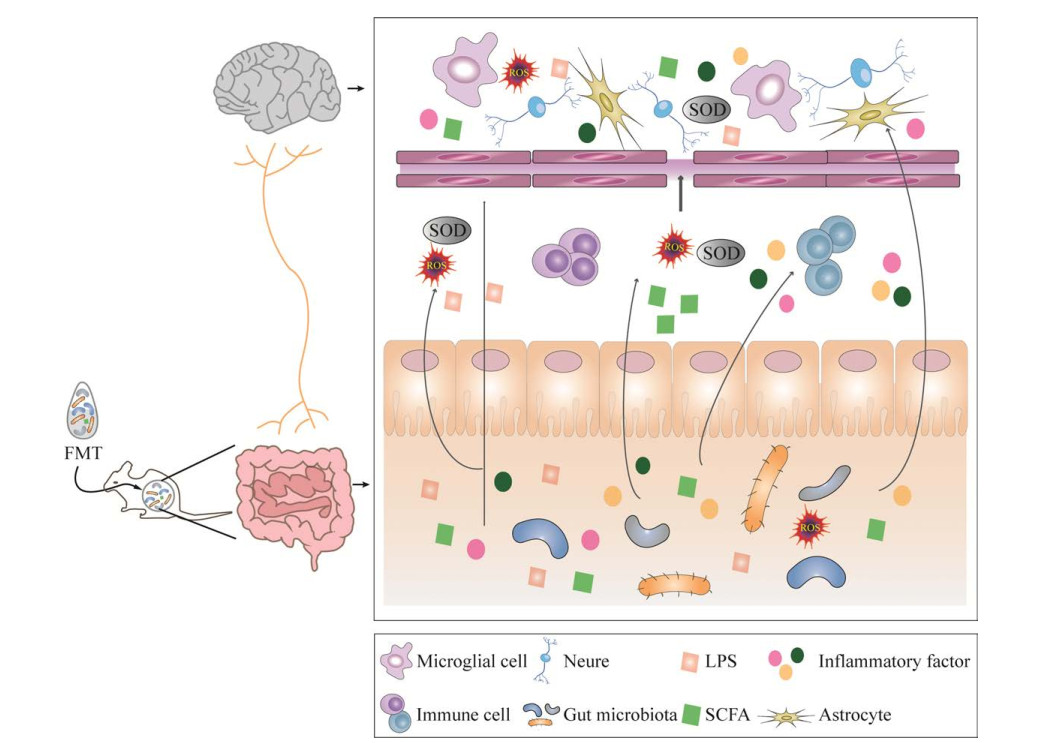
|
| 图 2 FMT的潜在作用机制 Fig. 2 Potential mechanism of the action of FMT. |
| |
肠道中条件致病菌导致肠道通透性增加,提高了循环中细菌及细菌成分(如LPS)水平,导致脑组织中炎症因子IL-1、IL-6、TNF-α及单核细胞趋化蛋白1的表达上升,引起宿主炎症及氧化应激。FMT通过增加短链脂肪酸(乙酸、丙酸和丁酸)的含量[66],调节炎症因子的释放,以游离脂肪酸受体(free fatty acid receptor 2, Ffar-2)依赖的方式保护肠道免受炎症的影响,从而减轻肠道炎症;同时SCFA还可以抑制小胶质细胞的激活和功能,保护血脑屏障,减少神经炎症[67]。也有研究报道,FMT通过重塑肠道菌群,下调促炎因子,抑制神经系统疾病相关蛋白及信号通路,例如阻止NLRP3炎性小体聚集,下调NF-κB信号通路,抑制α-突触核蛋白、β-淀粉样蛋白在大脑中的积聚,从而缓解炎症的发生[68]。FMT可能通过影响菌群代谢物如LPS和肽聚糖等免疫激动剂,使其水平降低,并抑制它们穿过血脑屏障,可能通过调控免疫相关蛋白和信号通路,例如增加CNTNAP4和减少TLR4的表达,以及通过增加肠道菌群代谢物如色氨酸的代谢功能和途径,从而改善机体的免疫应答,对神经起到保护作用[69]。FMT可以缓解机体内氧化应激的发生,降低活性氧(reactive oxygen species, ROS)和过氧化产物丙二醛等水平,增加还原性谷胱甘肽(glutathione, GSH)含量并提高抗氧化相关酶如过氧化物酶、超氧化物歧化酶(superoxide dismutase, SOD)等的活性[70],并改善了Nrf2、MAPK等神经系统中与氧化应激相关的信号通路,从而减轻神经系统疾病的症状。
尽管FMT在神经疾病治疗上主要通过上述途径发挥神经保护的作用,但现阶段的研究较难在多种肠道菌群-肠-脑轴交流途径中区分出每种途径的直接影响程度,因此,未来将FMT与微生物组、代谢物、宏基因组等多组学结合进行研究,可能有利于阐释FMT对治疗神经疾病的机制。
5 总结与展望本文对肠道微生物-肠-脑轴、FMT的作用及其在不同的神经系统疾病中的应用进行了综述,FMT通过重建肠道微生物组影响肠道微生态平衡,从而干预神经系统疾病患病体的紊乱肠道,使健康的肠道菌群在患病体中重新建立稳定的生态环境。许多动物实验和临床试验都证明FMT可以在一定程度上缓解抑郁症、AD、PD和ASD患者的症状,改善其肠道菌群的构成,因此可以考虑作为一种治疗神经疾病的备选方式。另外还有一些神经性疾病,例如多发性硬化、肌萎缩性脊髓侧索硬化等与本文提到的神经系统疾病具有相似性,科学家们也在对其的发病机制及治疗途径进行探索。
按照现在研究的发展趋势,FMT可能会作为治疗神经系统疾病的新方法,但是由于FMT对肠道无菌操作要求很高,利用抗生素处理较为困难,供体的选择以及移植菌体的处理都需要经过很严格的设计。另外,有研究报道两例炎症性肠病患者在进行FMT后出现严重腹泻,粪便检查结果为产气荚膜梭菌感染[71],所以在进行FMT前需要对供体进行严格筛选,以减少FMT带来的负面作用。除此之外,不同的疾病对应的特异性菌株不同,FMT这种治疗方法如果能够更加准确定位单菌株可能会提升其效果,还需进一步开展更多的工作,确保更有效地治疗对应病症。目前大多研究都集中于肠道菌群和各类神经疾病间的联系,以及饮食干预对疾病的影响,但是对具体的机制研究甚少,因此,未来需要采用多组学方法,深入探究通过肠道菌群治疗神经疾病的作用机制,筛选出更有针对性的菌株,为疾病的治疗提供理论依据。
| [1] |
