构建微生物细胞工厂利用木糖合成高值化学品
王彤
,
陆亮宇
,
申晓林
,
孙新晓
,
王佳
,
袁其朋
生物工程学报  2024, Vol. 40 2024, Vol. 40 Issue (8): 2695-2709 Issue (8): 2695-2709 |

中国作为一个农业大国,随着对粮食作物需求的不断增加,产生的农业废弃物的量也逐渐增加。据统计,中国农作物秸秆年产量近9亿t,约占全球秸秆产量的25%[1],如果将农业废弃物焚烧或随意丢弃,不仅会浪费资源,而且会对环境造成二次危害[2]。随着对碳排放的担忧日益加剧,人们越来越重视利用微生物细胞工厂将生物质转化为有价值的产品,如化学品、材料、生物燃料等,从而减轻对化石资源的依赖,减少二氧化碳的排放[3-4]。
木质纤维素是农业废弃物的主要成分,也是地球上最丰富的生物质资源。它主要存在于各种植物中,特别是木材和某些农作物的秸秆,如玉米秸秆或甘蔗渣等都含有丰富的木质纤维素。全球这些生物质资源每年的产量约为1 800亿t[5]。木质纤维素主要由纤维素(干物质重的30%−50%)、半纤维素(干物质重的20%−40%)及木质素(干物质重的15%−25%)三部分构成[6],这些资源不仅丰富,而且可再生[7-8]。因此利用木质纤维素为原料构建微生物细胞工厂合成目标产品不仅有助于替代传统化石资源,而且对实现“双碳”目标具有重要意义。就原料而言,木糖是木聚糖和半纤维素的主要成分,占木质纤维素水解产物总量的30%−40%[9]。以木糖为底物生物合成高值化学品具有较高的经济价值和广阔的应用前景。然而,相较于葡萄糖的广泛利用,微生物代谢木糖过程中存在转运效率低、碳分解代谢抑制的缺点,导致目标产品产量低、木糖衍生产品种类少等问题,极大地限制了木质纤维素的生物转化效率[10]。
近年来,随着合成生物学工具和代谢工程策略的进一步发展,微生物代谢木糖的效率得到了较大的提升,构建微生物细胞工厂利用木糖合成高附加值化合物取得了很大的进展。本文总结了目前发现的木糖微生物代谢途径及其衍生的代表性产品,综述了木糖与葡萄糖共利用工程菌株的构建策略,讨论了微生物直接利用木质纤维素水解物合成目标产物的最新进展。最后,本文分析了微生物代谢木糖目前面临的挑战,展望了未来的发展方向,为拓展以木糖为原料的衍生产物的种类、实现木质纤维素组分的综合利用提供借鉴和参考。
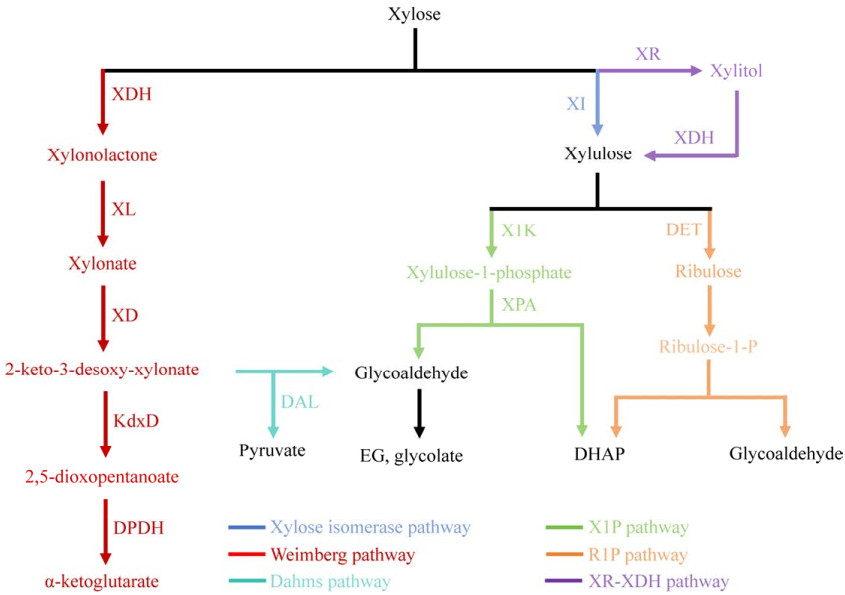
1 微生物中的木糖代谢途径迄今为止,已有多个微生物木糖代谢途径被发现和报道,包括木糖还原酶-木糖醇脱氢酶(xylose reductase and xylitol dehydrogenase, XR-XDH)途径和木糖异构酶(xylose isomerase, XI)途径,1-磷酸核酮糖途径(ribulose 1-phosphate, R1P)途径和1-磷酸木酮糖(xylulose-1-phosphatem, X1P)途径,Weimberg (WMB)途径和Dahms途径(图 1)。近年来,利用这些途径代谢木糖合成高附加值化学品取得了很大的进展。
|
| 图 1 微生物木糖代谢途径 Fig. 1 Overview of different xylose assimilation pathways. XI: Xylose isomerase; XR: Xylose reductase; XDH (orange): Xylitol dehydrogenase; X1K: Xylulose-1-kinase; DHAP: Dihydroxyacetone phosphate; XPA: Xylulose-1-phosphate; DTE: D-tagatose epimerase; R1K: Ribulose-1-kinase; RPA: Ribulose-1-phosphate aldolase; XDH (red): Xylose dehydrogenase; XL: Xylonolactonase; XD: Xylonate dehydratase; KdxD: 2-keto-3-deoxy-D-xylonate dehydratase; DPDH: 2, 5-dioxopentanoate dehydrogenase; DAL: 2-keto-3-deoxyxylonate aldolase. |
| |
XR-XDH途径和XI途径是最传统也是应用最广泛的木糖代谢途径。其中,XR-XDH途径主要存在于一些丝状真菌和酵母中。木糖先被XR还原为木糖醇。然后,木糖醇被XDH氧化为木酮糖[11],木酮糖激酶(xylulokinase, XK)将木酮糖磷酸化生成木糖-5-磷酸(xylose-5-phosphate, X5P),进入磷酸戊糖途径(pentose phosphate pathway, PPP)途径参与细胞生长与产物生产[12]。Promdonkoy等[13]过表达XR-XDH、XK、木糖特异性转运蛋白(HXT7F79S)和异丁醇合成途径相关酶的基因,敲除PHO13基因(编码对硝基苯基磷酸酶,能将X5P代谢为木酮糖)和GRE3基因(编码非特异性NADPH依赖性醛糖还原酶),构建了以木糖为碳源、可合成48.4 mg/L异丁醇的酿酒酵母(Saccharomyces cerevisiae)工程菌株。虽然应用XR-XDH途径能够利用木糖生产高值化学品,但依赖NADPH的XR和依赖NAD+的XDH所造成的辅因子失衡严重影响XR-XDH途径代谢效率。特别是在厌氧条件下,XR和XDH对不同辅助因子的偏好可导致NAD+缺乏或NADH过剩,从而导致木糖醇积累[14]。为了解决XR-XDH途径的辅因子失衡问题,Suzuki等[15]应用蛋白质工程策略改造XDH,使其可利用NADP+为辅因子以实现辅因子的供需平衡;改造后的马克斯克鲁维酵母(Kluyveromyces marxianus) DMB13能够快速将木糖转化为乙醇,乙醇最高得率可达0.402 g/g。类似地,Songdech等[16]构建了异丁醇高产菌株,以木糖为唯一碳源合成了14.8 g/L异丁醇。
XI途径主要存在于大肠杆菌(Escherichia coli)等细菌中,是由XI将木糖代谢为木酮糖后,经XK催化木酮糖磷酸化生成X5P,再经由PPP参与细胞代谢的过程。Qiu等[17-18]在生产D-乳酸的底盘菌株中引入XI途径,随后分别敲除ldh (编码L-乳酸脱氢酶)和ldhD (编码D-乳酸脱氢酶),在高密度发酵条件下生产了97.3 g/L的D-乳酸和130.8 g/L的L-乳酸。XI途径没有辅因子需求,将其引入到酿酒酵母中有望解决XR-XDH途径的辅因子失衡问题。然而,酿酒酵母中XI途径因翻译时蛋白质折叠错误等因素导致表达量很低[19]。应用位于细胞质或周质的伴侣有助于蛋白质正确盘曲折叠。Temer等[20]在酿酒酵母中过表达编码丙酸杆菌(Propionibacterium) XI的基因与编码大肠杆菌细胞质伴侣GroEL-GroES复合物的基因,重组菌株中XI能够有效折叠,并且能够快速将木糖生物转化为乙醇,产量为0.44 g/g。此外,基因修饰、密码子优化等手段也能够提高XI酶活性。Seike等[21]通过易错PCR进行随机突变获得XIT63I/V162A突变体,木糖代谢速率是野生菌株的1.7倍。
1.2 R1P途径和X1P途径R1P途径和X1P途径均是以木酮糖为底物生成磷酸二羟丙酮(dihydroxyacetone phosphate, DHAP)和乙醇醛的木糖代谢途径。两种途径均将C5直接裂解为C2和C3小分子,没有碳损失,是生产C2化合物的重要途径。
R1P途径利用D-塔格糖差向异构酶(d-tagatose 3-epimerase, DTE)将木酮糖差向异构化生成核酮糖,随后核酮糖-1-激酶(ribulose-1-kinase, R1K)将核酮糖磷酸化生成R1P,1-磷酸核酮糖醛缩酶(ribulose-1-phosphate aldolase, RPA)将R1P裂解为DHAP和乙醇醛[22]。R1P途径已经被应用到乙二醇(ethylene glycol, EG)和乙醇酸(glycolic acid, GA)的高效合成。Pereira等[23]在大肠杆菌中敲除xylB基因(编码木酮糖激酶)阻断了天然木糖代谢途径,引入R1P途径代谢木糖,通过过表达aldA基因(编码醛脱氢酶A)将乙醇醛转化为GA,通过敲除glcD基因(编码乙醇酸氧化酶亚基GlcD)阻止GA的分解代谢,合成了44 g/L GA,碳收率为0.44 g/g;为了提高该途径中间体DHAP的利用效率,Peraira等[23]在乙醇酸生产菌株中引入乙醛酸旁路途径,DHAP通过中心碳代谢进入三羧酸(tricarboxylic acid, TCA)循环后,由乙醛酸旁路途径生成乙醛酸,并在乙醛酸还原酶作用下生成GA,该重组菌株利用木糖合成了40 g/L GA,碳收率达0.52 g/g。
与R1P途径不同,X1P途径先将木酮糖在木酮糖-1-激酶(xylulose-1-kinase, X1K)的作用下磷酸化生成X1P,接着1-磷酸木酮糖醛缩酶(xylulose-1-phosphate aldolase, XPA)将其裂解为DHAP和乙醇醛[22]。X1P途径也是生产乙醇酸、乙二醇等二碳化合物的另一重要途径。Cam等[24]通过在大肠杆菌中引入X1P途径构建了GA工程菌株,利用木糖可合成4.3 g/L GA。X1P途径还可被引入到酿酒酵母中合成C2化合物。Uranukul等[25]在酿酒酵母中引入X1P途径构建能够合成EG的生产菌株,利用木糖合成了4.05 g/L EG。
1.3 WMB途径和Dahms途径与上述木糖代谢反应途径不同的是,来自新月柄杆菌(Caulobacter crescentus)的WMB途径是由5种酶催化的木糖氧化反应,首先木糖脱氢酶(xylose dehydrogenase, XDH)将木糖氧化成为D-木糖内酯,D-木糖内酯被木糖内酯酶(xylonolactonase, XL)水解为D-木糖酸,接着D-木糖酸在木糖酸脱水酶(xylonate dehydratase, XD)的作用下生成2-酮-3-脱氧木糖酸酯,再经由2-酮-3-脱氧-D-木糖酸脱水酶(2-keto-3-deoxy-D-xylonate dehydratase, KdxD)作用生成2, 5-二氧代戊酸;最后被2, 5-二氧代戊酸脱氢酶(2, 5-dioxopentanoate dehydrogenase, DPDH)氧化生成α-酮戊二酸;该途径合成α-酮戊二酸的过程中没有碳损失,因此是一条合成TCA循环衍生化合物更高效、更短的途径[26]。Brüsseler等[27]在谷氨酸棒状杆菌(Corynebacterium glutamicum)中表达WMB途径使其能够利用木糖生长,进一步构建α-酮戊二酸工程菌株生产了7.92 g/L α-酮戊二酸。应用相同策略,Zhao等[28]在大肠杆菌中表达WMB途径,并引入α-酮戊二酸生物下游产物γ-氨基丁酸的合成途径,γ-氨基丁酸产量达到3.95 g/L。
在假单胞菌(Pseudomonas)中发现的Dahms途径是通过2-酮-3-脱氧木糖醛缩酶(2-keto-3-deoxyxyloaldolase, DAL)将WMB途径中间体2-酮-3-脱氧-D-木糖酸裂解为丙酮酸和乙醇醛,后者可被醛脱氢酶转化为GA[29]。Dahms途径是将C5裂解成为C3和C2小分子的过程,也是C2化合物生物合成的重要途径之一。Cabulong等[30]将Dahms途径与乙醛酸旁路相结合,以木糖为底物,最终生产了4.57 g/L乙醛酸(0.46 g/g)。Wang等[31]在大肠杆菌中引入Dahms途径并过表达FucO基因(编码NADH依赖的醛还原酶)将乙醇醛转化为EG,分批补料发酵产量达到72 g/L。然而在应用WMB途径和Dahms途径生产高值化合物的同时,其毒性中间体木糖酸的大量积累会严重影响细胞的生长和产物的生产。为了减少木糖酸的积累,Cabulong等[32]将强启动子T7替换为较弱的tac启动子来减弱xdh (编码木糖脱氢酶)的表达,减缓木糖代谢,96 h后EG产量为7.72 g/L,达到理论产量的95%。
1.4 木糖代谢途径的协同效应木糖代谢途径之间,木糖代谢途径与其他生物代谢途径之间并不是独立存在而是相互关联的,如Dahms途径和X1P途径均能生成GA,木糖代谢途径的产物最终均会进入中心碳代谢。因此,同一种化合物有时可经多条代谢途径合成。当不同合成途径间存在代谢中间体、能量或还原力等的互补关系时,通常会出现代谢协同效应,即组合使用互补途径合成目标化合物的效率比单独使用任意一条途径高[33]。例如木糖经WMB途径合成α-酮戊二酸步骤短、效率高且没有碳损失,但该途径合成乙酰辅酶A的能力较差。木糖经XI途径合成α-酮戊二酸存在步骤长、碳收率低的缺陷,但合成乙酰辅酶A的效率高。Wang等[34]通过在大肠杆菌内表达XI途径来增加乙酰辅酶A的供应,并协同表达WMB途径更直接地增加前体α-酮戊二酸的供应,同时引入α-酮戊二酸下游产物戊二酸异源合成途径,合成了602 mg/L戊二酸,比单独使用XI途径(104 mg/L)和WMB途径(209 mg/L)合成戊二酸的产量都高。Xia等[35]利用木糖XR-XDH途径在酿酒酵母中合成乙醇。但过程中释放大量二氧化碳,导致碳收率不高;通过额外引入卡尔文循环、核酮糖-1, 5-二磷酸羧化酶/加氧酶和磷酸核糖激酶,将释放的二氧化碳重新固定到乙醇合成途径,将碳收率从0.324 g/g提高到0.336 g/g (表 1)。
| Host | Substrate | Target compound | Titer (g/L) | Pathway |
| Saccharomyces cerevisiae | Xylose | Isobutanol | 0.05 | XR-XDH pathway[13] |
| Kluyveromyces marxianus | Xylose | Ethanol | 0.40* | XR-XDH pathway[15] |
| Saccharomyces cerevisiae | Xylose | Isobutanol | 14.80 | XR-XDH pathway[16] |
| Escherichia coli | Xylose | d-lactate | 97.30 | XI pathway[17] |
| Escherichia coli | Xylose | d-lactate | 130.80 | XI pathway[18] |
| Saccharomyces cerevisiae | Xylose | Ethanol | 0.44* | XI pathway[20] |
| Escherichia | Xylose | GA | 44.00 | R1P pathway[23] |