谢雅静, 时晓敏, 颜世敢, 朱丽萍. 肠道菌群与精神类疾病相关性研究进展[J]. 中国药理学通报, 2022, 38(11): 1617-1622. XIE YJ, SHI XM, YAN SG, ZHU LP. Progress on correlation between intestinal flora and mental diseases[J]. Chinese Pharmacological Bulletin, 2022, 38(11): 1617-1622 (in Chinese). |
| [2] |
KIM YK, SHIN C. The microbiota-gut-brain axis in neuropsychiatric disorders: pathophysiological mechanisms and novel treatments[J]. Current Neuropharmacology, 2018, 16(5): 559-573. DOI:10.2174/1570159X15666170915141036
|
| [3] |
WANG Y, ZHENG FL, LIU S, LUO HH. Research progress in fecal microbiota transplantation as treatment for irritable bowel syndrome[J]. Gastroenterology Research and Practice, 2019, 2019, 9759138.
|
| [4] |
HERTLI S, ZIMMERMANN P. Molecular interactions between the intestinal microbiota and the host[J]. Molecular Microbiology, 2022, 117(6): 1297-1307. DOI:10.1111/mmi.14905
|
| [5] |
FLEMER B, LYNCH DB, BROWN JMR, JEFFERY IB, RYAN FJ, CLAESSON MJ, O'RIORDAIN M, SHANAHAN F, O'TOOLE PW. Tumour-associated and non-tumour-associated microbiota in colorectal cancer[J]. Gut, 2017, 66(4): 633-643. DOI:10.1136/gutjnl-2015-309595
|
| [6] |
WEERSMA RK, ZHERNAKOVA A, FU JY. Interaction between drugs and the gut microbiome[J]. Gut, 2020, 69(8): 1510-1519. DOI:10.1136/gutjnl-2019-320204
|
| [7] |
CLEMENTE JC, URSELL LK, PARFREY LW, KNIGHT R. The impact of the gut microbiota on human health: an integrative view[J]. Cell, 2012, 148(6): 1258-1270. DOI:10.1016/j.cell.2012.01.035
|
| [8] |
CARABOTTI M, SCIROCCO A, MASELLI MA, SEVERI C. The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems[J]. Annals of Gastroenterology, 2015, 28(2): 203-209.
|
| [9] |
SENDER R, MILO R. The distribution of cellular turnover in the human body[J]. Nature Medicine, 2021, 27: 45-48. DOI:10.1038/s41591-020-01182-9
|
| [10] |
MA QQ, XING CS, LONG WY, WANG HY, LIU Q, WANG RF. Impact of microbiota on central nervous system and neurological diseases: the gut-brain axis[J]. Journal of Neuroinflammation, 2019, 16(1): 53. DOI:10.1186/s12974-019-1434-3
|
| [11] |
YU CD, XU QJ, CHANG RB. Vagal sensory neurons and gut-brain signaling[J]. Current Opinion in Neurobiology, 2020, 62: 133-140. DOI:10.1016/j.conb.2020.03.006
|
| [12] |
BRAVO JA, FORSYTHE P, CHEW MV, ESCARAVAGE E, SAVIGNAC HM, DINAN TG, BIENENSTOCK J, CRYAN JF. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve[J]. Proceedings of the National Academy of Sciences of the United States of America, 2011, 108(38): 16050-16055.
|
| [13] |
SITTIPO P, CHOI J, LEE S, LEE YK. The function of gut microbiota in immune-related neurological disorders: a review[J]. Journal of Neuroinflammation, 2022, 19(1): 154. DOI:10.1186/s12974-022-02510-1
|
| [14] |
AGIRMAN G, YU KB, HSIAO EY. Signaling inflammation across the gut-brain axis[J]. Science, 2021, 374(6571): 1087-1092. DOI:10.1126/science.abi6087
|
| [15] |
ALLENDORF DH, FRANSSEN EH, BROWN GC. Lipopolysaccharide activates microglia via neuraminidase 1 desialylation of Toll-like receptor 4[J]. Journal of Neurochemistry, 2020, 155(4): 403-416. DOI:10.1111/jnc.15024
|
| [16] |
CORRÊA-OLIVEIRA R, FACHI JL, VIEIRA A, SATO FT, VINOLO MAR. Regulation of immune cell function by short-chain fatty acids[J]. Clinical & Translational Immunology, 2016, 5(4): e73.
|
| [17] |
GOSWAMI C, IWASAKI Y, YADA T. Short-chain fatty acids suppress food intake by activating vagal afferent neurons[J]. The Journal of Nutritional Biochemistry, 2018, 57: 130-135. DOI:10.1016/j.jnutbio.2018.03.009
|
| [18] |
STRANDWITZ P. Neurotransmitter modulation by the gut microbiota[J]. Brain Research, 2018, 1693, 128-133.
|
| [19] |
ZIPP F, BITTNER S, SCHAFER DP. Cytokines as emerging regulators of central nervous system synapses[J]. Immunity, 2023, 56(5): 914-925. DOI:10.1016/j.immuni.2023.04.011
|
| [20] |
YANG HL, LI MM, ZHOU MF, XU HS, HUAN F, LIU N, GAO R, WANG J, ZHANG N, JIANG L. Links between gut dysbiosis and neurotransmitter disturbance in chronic restraint stress-induced depressive behaviours: the role of inflammation[J]. Inflammation, 2021, 44(6): 2448-2462. DOI:10.1007/s10753-021-01514-y
|
| [21] |
LIU SH, GUO RJ, LIU F, YUAN QJ, YU Y, REN FF. Gut microbiota regulates depression-like behavior in rats through the neuroendocrine-immune-mitochondrial pathway[J]. Neuropsychiatric Disease and Treatment, 2020, 16: 859-869. DOI:10.2147/NDT.S243551
|
| [22] |
LIN CH, CHEN CC, CHIANG HL, LIOU JM, CHANG CM, LU TP, CHUANG EY, TAI YC, CHENG C, LIN HY, WU MS. Altered gut microbiota and inflammatory cytokine responses in patients with Parkinson's disease[J]. Journal of Neuroinflammation, 2019, 16(1): 129. DOI:10.1186/s12974-019-1528-y
|
| [23] |
GAREAU MG, WINE E, RODRIGUES DM, CHO JH, WHARY MT, PHILPOTT DJ, MACQUEEN G, SHERMAN PM. Bacterial infection causes stress-induced memory dysfunction in mice[J]. Gut, 2011, 60(3): 307-317. DOI:10.1136/gut.2009.202515
|
| [24] |