| Escherichia | Xylose | GA | 4.30 | X1P pathway[24] |
| Saccharomyces cerevisiae | Xylose | EG | 4.05 | X1P pathway[25] |
| Corynebacterium glutamicum | Xylose | α-ketoglutarate | 7.92 | WMB pathway[27] |
| Escherichia coli | Xylose | γ-aminobutyric acid | 3.95 | WMB pathway[28] |
| Escherichia coli | Xylose | Glyoxylic acid | 4.57 | Dahms pathway[30] |
| Bacillus subtilis | Xylose | EG | 72.00 | Dahms pathway[31] |
| Escherichia coli | Xylose | EG | 7.72 | Dahms pathway[32] |
| Escherichia coli | Xylose | Glutarate | 0.60 | XI pathway and WMB pathway[34] |
| * represents g/g. | ||||
微生物异源表达木糖代谢途径的研究日益成熟,推动了构建微生物细胞工厂高效利用木糖生产高值化学品。在此基础上,大量研究聚焦于开发微生物共利用葡萄糖和木糖的代谢工程策略,以实现木质纤维素的充分利用。
2.1 改造糖转运系统实现葡萄糖-木糖共利用当供给大肠杆菌等微生物多种糖时,它们会优先消耗葡萄糖,葡萄糖耗尽后才会开始消耗乳糖、阿拉伯糖、木糖等其他碳源,即碳分解代谢物抑制效应(carbon catabolite repression, CCR)[36]。木糖的转运和代谢受到转录激活因子XylR (由xylR编码)和cAMP-CRP的调控。当葡萄糖在培养基中存在时,葡萄糖转运蛋白EIIBCGlc (由ptsG编码)消耗cAMP,从而减少cAMP-CRP复合物的形成,抑制了木糖的转运和代谢。因此破坏葡萄糖磷酸转移酶系统(phosphotransferase system, PTS),能够削弱微生物的CCR信号通路,减缓微生物对葡萄糖的摄取和利用效率,从而达到葡萄糖-木糖共利用的目的。例如Zhu等[37]敲除大肠杆菌ptsG基因,构建了能够共利用葡萄糖和木糖生产琥珀酸盐的菌株,产量达43 g/L;进一步优化葡萄糖与木糖的配比,琥珀酸滴度提高到107 g/L。应用相同策略,Li等[38]敲除大肠杆菌ptsG基因实现葡萄糖和木糖共利用,构建了4-羟基扁桃酸(4-hydroxymandelic acid, 4-HMA)生产菌株,4-HMA滴度为1.11 g/L,与单独使用葡萄糖或木糖作为唯一碳源的菌株相比,产量分别提高了48.6%和35.3%。破坏PTS系统是实现葡萄糖和木糖共利用的有效手段之一。然而,PTS系统的失活也会限制葡萄糖运输速率,导致细胞生长缓慢和生产力低下。这可以通过表达galP (编码半乳糖渗透酶)和Zmglf (编码葡萄糖转运促进蛋白)的葡萄糖转运系统来改善[39]。Saini等[40]敲除大肠杆菌ptsG和过表达Zmglf基因以葡萄糖和木糖为碳源生产2.6 g/L正丁醇,碳收率达到理论产率的32%。Wei等[41]在工程大肠杆菌中将内源的XylR,替换为不受CCR控制的XylRR121C/P363S,激活了xylA (编码木糖异构酶)、xylB和xylFGH (编码ATP依赖性木糖转运蛋白)的转录,利用葡萄糖和木糖生产了29.16 g/L 4-羟基异亮氨酸(4-hydroxyisoleucine, 4-HIL),产率为0.91 g/(L·h)。xylR的表达除了受葡萄糖抑制,还受阿拉伯糖抑制,araC编码的阿拉伯糖转录调节因子AraC能够抑制木糖转录因子xylR的表达。Kim等[42]通过敲除araC基因,解除CCR,成功构建了能利用木糖和葡萄糖生产4.8 g/L木糖醇的大肠杆菌突变菌株。
实现葡萄糖和木糖共利用还可通过增强木糖的转运及木糖代谢途径基因的表达水平,以提高木糖的摄取率和利用效率。如Wang等[43]通过组成型表达参与木糖转运和代谢的xylF (编码木糖转运蛋白XylF)和xylA基因实现共利用葡萄糖和木糖生产了约900 mg/L甲基酮。Li等[44]通过过表达araE (编码戊糖转运蛋白)和xylAB实现谷氨酸棒杆菌共利用葡萄糖-木糖,并构建1, 3-丙二醇(1, 3-propanediol, 1, 3-PDO)合成途径,敲除1, 3-PDO的竞争途径基因,合成了98.2 g/L 1, 3-PDO。Su等[45]在酿酒酵母中敲除PHO13基因和表达能转运木糖且不能转运葡萄糖的转运蛋白Gal2N376F以实现葡萄糖和木糖共利用,在5 L发酵罐中生产了903 mg/L类胡萝卜素。
除了改造PTS系统和过表达木糖摄取代谢等相关基因等策略,适应性实验室进化技术(adaptive laboratory evolution, ALE)也在葡萄糖-木糖的共利用方面显示出巨大的潜力。Ling等[46]在表达XI途径的恶臭假单胞菌(Pseudomonas putida) KT2440中应用ALE,筛选出携带XylEA62V/A455V的突变体QP478,该菌株能够利用葡萄糖、木糖快速生长并生产粘康酸(mucoconic acid, MA),优化的工程菌株能够生产33.7 g/L MA。Li等[47]对酿酒酵母进行定向进化,从突变菌株筛选出葡萄糖/木糖共转运蛋白,该共转运蛋白对木糖表现出高亲和力。当培养基中葡萄糖和木糖浓度相同时,木糖运输速率更是超过了葡萄糖。
2.2 开发共培养体系实现葡萄糖-木糖共利用开发专用葡萄糖菌株和专用木糖菌株的共培养体系不仅能将不同碳源的代谢途径区室化缓解CCR,而且能将复杂的生物合成通路模块化,将通路的子模块划分到各个菌株中,减少菌株的生长代谢负担,以提高葡萄糖、木糖共利用效率和目标产物的产量。Zhang等[48]分别构建了利用木糖合成3-脱氢莽草酸(3-dehydroshikimic acid, DHS)的菌株和利用葡萄糖转化DHS合成MA的菌株,将两株菌进行共培养生产了850 mg/L MA。应用同一策略,Seo等[49]构建了以葡萄糖为底物合成异丁醇的底盘菌株和以木糖为底物生产丁酰辅酶A的菌株,在此基础上构建了共培养体系合成丁酸异丁酯,产量达392 mg/L (图 2)。应用共培养技术还能够同时生产两种化合物。Flores等[50]构建了利用葡萄糖生产乙醇和利用木糖生产琥珀酸的共培养体系。消耗葡萄糖的菌株中丙酮酸被丙酮酸脱羧酶(pyruvate decarboxylase, PDC)代谢为乙醛和1分子CO2,前者被乙醇脱氢酶(alcohol dehydrogenase, Adh)转化为乙醇;而消耗木糖的菌株则是利用乙醇生产途径产生的CO2与磷酸烯醇式丙酮酸PEP在磷酸烯醇丙酮酸羧激酶(phosphoenolpyruvate carboxykinase, Pck)的作用下生成草酰乙酸,进一步生产琥珀酸,同时实现CO2固定化,初始接种比例为1:1的条件下生产了2.7 g/L乙醇和2.7 g/L琥珀酸[50]。
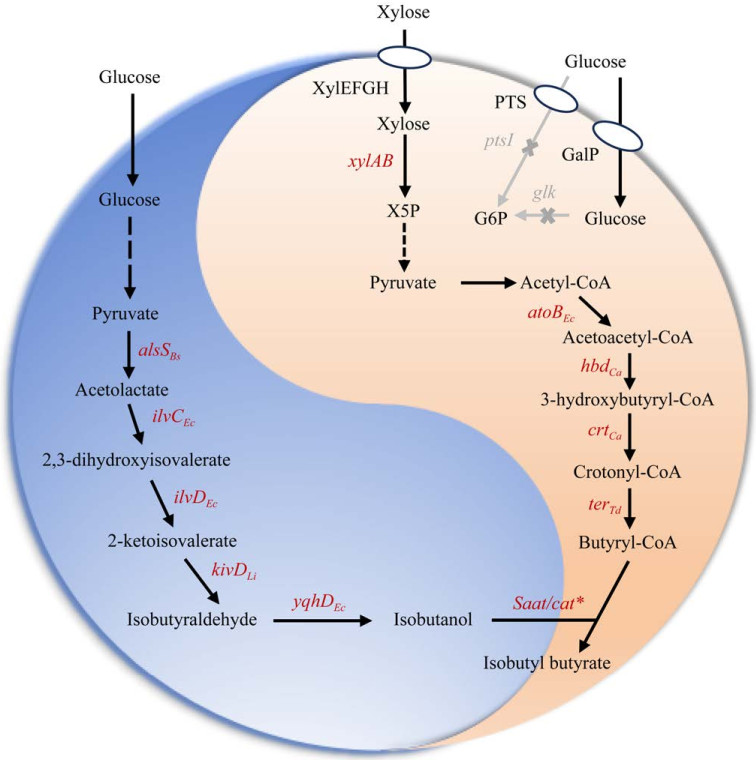
|