KELLY JR, BORRE Y, O'BRIEN C, PATTERSON E, EL AIDY S, DEANE J, KENNEDY PJ, BEERS S, SCOTT K, MOLONEY G, HOBAN AE, SCOTT L, FITZGERALD P, ROSS P, STANTON C, CLARKE G, CRYAN JF, DINAN TG. Transferring the blues: depression-associated gut microbiota induces neurobehavioural changes in the rat[J]. Journal of Psychiatric Research, 2016, 82: 109-118. DOI:10.1016/j.jpsychires.2016.07.019
|
| [25] |
RONG H, XIE XH, ZHAO J, LAI WT, WANG MB, XU D, LIU YH, GUO YY, XU SX, DENG WF, YANG QF, XIAO L, ZHANG YL, HE FS, WANG S, LIU TB. Similarly in depression, nuances of gut microbiota: evidences from a shotgun metagenomics sequencing study on major depressive disorder versus bipolar disorder with current major depressive episode patients[J]. Journal of Psychiatric Research, 2019, 113: 90-99. DOI:10.1016/j.jpsychires.2019.03.017
|
| [26] |
LIU P, WU L, PENG GP, HAN YQ, TANG RQ, GE JP, ZHANG LJ, JIA LF, YUE SQ, ZHOU K, LI LJ, LUO BY, WANG BH. Altered microbiomes distinguish Alzheimer's disease from amnestic mild cognitive impairment and health in a Chinese cohort[J]. Brain, Behavior, and Immunity, 2019, 80: 633-643. DOI:10.1016/j.bbi.2019.05.008
|
| [27] |
PARK SH, LEE JH, SHIN J, KIM JS, CHA B, LEE S, KWON KS, SHIN YW, CHOI SH. Cognitive function improvement after fecal microbiota transplantation in Alzheimer's dementia patient: a case report[J]. Current Medical Research and Opinion, 2021, 37(10): 1739-1744. DOI:10.1080/03007995.2021.1957807
|
| [28] |
SCHEPERJANS F, AHO V, PEREIRA PAB, KOSKINEN K, PAULIN L, PEKKONEN E, HAAPANIEMI E, KAAKKOLA S, EEROLA- RAUTIO J, POHJA M, KINNUNEN E, MURROS K, AUVINEN P. Gut microbiota are related to Parkinson's disease and clinical phenotype[J]. Movement Disorders: Official Journal of the Movement Disorder Society, 2015, 30(3): 350-358. DOI:10.1002/mds.26069
|
| [29] |
de ANGELIS M, FRANCAVILLA R, PICCOLO M, de GIACOMO A, GOBBETTI M. Autism spectrum disorders and intestinal microbiota[J]. Gut Microbes, 2015, 6(3): 207-213. DOI:10.1080/19490976.2015.1035855
|
| [30] |
FINEGOLD SM, DOWNES J, SUMMANEN PH. Microbiology of regressive autism[J]. Anaerobe, 2012, 18(2): 260-262. DOI:10.1016/j.anaerobe.2011.12.018
|
| [31] |
CAPUTI V, MARSILIO I, FILPA V, CERANTOLA S, ORSO G, BISTOLETTI M, PACCAGNELLA N, de MARTIN S, MONTOPOLI M, DALLACQUA S, CREMA F, Di GANGI IM, GALUPPINI F, LANTE I, BOGIALLI S, RUGGE M, DEBETTO P, GIARONI C, GIRON MC. Antibiotic-induced dysbiosis of the microbiota impairs gut neuromuscular function in juvenile mice[J]. British Journal of Pharmacology, 2017, 174(20): 3623-3639. DOI:10.1111/bph.13965
|
| [32] |
YAMANBAEVA G, SCHAUB AC, SCHNEIDER E, SCHWEINFURTH N, KETTELHACK C, DOLL JPK, MÄHLMANN L, BRAND S, BEGLINGER C, BORGWARDT S, LANG UE, SCHMIDT A. Effects of a probiotic add-on treatment on fronto-limbic brain structure, function, and perfusion in depression: secondary neuroimaging findings of a randomized controlled trial[J]. Journal of Affective Disorders, 2023, 324: 529-538. DOI:10.1016/j.jad.2022.12.142
|
| [33] |
GENG SJ, CHENG SS, LI Y, WEN ZS, MA X, JIANG XM, WANG YZ, HAN XY. Faecal microbiota transplantation reduces susceptibility to epithelial injury and modulates tryptophan metabolism of the microbial community in a piglet model[J]. Journal of Crohn's and Colitis, 2018, 12(11): 1359-1374.