| 图 2 构建大肠杆菌共培养体系生产丁酸异丁酯 Fig. 2 Constructing an Escherichia coli co-culture system to produce isobutyl butyrate. Gray arrow indicates knockout genes; Red arrow indicates overexpressed genes; Bs: Bacillus subtilis; Ec: Escherichia coli; Li: Lactococcus lactis; Ca: Clostridium acetobutylicum; Td: Treponema denticola; alsS encoding acetolactate synthase; ilvC encoding ketol-acid reductoisomerase; ilvD encoding dihydroxy-acid dehydratase; kivD encoding α-ketoisovalerate decarboxylase; yqhD encoding alcohol dehydrogenase; Saat/cat* encoding alcohol acyltransferase; atoB encoding acetyl-CoA acetyltransferase; hbd encoding hydroxybutyryl-CoA dehydrogenase; crt encoding 3-hydroxybutyryl-CoA dehydratase; ter encoding crotonyl-CoA dehydratase. |
| |
平行代谢途径工程(parallel metabolic pathway engineering, PMPE)也是实现葡萄糖和木糖共利用的有效方法之一。该方法的原理是设计改造代谢网络,将一种碳源专用于目标产物的生产,而另一种碳源专用于细胞生长,以达到将细胞生长和产物合成过程解耦的目的。在该方法中,Fujiwara等[51]利用半乳糖渗透酶/葡萄糖激酶系统(GalP/Glk系统)取代内源性PTS系统以缓解CCR效应;通过敲除pykA和pykF基因(编码丙酮酸激酶),将PEP流向TCA循环的路径阻断,从而实现将葡萄糖只用于合成莽草酸途径衍生产物MA;通过引入Dahms途径将木糖导入TCA循环,用于细胞生长,应用该策略可以生产4.09 g/L MA (图 3)。同样地,Wu等[52]利用葡萄糖生产海藻糖的合成途径短且不需要经过分解代谢的优势,阻断大肠杆菌葡萄糖的分解代谢途径使其专用于海藻糖的生产,并引入木糖代谢途径用于大肠杆菌的生长,应用该策略生产了5.55 g/L海藻糖。研究人员还构建了以木糖作为底物合成目标产品,以葡萄糖作为碳源支持细胞生长的细胞工厂。Tai等[53]在大肠杆菌中设计并构建了以木糖为底物生产1, 4-丁二醇(1, 4-butanediol, 1, 4-BDO)的途径,以葡萄糖为碳源工程大肠杆菌合成了12 g/L 1, 4-BDO (表 2)。
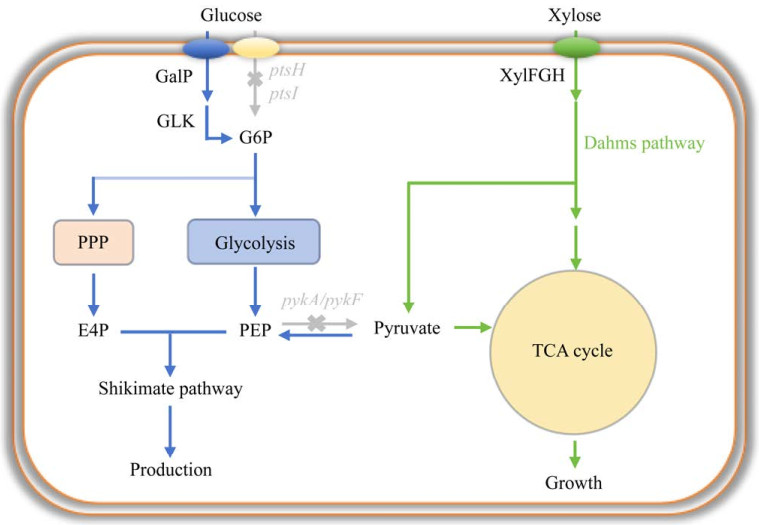
|
| 图 3 平行代谢途径工程策略 Fig. 3 Parallel metabolic pathway engineering strategy. Gray indicates knockout genes; PPP: Pentose phosphate pathway; TCA cycle: Tricarboxylic acid cycle; ptsH, ptsI encoding phosphotransferase system; pykA, pykF encoding pyruvate kinase; GalP: Galactose: H(+) symporter; GLK: Glucokinase; XylFGH: D-xylose transporter. |
| |
| Host | Substrate | Target compound | Titer (g/L) | Strategies |
| Escherichia coli | Glucose and xylose | Succinate | 107.00 | Knockout ptsG gene[37] |
| Escherichia coli | Glucose and xylose | 4-HMA | 1.11 | Knockout ptsG gene[38] |
| Escherichia coli | Glucose and xylose | n-butanol | 2.60 | Knockout ptsG gene; Overexpress Zmglf gene[40] |
| Escherichia coli | Glucose and xylose | 4-HIL | 29.20 | Overexpress XylRR121C/P363S mutants[41] |
| Escherichia coli | Glucose and xylose | Xylitol | 4.80 | Knockout araC gene[42] |
| Escherichia coli | Glucose and xylose | methyl ketone | 0.90 | Knockout pgi gene; downregulation of CRP[43] |
| Escherichia coli | Glucose and xylose | 1, 3-PDO | 98.20 | Overexpress araE, xylA, xylB gene[44] |
| Pseudomonas putida KT2440 | Glucose and xylose | MA | 33.70 | ALE, overexpress XylEA62V/A455V mutants[46] |
| Escherichia coli | Glucose and xylose | MA | 0.85 | Co-culture[48] |
| Escherichia coli | Glucose and xylose | Isobutyl butyrate | 0.39 | Co-culture[49] |
| Escherichia coli | Glucose and xylose | Ethanol Succinate |
2.70 2.70 |
Co-culture, CO2 fixation[50] |
| Escherichia coli | Glucose and xylose | MA | 4.09 | Glucose for growth, xylose for production[51] |
| Escherichia coli | Glucose and xylose | Trehalose | 5.55 | Glucose for growth, xylose for production[52] |
| Escherichia coli | Glucose and xylose | 1, 4-BDO | 12.00 | xylose for growth, glucose for production[53] |
天然木质纤维素具有复杂的结构,大多数不能被微生物直接降解。因此,木质纤维素需经化学或物理化学方法预处理后得到水解液才能被微生物利用[54-55]。在预处理过程中,木质纤维素不可避免地会生成能够抑制细胞代谢的有毒有害物质,主要包括呋喃衍生物(糠醛和5-羟甲基糠醛(5-Hydroxymethylfurfural, HMF)[56]、有机弱酸(乙酸和甲酸)[57]和酚类化合物(4-羟基苯甲醛、香草醛、丁香醛)等[58]。
克服木质纤维素水解液中毒性物质对细胞生长代谢的抑制是充分利用木质纤维素水解产物的关键。通过外源直接添加物理或化学解毒剂是常用的策略之一。例如Yegin等[59]发现CaCO3为主要成分的蛋壳可以中和秸秆水解液中的乙酸。通过添加40 g/L蛋壳粉,工程菌株利用秸秆水解物生产了43.54 g/L聚苹果酸(polymalic acid, PMA)。Gao等[60]通过稀释木质纤维素水解液以降低有毒物质的浓度,生产了7 g/L游离脂肪酸(free fatty acid, FFA)。虽然对木质纤维素水解液以物理或化学方法进行解毒处理能够减弱或去除有毒物质的影响,但是这也同时增加了生产成本和环境负担。通过对菌株进行改造,增加其解毒能力或耐受性是更好的选择。Suo等[61]在酪丁酸梭菌(Clostridium tyrobutyricum)中过表达来源于拜氏梭菌(Clostridium beijerinckii)的可降解呋喃醛和酚类化合物的醛/酮还原酶,获得的重组菌株可以利用玉米芯酸水解产物生产18.3 g/L丁酸。除了在胞内构建解毒系统,应用ALE等策略筛选耐受性强的菌株也是提高木质纤维素水解液利用率的重要策略之一。例如Lam等[62]对酿酒酵母来源的还原酶GRE2定向进化,以提高其对糠醛和羟甲基糠醛的解毒活性。重组酿酒酵母菌株可以利用麦秆、低酸玉米秸秆水解液产生近50 g/L的D-乳酸。Yan等[63]以玉米秸秆水解液为底物对运动发酵单胞菌(Zymomonas mobilis)进行适应性进化,获得的突变菌株利用玉米秸秆水解液生产乙醇的产量提高了21.8%。进一步研究发现,该突变菌株中编码氧化还原酶基因ZMO3_RS07160的表达水平上调,增强了菌株代谢香草醛的能力。
4 总结与展望木质纤维素是农业废弃物的主要成分,也是地球上最丰富的生物质资源。木质纤维素水解产物中含有大量的葡萄糖、木糖等单糖。近年来以葡萄糖为碳源构建微生物细胞工厂合成高附加值化合物取得了巨大的成就。与之相比,微生物代谢利用木糖的研究虽然已取得了一些进展,但现阶段仍面临很多挑战。首先,虽然有多条木糖代谢途径被发现和报道,但与葡萄糖高效的内源代谢途径相比,木糖代谢途径中存在较多代谢瓶颈,如XI途径中的木糖异构酶,XR-XDH途径中由于两种酶对辅因子的需求不同造成的代谢失衡,WMB途径中的木糖酸脱水酶等限制了木糖代谢途径的效率等。随着微流控平台、Cytoscape等生物分子交互可视化工具的开发,这些方法有望用于筛选获得更高效的替代酶[64]。其次,随着数据库的不断完善,利用机器学习的方法从头创制新酶取得了令人瞩目的进展[65],未来可通过设计创制高效的酶分子解除木糖代谢途径中的瓶颈。除此之外,CCR效应目前仍然是限制葡萄糖和木糖共利用效率的主要制约因素[10]。开发不受葡萄糖抑制的木糖新型转运蛋白、创制不受cAMP-CRP复合物调控的新型启动子控制木糖代谢途径基因的表达有望成为解决这些问题的办法。精确调控发酵条件和过程,如在微生物生长的不同阶段改变培养基的组成,避免长时间暴露于单一碳源也是潜在的有效策略。最后,利用ALE技术虽然可以获得针对木质纤维素水解产物中有毒物质的耐受菌株,但该方法存在周期长、工作量大、突变菌株遗传稳定性差、进化表型背后的遗传机制难解析等问题。未来可通过开发计算模型预测可提高菌株耐受性的突变,并指导ALE实验的设计,简化对有益突变的识别,减少试错成本。此外,一些极端微生物在恶劣环境的长期驯化下,进化出了高效的胁迫响应机制。借助多组学技术和高通量筛选方法,开发和应用热激蛋白、DNA修复蛋白、氧化应激蛋白等抗逆元件[66],有望进一步提高菌株对有毒物质的耐受性。
| [1] |
YAO XF, ZHOU HB, MENG HB, DING JT, SHEN YJ, CHENG HS, ZHANG X, LI R, FAN SY. Amino acid profile characterization during the co-composting of a livestock manure and maize straw mixture[J]. Journal of Cleaner Production, 2021, 278: 123494. DOI:10.1016/j.jclepro.2020.123494
|
| [2] |
LI HJ, HE YH, YAN Z, YANG ZH, TIAN F, LIU XC, WU ZS. Insight into the microbial mechanisms for the improvement of spent mushroom substrate composting efficiency driven by phosphate-solubilizing Bacillus subtilis[J]. Journal of Environmental Management, 2023, 336: 117561. DOI:10.1016/j.jenvman.2023.117561
|
| [3] |
YADAV VG, YADAV GD, PATANKAR SC. The production of fuels and chemicals in the new world: critical analysis of the choice between crude oil and biomass vis-à-vis sustainability and the environment[J]. Clean Technologies and Environmental Policy, 2020, 22(9): 1757-1774. DOI:10.1007/s10098-020-01945-5
|
| [4] |
LIU ZH, WANG K, CHEN Y, TAN TW, NIELSEN J. Third-generation biorefineries as the means to produce fuels and chemicals from CO2[J]. Nature Catalysis, 2020, 3: 274-288. DOI:10.1038/s41929-019-0421-5
|
| [5] |
DENG WP, FENG YC, FU J, GUO HW, GUO Y, HAN BX, JIANG ZC, KONG LC, LI CZ, LIU HC, Nguyen PTT, REN PN, WANG F, WANG S, WANG YQ, WANG Y, WONG SS, YAN K, YAN N, YANG XF, et al. Catalytic conversion of lignocellulosic biomass into chemicals and fuels[J]. Green Energy & Environment, 2023, 8(1): 10-114.