|
| [34] |
FAREED S, SARODE N, STEWART FJ, MALIK A, LAGHAIE E, KHIZER S, YAN FX, PRATTE Z, LEWIS J, IMMERGLUCK LC. Applying fecal microbiota transplantation (FMT) to treat recurrent Clostridium difficile infections (rCDI) in children[J]. PeerJ, 2018, 6: e4663. DOI:10.7717/peerj.4663
|
| [35] |
PARAMSOTHY S, NIELSEN S, KAMM MA, DESHPANDE NP, FAITH JJ, CLEMENTE JC, PARAMSOTHY R, WALSH AJ, van den BOGAERDE J, SAMUEL D, LEONG RWL, CONNOR S, NG W, LIN E, BORODY TJ, WILKINS MR, COLOMBEL JF, MITCHELL HM, KAAKOUSH NO. Specific bacteria and metabolites associated with response to fecal microbiota transplantation in patients with ulcerative colitis[J]. Gastroenterology, 2019, 156(5): 1440-1454.e2. DOI:10.1053/j.gastro.2018.12.001
|
| [36] |
HERRMAN H, KIELING C, McGORRY P, HORTON R, SARGENT J, PATEL V. Reducing the global burden of depression: a Lancet-World Psychiatric Association Commission[J]. Lancet, 2019, 393(10189): e42-e43. DOI:10.1016/S0140-6736(18)32408-5
|
| [37] |
尹一淑, 刘军莲, 王佳平, 朱元兵, 李勇枝, 卢卫红. 抑郁症相关发病机制研究进展[J]. 医学综述, 2022, 28(12): 2368-2372. YIN YS, LIU JL, WANG JP, ZHU YB, LI YZ, LU WH. Research progress in pathogenesis of depression[J]. Medical Recapitulate, 2022, 28(12): 2368-2372 (in Chinese). |
| [38] |
CRUZ-PEREIRA JS, REA K, NOLAN YM, O'LEARY OF, DINAN TG, CRYAN JF. Depression's unholy trinity: dysregulated stress, immunity, and the microbiome[J]. Annual Review of Psychology, 2020, 71: 49-78. DOI:10.1146/annurev-psych-122216-011613
|
| [39] |
张楠, 许二平, 陈玉龙. NLRP3炎症小体与抑郁症的关系及中医药的干预作用[J]. 中国实验方剂学杂志, 2023, 29(3): 186-193. ZHANG N, XU EP, CHEN YL. Relationship between NLRP3 inflammasome and depression and intervention effect of traditional Chinese medicine: a review[J]. Chinese Journal of Experimental Traditional Medical Formulae, 2023, 29(3): 186-193 (in Chinese). |
| [40] |
ZHANG Y, HUANG RR, CHENG MJ, WANG LR, CHAO J, LI JX, ZHENG P, XIE P, ZHANG ZJ, YAO HH. Gut microbiota from NLRP3-deficient mice ameliorates depressive-like behaviors by regulating astrocyte dysfunction via circHIPK2[J]. Microbiome, 2019, 7(1): 116. DOI:10.1186/s40168-019-0733-3
|
| [41] |
MARCONDES ÁVILA PR, FIOROT M, MICHELS M, DOMINGUINI D, ABATTI M, VIEIRA A, de MOURA AB, BEHENCK JP, BORBA LA, BOTELHO MEM, RÉUS GZ, DAL-PIZZOL F, RITTER C. Effects of microbiota transplantation and the role of the vagus nerve in gut-brain axis in animals subjected to chronic mild stress[J]. Journal of Affective Disorders, 2020, 277: 410-416. DOI:10.1016/j.jad.2020.08.013
|
| [42] |
RAO JJ, XIE RN, LIN L, JIANG J, DU L, ZENG XD, LI GY, WANG CM, QIAO Y. Fecal microbiota transplantation ameliorates gut microbiota imbalance and intestinal barrier damage in rats with stress-induced depressive-like behavior[J]. The European Journal of Neuroscience, 2021, 53(11): 3598-3611. DOI:10.1111/ejn.15192
|
| [43] |