|
| [6] |
UPTON BM, KASKO AM. Strategies for the conversion of lignin to high-value polymeric materials: review and perspective[J]. Chemical Reviews, 2016, 116(4): 2275-2306. DOI:10.1021/acs.chemrev.5b00345
|
| [7] |
CHANDEL AK, GARLAPATI VK, SINGH AK, ANTUNES FAF, Da SILVA SS. The path forward for lignocellulose biorefineries: bottlenecks, solutions, and perspective on commercialization[J]. Bioresource Technology, 2018, 264: 370-381. DOI:10.1016/j.biortech.2018.06.004
|
| [8] |
LI CZ, ZHAO XC, WANG AQ, HUBER GW, ZHANG T. Catalytic transformation of lignin for the production of chemicals and fuels[J]. Chemical Reviews, 2015, 115(21): 11559-11624. DOI:10.1021/acs.chemrev.5b00155
|
| [9] |
KIM SR, HA SJ, WEI N, OH EJ, JIN YS. Simultaneous co-fermentation of mixed sugars: a promising strategy for producing cellulosic ethanol[J]. Trends in Biotechnology, 2012, 30(5): 274-282. DOI:10.1016/j.tibtech.2012.01.005
|
| [10] |
ZHAO Z, XIAN M, LIU M, ZHAO G. Biochemical routes for uptake and conversion of xylose by microorganisms[J]. Biotechnology for Biofuels, 2020, 13: 21. DOI:10.1186/s13068-020-1662-x
|
| [11] |
RIZZI M, KLEIN C, SCHULZE C, BUI-THANH NA, DELLWEG H. Xylose fermentation by yeasts. 5. Use of ATP balances for modeling oxygen-limited growth and fermentation of yeast Pichia stipitis with xylose as carbon source[J]. Biotechnology and Bioengineering, 1989, 34(4): 509-514. DOI:10.1002/bit.260340411
|
| [12] |
CUNHA JT, SOARES PO, ROMANÍ A, THEVELEIN JM, DOMINGUES L. Xylose fermentation efficiency of industrial Saccharomyces cerevisiae yeast with separate or combined xylose reductase/xylitol dehydrogenase and xylose isomerase pathways[J]. Biotechnology for Biofuels, 2019, 12: 20. DOI:10.1186/s13068-019-1360-8
|
| [13] |
PROMDONKOY P, SIRIPONG W, DOWNES JJ, TANAPONGPIPAT S, RUNGUPHAN W. Systematic improvement of isobutanol production from D-xylose in engineered Saccharomyces cerevisiae[J]. AMB Express, 2019, 9(1): 160. DOI:10.1186/s13568-019-0885-3
|
| [14] |
JO JH, PARK YC, JIN YS, SEO JH. Construction of efficient xylose-fermenting Saccharomyces cerevisiae through a synthetic isozyme system of xylose reductase from Scheffersomyces stipitis[J]. Bioresource Technology, 2017, 241: 88-94. DOI:10.1016/j.biortech.2017.05.091
|
| [15] |
SUZUKI T, HOSHINO T, MATSUSHIKA A. High-temperature ethanol production by a series of recombinant xylose-fermenting Kluyveromyces marxianus strains[J]. Enzyme and Microbial Technology, 2019, 129: 109359. DOI:10.1016/j.enzmictec.2019.109359
|
| [16] |
SONGDECH P, BUTKINAREE C, YINGCHUTRAKUL Y, PROMDONKOY P, RUNGUPHAN W, SOONTORNGUN N. Increased production of isobutanol from xylose through metabolic engineering of Saccharomyces cerevisiae overexpressing transcription factor Znf1 and exogenous genes[J]. FEMS Yeast Research, 2024, 24: foae006. DOI:10.1093/femsyr/foae006
|
| [17] |
QIU ZY, GAO QQ, BAO J. Constructing xylose-assimilating pathways in Pediococcus acidilactici for high titer D-lactic acid fermentation from corn stover feedstock[J]. Bioresource Technology, 2017, 245(Pt B): 1369-1376.