CAI T, SHI X, YUAN LZ, TANG D, WANG F. Fecal microbiota transplantation in an elderly patient with mental depression[J]. International Psychogeriatrics, 2019, 31(10): 1525-1526. DOI:10.1017/S1041610219000115
|
| [44] |
YANG CL, HU TJ, XUE X, SU XH, ZHANG X, FAN YH, SHEN XB, DONG XS. Multi-omics analysis of fecal microbiota transplantation's impact on functional constipation and comorbid depression and anxiety[J]. BMC Microbiology, 2023, 23(1): 389. DOI:10.1186/s12866-023-03123-1
|
| [45] |
SCHELTENS P, de STROOPER B, KIVIPELTO M, HOLSTEGE H, CHÉTELAT G, TEUNISSEN CE, CUMMINGS J, van der FLIER WM. Alzheimer's disease[J]. Lancet, 2021, 397(10284): 1577-1590. DOI:10.1016/S0140-6736(20)32205-4
|
| [46] |
WANG XY, SUN GQ, FENG T, ZHANG J, HUANG X, WANG T, XIE ZQ, CHU XK, YANG J, WANG H, CHANG SS, GONG YX, RUAN LF, ZHANG GQ, YAN SY, LIAN W, DU C, YANG DB, ZHANG QL, LIN FF, et al. Sodium oligomannate therapeutically remodels gut microbiota and suppresses gut bacterial amino acids-shaped neuroinflammation to inhibit Alzheimer's disease progression[J]. Cell Research, 2019, 29: 787-803. DOI:10.1038/s41422-019-0216-x
|
| [47] |
PISTOLLATO F, SUMALLA CANO S, ELIO I, MASIAS VERGARA M, GIAMPIERI F, BATTINO M. Role of gut microbiota and nutrients in amyloid formation and pathogenesis of Alzheimer disease[J]. Nutrition Reviews, 2016, 74(10): 624-634. DOI:10.1093/nutrit/nuw023
|
| [48] |
SUN J, XU JX, LING Y, WANG FY, GONG TY, YANG CW, YE SQ, YE KY, WEI DH, SONG ZQ, CHEN DN, LIU JM. Fecal microbiota transplantation alleviated Alzheimer's disease-like pathogenesis in APP/PS1 transgenic mice[J]. Translational Psychiatry, 2019, 9: 189. DOI:10.1038/s41398-019-0525-3
|
| [49] |
KIM MS, KIM Y, CHOI H, KIM W, PARK S, LEE D, KIM DK, KIM HJ, CHOI H, HYUN DW, LEE JY, CHOI EY, LEE DS, BAE JW, MOOK-JUNG I. Transfer of a healthy microbiota reduces amyloid and tau pathology in an Alzheimer's disease animal model[J]. Gut, 2020, 69(2): 283-294. DOI:10.1136/gutjnl-2018-317431
|
| [50] |
ZHANG T, GAO GQ, KWOK LY, SUN ZH. Gut microbiome-targeted therapies for Alzheimer's disease[J]. Gut Microbes, 2023, 15(2): 2271613. DOI:10.1080/19490976.2023.2271613
|
| [51] |
DODIYA HB, KUNTZ T, SHAIK SM, BAUFELD C, LEIBOWITZ J, ZHANG XL, GOTTEL N, ZHANG XQ, BUTOVSKY O, GILBERT JA, SISODIA SS. Sex-specific effects of microbiome perturbations on cerebral Aβ amyloidosis and microglia phenotypes[J]. The Journal of Experimental Medicine, 2019, 216(7): 1542-1560. DOI:10.1084/jem.20182386
|
| [52] |
LIM SY, TAN AH, AHMAD-ANNUAR A, KLEIN C, TAN LCS, ROSALES RL, BHIDAYASIRI R, WU YR, SHANG HF, EVANS AH, PAL PK, HATTORI N, TAN CT, JEON B, TAN EK, LANG AE. Parkinson's disease in the western Pacific region[J]. The Lancet Neurology, 2019, 18(9): 865-879. DOI:10.1016/S1474-4422(19)30195-4
|
| [53] |