|
| [18] |
[QIU ZY, GAO QQ, BAO J. Engineering Pediococcus acidilactici with xylose assimilation pathway for high titer cellulosic L-lactic acid fermentation[J]. Bioresource Technology, 2018, 249: 9-15. DOI:10.1016/j.biortech.2017.09.117
|
| [19] |
PRABHU AA, BHARALI B, SINGH A, ALLAKA M, SUKUMAR P, VEERANKI VD. Engineering folding mechanism through Hsp70 and Hsp40 chaperones for enhancing the production of recombinant human interferon gamma (rhIFN-γ) in Pichia pastoris cell factory[J]. Chemical Engineering Science, 2018, 181: 58-67. DOI:10.1016/j.ces.2018.02.003
|
| [20] |
TEMER B, dos SANTOS LV, NEGRI VA, GALHARDO JP, MAGALHÃES PHM, JOSÉ J, MARSCHALK C, CORRÊA TLR, CARAZZOLLE MF, PEREIRA GAG. Conversion of an inactive xylose isomerase into a functional enzyme by co-expression of GroEL-GroES chaperonins in Saccharomyces cerevisiae[J]. BMC Biotechnology, 2017, 17(1): 71. DOI:10.1186/s12896-017-0389-7
|
| [21] |
SEIKE T, KOBAYASHI Y, SAHARA T, OHGIYA S, KAMAGATA Y, FUJIMORI KE. Molecular evolutionary engineering of xylose isomerase to improve its catalytic activity and performance of micro-aerobic glucose/xylose co-fermentation in Saccharomyces cerevisiae[J]. Biotechnology for Biofuels, 2019, 12: 139. DOI:10.1186/s13068-019-1474-z
|
| [22] |
Tan BW, Zheng YM, Yan HJ, Liu YZ, Li ZJ. Metabolic engineering of Halomonas bluephagenesis to metabolize xylose for poly-3-hydroxybutyrate production[J]. Biochemical Engineering Journal, 2022, 187: 108623. DOI:10.1016/j.bej.2022.108623
|
| [23] |
PEREIRA B, LI ZJ, de MEY M, LIM CG, ZHANG HR, HOELTGEN C, STEPHANOPOULOS G. Efficient utilization of pentoses for bioproduction of the renewable two-carbon compounds ethylene glycol and glycolate[J]. Metabolic Engineering, 2016, 34: 80-87. DOI:10.1016/j.ymben.2015.12.004
|
| [24] |
CAM Y, ALKIM C, TRICHEZ D, TREBOSC V, VAX A, BARTOLO F, BESSE P, FRANÇOIS JM, WALTHER T. Engineering of a synthetic metabolic pathway for the assimilation of (d)-xylose into value-added chemicals[J]. ACS Synthetic Biology, 2016, 5(7): 607-618. DOI:10.1021/acssynbio.5b00103
|
| [25] |
URANUKUL B, WOOLSTON BM, FINK GR, STEPHANOPOULOS G. Biosynthesis of monoethylene glycol in Saccharomyces cerevisiae utilizing native glycolytic enzymes[J]. Metabolic Engineering, 2019, 51: 20-31. DOI:10.1016/j.ymben.2018.09.012
|
| [26] |
ROSSONI L, CARR R, BAXTER S, CORTIS R, THORPE T, EASTHAM G, STEPHENS G. Engineering Escherichia coli to grow constitutively on D-xylose using the carbon-efficient Weimberg pathway[J]. Microbiology, 2018, 164(3): 287-298. DOI:10.1099/mic.0.000611
|
| [27] |
BRÜSSELER C, SPÄTH A, SOKOLOWSKY S, MARIENHAGEN J. Alone at last!-heterologous expression of a single gene is sufficient for establishing the five-step Weimberg pathway in Corynebacterium glutamicum[J]. Metabolic Engineering Communications, 2019, 9: e00090. DOI:10.1016/j.mec.2019.e00090
|
| [28] |
ZHAO AQ, HU XQ, WANG XY. Metabolic engineering of Escherichia coli to produce gamma-aminobutyric acid using xylose[J]. Applied Microbiology and Biotechnology, 2017, 101(9): 3587-3603. DOI:10.1007/s00253-017-8162-3
|
| [29] |
REN YX, ERONEN V, BLOMSTER ANDBERG M, KOIVULA A, HAKULINEN N. Structure and function of aldopentose catabolism enzymes involved in oxidative non-phosphorylative pathways[J]. Biotechnology for Biofuels and Bioproducts, 2022, 15(1): 147. DOI:10.1186/s13068-022-02252-5
|
| [30] |
CABULONG RB, LEE WK, BAÑARES AB, RAMOS KRM, NISOLA GM, VALDEHUESA KNG, CHUNG WJ. Engineering Escherichia coli for glycolic acid production from D-xylose through the Dahms pathway and glyoxylate bypass[J]. Applied Microbiology and Biotechnology, 2018, 102(5): 2179-2189. DOI:10.1007/s00253-018-8744-8
|
| [31] |
WANG YH, XIAN M, FENG XJ, LIU M, ZHAO G. Biosynthesis of ethylene glycol from D-xylose in recombinant Escherichia coli[J]. Bioengineered, 2018, 9(1): 233-241. DOI:10.1080/21655979.2018.1478489
|
| [32] |
CABULONG RB, VALDEHUESA KNG, RAMOS KRM, NISOLA GM, LEE WK, LEE CR, CHUNG WJ. Enhanced yield of ethylene glycol production from D-xylose by pathway optimization in Escherichia coli[J]. Enzyme and Microbial Technology, 2017, 97: 11-20. DOI:10.1016/j.enzmictec.2016.10.020
|
| [33] |
SHEN CR, LIAO JC. Synergy as design principle for metabolic engineering of 1-propanol production in Escherichia coli[J]. Metabolic Engineering, 2013, 17: 12-22. DOI:10.1016/j.ymben.2013.01.008
|
| [34] |
WANG J, SHEN XL, LIN YH, CHEN ZY, YANG YP, YUAN QP, YAN YJ. Investigation of the synergetic effect of xylose metabolic pathways on the production of glutaric acid[J]. ACS Synthetic Biology, 2018, 7(1): 24-29. DOI:10.1021/acssynbio.7b00271
|
| [35] |
XIA PF, ZHANG GC, WALKER B, SEO SO, KWAK S, LIU JJ, KIM H, ORT DR, WANG SG, JIN YS. Recycling carbon dioxide during xylose fermentation by engineered Saccharomyces cerevisiae[J]. ACS Synthetic Biology, 2017, 6(2): 276-283. DOI:10.1021/acssynbio.6b00167
|
| [36] |
LU CZ, RAMALHO TP, BISSCHOPS MMM, WIJFFELS RH, MARTINS dos SANTOS VAP, WEUSTHUIS RA. Crossing bacterial boundaries: the carbon catabolite repression system Crc-Hfq of Pseudomonas putida KT2440 as a tool to control translation in E. coli[J]. New Biotechnology, 2023, 77: 20-29. DOI:10.1016/j.nbt.2023.06.004
|
| [37] |
ZHU FY, WANG CQ, SAN KY, BENNETT GN. Metabolic engineering of Escherichia coli to produce succinate from woody hydrolysate under anaerobic conditions[J]. Journal of Industrial Microbiology & Biotechnology, 2020, 47(2): 223-232.
|
| [38] |
LI FF, ZHAO Y, LI BZ, QIAO JJ, ZHAO GR. Engineering Escherichia coli for production of 4-hydroxymandelic acid using glucose-xylose mixture[J]. Microbial Cell Factories, 2016, 15: 90. DOI:10.1186/s12934-016-0489-4
|
| [39] |
ZHAO CH, ZHANG YP, LI Y. Production of fuels and chemicals from renewable resources using engineered Escherichia coli[J]. Biotechnology Advances, 2019, 37(7): 107402. DOI:10.1016/j.biotechadv.2019.06.001
|
| [40] |
SAINI M, LIN LJ, CHIANG CJ, CHAO YP. Synthetic consortium of Escherichia coli for n-butanol production by fermentation of the glucose-xylose mixture[J]. Journal of Agricultural and Food Chemistry, 2017, 65(46): 10040-10047. DOI:10.1021/acs.jafc.7b04275
|
| [41] |
WEI MH, LI GR, XIE HX, YANG WJ, XU HR, HAN SB, WANG JZ, MENG Y, XU QY, LI YJ, CHEN N, ZHANG CL. Sustainable production of 4-hydroxyisoleucine with minimised carbon loss by simultaneously utilising glucose and xylose in engineered Escherichia coli[J]. Bioresource Technology, 2022, 354: 127196. DOI:10.1016/j.biortech.2022.127196
|
| [42] |
KIM SM, CHOI BY, RYU YS, JUNG SH, PARK JM, KIM GH, LEE SK. Simultaneous utilization of glucose and xylose via novel mechanisms in engineered Escherichia coli[J]. Metabolic Engineering, 2015, 30: 141-148. DOI:10.1016/j.ymben.2015.05.002
|
| [43] |
WANG X, GOH EB, BELLER HR. Engineering E. coli for simultaneous glucose-xylose utilization during methyl ketone production[J]. Microbial Cell Factories, 2018, 17(1): 12. DOI:10.1186/s12934-018-0862-6
|
| [44] |
LI ZH, DONG YF, LIU Y, CEN XC, LIU DH, CHEN Z. Systems metabolic engineering of Corynebacterium glutamicum for high-level production of 1, 3-propanediol from glucose and xylose[J]. Metabolic Engineering, 2022, 70: 79-88. DOI:10.1016/j.ymben.2022.01.006
|
| [45] |
SU BL, SONG DD, ZHU HH. Metabolic engineering of Saccharomyces cerevisiae for enhanced carotenoid production from xylose-glucose mixtures[J]. Frontiers in Bioengineering and Biotechnology, 2020, 8: 435. DOI:10.3389/fbioe.2020.00435
|
| [46] |
LING C, PEABODY GL, SALVACHÚA D, KIM YM, KNEUCKER CM, CALVEY CH, MONNINGER MA, MUNOZ NM, POIRIER BC, RAMIREZ KJ, ST JOHN PC, WOODWORTH SP, MAGNUSON JK, BURNUM-JOHNSON KE, GUSS AM, JOHNSON CW, BECKHAM GT. Muconic acid production from glucose and xylose in Pseudomonas putida via evolution and metabolic engineering[J]. Nature Communications, 2022, 13: 4925. DOI:10.1038/s41467-022-32296-y
|
| [47] |
LI HB, SCHMITZ O, ALPER HS. Enabling glucose/xylose co-transport in yeast through the directed evolution of a sugar transporter[J]. Applied Microbiology and Biotechnology, 2016, 100(23): 10215-10223. DOI:10.1007/s00253-016-7879-8
|
| [48] |
ZHANG HR, PEREIRA B, LI ZJ, STEPHANOPOULOS G. Engineering Escherichia coli coculture systems for the production of biochemical products[J]. Proceedings of the National Academy of Sciences of the United States of America, 2015, 112(27): 8266-8271.