WALLEN ZD, APPAH M, DEAN MN, SESLER CL, FACTOR SA, MOLHO E, ZABETIAN CP, STANDAERT DG, PAYAMI H. Characterizing dysbiosis of gut microbiome in PD: evidence for overabundance of opportunistic pathogens[J]. NPJ Parkinson's Disease, 2020, 6: 11. DOI:10.1038/s41531-020-0112-6
|
| [54] |
SAMPSON TR, DEBELIUS JW, THRON T, JANSSEN S, SHASTRI GG, ILHAN ZE, CHALLIS C, SCHRETTER CE, ROCHA S, GRADINARU V, CHESSELET MF, KESHAVARZIAN A, SHANNON KM, KRAJMALNIK-BROWN R, WITTUNG- STAFSHEDE P, KNIGHT R, MAZMANIAN SK. Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson's disease[J]. Cell, 2016, 167(6): 1469-1480.e12. DOI:10.1016/j.cell.2016.11.018
|
| [55] |
ZHAO Z, NING JW, BAO XQ, SHANG MY, MA JW, LI G, ZHANG D. Fecal microbiota transplantation protects rotenone-induced Parkinson's disease mice via suppressing inflammation mediated by the lipopolysaccharide-TLR4 signaling pathway through the microbiota-gut-brain axis[J]. Microbiome, 2021, 9(1): 226. DOI:10.1186/s40168-021-01107-9
|
| [56] |
SUN MF, ZHU YL, ZHOU ZL, JIA XB, XU YD, YANG Q, CUI C, SHEN YQ. Neuroprotective effects of fecal microbiota transplantation on MPTP-induced Parkinson's disease mice: gut microbiota, glial reaction and TLR4/TNF-α signaling pathway[J]. Brain, Behavior, and Immunity, 2018, 70: 48-60. DOI:10.1016/j.bbi.2018.02.005
|
| [57] |
CHENG Y, TAN GH, ZHU QH, WANG C, RUAN GC, YING SH, QIE JL, HU XF, XIAO ZF, XU FH, CHEN L, CHEN MJ, PEI Y, ZHANG H, TIAN YT, CHEN DF, LIU XY, HUANG HQ, WEI YL. Efficacy of fecal microbiota transplantation in patients with Parkinson's disease: clinical trial results from a randomized, placebo-controlled design[J]. Gut Microbes, 2023, 15(2): 2284247. DOI:10.1080/19490976.2023.2284247
|
| [58] |
薛刘军, 欧洲, 王丽君, 魏明, 杨秀, 郑金龙, 佟强. 粪菌移植替代多巴胺能药物治疗帕金森病案例分析[J]. 临床神经病学杂志, 2019, 32(5): 329-332. XUE LJ, OU Z, WANG LJ, WEI Ming, YANG Xiu, ZHENG Jinlong, TONG Qiang. A case analyse of Parkinson's disease treated with fecal microbiota transplantation instead of dopaminergic drugs[J]. Journal of Clinical Neurology, 2019, 32(5): 329-332 (in Chinese). |
| [59] |
MEIMAND SE, ROSTAM-ABADI Y, REZAEI N. Autism spectrum disorders and natural killer cells: a review on pathogenesis and treatment[J]. Expert Review of Clinical Immunology, 2021, 17(1): 27-35. DOI:10.1080/1744666X.2020.1850273
|
| [60] |
GRIMALDI R, GIBSON GR, VULEVIC J, GIALLOUROU N, CASTRO-MEJÍA JL, HANSEN LH, GIBSON EL, NIELSEN DS, COSTABILE A. A prebiotic intervention study in children with autism spectrum disorders (ASDs)[J]. Microbiome, 2018, 6(1): 133. DOI:10.1186/s40168-018-0523-3
|
| [61] |
GUO PF, YANG XY, GUO XM, YANG HE, PAN J, LI Y. Dietary fish oil improves autistic behaviors and gut homeostasis by altering the gut microbial composition in a mouse model of fragile X syndrome[J]. Brain, Behavior, and Immunity, 2023, 110: 140-151. DOI:10.1016/j.bbi.2023.02.019
|
| [62] |
ZHANG WL, HUANG J, GAO F, YOU QL, DING LY, GONG JW, ZHANG MR, MA RF, ZHENG SH, SUN XD, ZHANG YL. Lactobacillus reuteri normalizes altered fear memory in male Cntnap4 knockout mice[J]. eBioMedicine, 2022, 86: 104323. DOI:10.1016/j.ebiom.2022.104323
|
| [63] |
KANG DW, ADAMS JB, GREGORY AC, BORODY T, CHITTICK L, FASANO A, KHORUTS A, GEIS E, MALDONADO J, MCDONOUGH-MEANS S, POLLARD EL, ROUX S, SADOWSKY MJ, LIPSON KS, SULLIVAN MB, CAPORASO JG, KRAJMALNIK-BROWN R. Microbiota transfer therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: an open-label study[J]. Microbiome, 2017, 5(1): 10. DOI:10.1186/s40168-016-0225-7
|
| [64] |
KANG DW, ADAMS JB, VARGASON T, SANTIAGO M, HAHN J, KRAJMALNIK-BROWN R, MARCO ML. Distinct fecal and plasma metabolites in children with autism spectrum disorders and their modulation after microbiota transfer therapy[J]. mSphere, 2020, 5(5): e00314-20.
|
| [65] |
KHORUTS A, SADOWSKY MJ. Understanding the mechanisms of faecal microbiota transplantation[J]. Nature Reviews Gastroenterology & Hepatology, 2016, 13: 508-516.
|
| [66] |
VARELA RB, VALVASSORI SS, LOPES-BORGES J, MARIOT E, DAL-PONT GC, AMBONI RT, BIANCHINI G, QUEVEDO J. Sodium butyrate and mood stabilizers block ouabain-induced hyperlocomotion and increase BDNF, NGF and GDNF levels in brain of Wistar rats[J]. Journal of Psychiatric Research, 2015, 61: 114-121. DOI:10.1016/j.jpsychires.2014.11.003
|
| [67] |
WANG Q, LUO YQ, RAY CHAUDHURI K, REYNOLDS R, TAN EK, PETTERSSON S. The role of gut dysbiosis in Parkinson's disease: mechanistic insights and therapeutic options[J]. Brain, 2021, 144(9): 2571-2593. DOI:10.1093/brain/awab156
|
| [68] |
JING YL, YU Y, BAI F, WANG LM, YANG DG, ZHANG C, QIN C, YANG ML, ZHANG D, ZHU YB, LI JJ, CHEN ZG. Effect of fecal microbiota transplantation on neurological restoration in a spinal cord injury mouse model: involvement of brain-gut axis[J]. Microbiome, 2021, 9(1): 59. DOI:10.1186/s40168-021-01007-y
|
| [69] |
PLATTEN M, NOLLEN EAA, RÖHRIG UF, FALLARINO F, OPITZ CA. Tryptophan metabolism as a common therapeutic target in cancer, neurodegeneration and beyond[J]. Nature Reviews Drug Discovery, 2019, 18: 379-401. DOI:10.1038/s41573-019-0016-5
|
| [70] |
LI J, WANG HD, QING W, LIU FT, ZENG NY, WU F, SHI YY, GAO XX, CHENG M, LI HL, SHEN W, MENG FG, HE Y, CHEN MX, LI Z, ZHOU HW, WANG Q. Congenitally underdeveloped intestine drives autism-related gut microbiota and behavior[J]. Brain, Behavior, and Immunity, 2022, 105: 15-26. DOI:10.1016/j.bbi.2022.06.006
|
| [71] |
AZIMIRAD M, YADEGAR A, ASADZADEH AGHDAEI H, KELLY CR. Enterotoxigenic Clostridium perfringens infection as an adverse event after faecal microbiota transplantation in two patients with ulcerative colitis and recurrent Clostridium difficile infection: a neglected agent in donor screening[J]. Journal of Crohn's and Colitis, 2019, 13(7): 960-961. DOI:10.1093/ecco-jcc/jjz006
|
 2024, Vol. 40
2024, Vol. 40