|
| [49] |
SEO H, CASTRO G, TRINH CT. Engineering a synthetic Escherichia coli coculture for compartmentalized de novo biosynthesis of isobutyl butyrate from mixed sugars[J]. ACS Synthetic Biology, 2024, 13(1): 259-268. DOI:10.1021/acssynbio.3c00493
|
| [50] |
FLORES AD, HOLLAND SC, MHATRE A, SARNAIK AP, GODAR A, ONYEABOR M, VARMAN AM, WANG X, NIELSEN DR. A coculture-coproduction system designed for enhanced carbon conservation through inter-strain CO2 recycling[J]. Metabolic Engineering, 2021, 67: 387-395. DOI:10.1016/j.ymben.2021.08.001
|
| [51] |
FUJIWARA R, NODA S, TANAKA T, KONDO A. Metabolic engineering of Escherichia coli for shikimate pathway derivative production from glucose-xylose co-substrate[J]. Nature Communications, 2020, 11: 279. DOI:10.1038/s41467-019-14024-1
|
| [52] |
WU YF, WANG J, SHEN XL, WANG J, CHEN ZY, SUN XX, YUAN QP, YAN YJ. Investigating the strategies for microbial production of trehalose from lignocellulosic sugars[J]. Biotechnology and Bioengineering, 2018, 115(3): 785-790. DOI:10.1002/bit.26505
|
| [53] |
TAI YS, XIONG MY, JAMBUNATHAN P, WANG JY, WANG JL, STAPLETON C, ZHANG KC. Engineering nonphosphorylative metabolism to generate lignocellulose-derived products[J]. Nature Chemical Biology, 2016, 12: 247-253. DOI:10.1038/nchembio.2020
|
| [54] |
QASIM U, RAFIQ S, JAMIL F, AHMED A, ALI T, KERS J, KHURRAM MS, HUSSAIN M, INAYAT A, PARK YK. Processing of lignocellulose in ionic liquids: a cleaner and sustainable approach[J]. Journal of Cleaner Production, 2021, 323: 129189. DOI:10.1016/j.jclepro.2021.129189
|
| [55] |
YAN M, WU T, MA JX, LU HL, ZHOU XF. A systematic study of lignocellulose nanofibrils (LCNF) prepared from wheat straw by varied acid pretreatments[J]. Industrial Crops and Products, 2022, 185: 115126. DOI:10.1016/j.indcrop.2022.115126
|
| [56] |
UJOR VC, OKONKWO CC. Microbial detoxification of lignocellulosic biomass hydrolysates: biochemical and molecular aspects, challenges, exploits and future perspectives[J]. Frontiers in Bioengineering and Biotechnology, 2022, 10: 1061667. DOI:10.3389/fbioe.2022.1061667
|
| [57] |
GUO HL, ZHAO Y, CHANG JS, LEE DJ. Inhibitor formation and detoxification during lignocellulose biorefinery: a review[J]. Bioresource Technology, 2022, 361: 127666. DOI:10.1016/j.biortech.2022.127666
|
| [58] |
AGRAWAL R, VERMA A, SINGHANIA RR, VARJANI S, DONG CD, KUMAR PATEL A. Current understanding of the inhibition factors and their mechanism of action for the lignocellulosic biomass hydrolysis[J]. Bioresource Technology, 2021, 332: 125042. DOI:10.1016/j.biortech.2021.125042
|
| [59] |
YEGIN S, SAHA BC, KENNEDY GJ, LEATHERS TD. Valorization of egg shell as a detoxifying and buffering agent for efficient polymalic acid production by Aureobasidium pullulans NRRL Y-2311-1 from barley straw hydrolysate[J]. Bioresource Technology, 2019, 278: 130-137. DOI:10.1016/j.biortech.2018.12.119
|
| [60] |
GAO JQ, YU W, LI YX, JIN MJ, YAO L, ZHOU YJ. Engineering co-utilization of glucose and xylose for chemical overproduction from lignocellulose[J]. Nature Chemical Biology, 2023, 19: 1524-1531. DOI:10.1038/s41589-023-01402-6
|
| [61] |
SUO YK, LIAO ZP, QU CY, FU HX, WANG JF. Metabolic engineering of Clostridium tyrobutyricum for enhanced butyric acid production from undetoxified corncob acid hydrolysate[J]. Bioresource Technology, 2019, 271: 266-273. DOI:10.1016/j.biortech.2018.09.095
|
| [62] |
LAM FH, TURANLı-YıLDıZ B, LIU D, RESCH MG, FINK GR, STEPHANOPOULOS G. Engineered yeast tolerance enables efficient production from toxified lignocellulosic feedstocks[J]. Science Advances, 2021, 7(26): eabf7613. DOI:10.1126/sciadv.abf7613
|
| [63] |
YAN Z, ZHANG J, BAO J. Increasing cellulosic ethanol production by enhancing phenolic tolerance of Zymomonas mobilis in adaptive evolution[J]. Bioresource Technology, 2021, 329: 124926. DOI:10.1016/j.biortech.2021.124926
|
| [64] |
SHAO FC, LEE PW, LI H, HSIEH K, WANG TH. Emerging platforms for high-throughput enzymatic bioassays[J]. Trends in Biotechnology, 2023, 41(1): 120-133. DOI:10.1016/j.tibtech.2022.06.006
|
| [65] |
VANELLA R, KOVACEVIC G, DOFFINI V, FERNÁNDEZ de SANTAELLA J, NASH MA. High-throughput screening, next generation sequencing and machine learning: advanced methods in enzyme engineering[J]. Chemical Communications, 2022, 58(15): 2455-2467. DOI:10.1039/D1CC04635G
|
| [66] |
HIRASAWA T, MAEDA T. Adaptive laboratory evolution of microorganisms: methodology and application for bioproduction[J]. Microorganisms, 2022, 11(1): 92. DOI:10.3390/microorganisms11010092
|