中国科学院微生物研究所、中国微生物学会主办
文章信息
- 王倩, 高教琪, 周雍进
- WANG Qian, GAO Jiaoqi, ZHOU Yongjin
- 葡萄糖和木糖高效共利用代谢工程研究进展
- Metabolic engineering for the efficient co-utilization of glucose and xylose
- 生物工程学报, 2024, 40(8): 2710-2730
- Chinese Journal of Biotechnology, 2024, 40(8): 2710-2730
- 10.13345/j.cjb.240199
-
文章历史
- Received: March 7, 2024
- Accepted: May 21, 2024
- Published: May 27, 2024
2. 大连市能源生物技术重点实验室, 辽宁 大连 116023
2. Dalian Key Laboratory of Energy Biotechnology, Dalian 116023, Liaoning, China
木质纤维素生物质作为可再生原料,常见于农作物秸秆、杂草、木材废料及其他固体废弃物中,通过水解和发酵释放糖,可用于生产醇类、微生物油脂和有机酸等各类化学品[1-3]。木质纤维素生物质主要由纤维素、半纤维素和木质素构成,其水解产物是各种己糖和戊糖的混合物,主要包括葡萄糖(占比60%–70%)和木糖(占比30%–40%)[4]。与纤维素水解生成的葡萄糖相比,半纤维素水解产生的木糖很难被微生物有效利用,这极大地限制了木质纤维素资源的应用潜力。因此,实现微生物对葡萄糖和木糖的高效共利用对于木质纤维素生物质有效转化为燃料和化学品至关重要[5]。但是截至目前,只有少数天然微生物具有木糖代谢途径[6],并且这些微生物对木糖和葡萄糖的共利用普遍存在底物偏好性强、碳分解代谢物阻遏(carbon catabolite repression, CCR)、产量低等问题[7]。此外,微生物对木糖的转运效率远低于葡萄糖也限制了下游途径的通量。因此,构建能够高效共利用混合糖特别是木糖和葡萄糖的工程菌株,对于木质纤维素的规模化工业利用至关重要[8]。
本文综述了近年来微生物共利用木质纤维素中葡萄糖和木糖的研究进展,详细讨论了用于改善微生物对葡萄糖和木糖共利用的代谢工程策略,包括:(1) 碳分解代谢阻遏效应缓解;(2)构建工程化木糖转运体,增加对木糖的亲和力;(3) 设计内源性代谢途径,包括采用自适应进化等非理性工程方法,以识别提高木糖代谢的关键靶点;(4) 利用人工多菌体系的模块化构建策略,将功能性代谢途径分配到不同宿主菌中,通过混菌共发酵技术实现葡萄糖和木糖共利用。最后,总结了基于以上工程策略生产的具有工业化前景的主要生物基产品。
1 微生物天然木糖及葡萄糖代谢途径迄今为止,研究发现自然界中具有木糖代谢途径的天然微生物有200余种,主要包括细菌、酵母菌和丝状真菌。其中既能利用木糖又能利用葡萄糖的酵母菌是研究重点,主要包括树干毕赤酵母(Scheffersomyces stipites)、马克斯克鲁维酵母(Kluyveromyces marxianus)、产朊假丝酵母(Candida utilis)和葡萄牙假丝酵母(Candida lusitaniae)等[9-10]。
微生物为了在生存环境中充分利用木糖,经过长时间进化形成了不同的木糖利用途径(图 1)。木糖最初在不同的微生物体内通过不同的酶转化为木酮糖。在细菌中,木糖异构化通常由xylA基因编码的木糖异构酶(xylose isomerase, XI)直接催化[11-12],而大多数真菌和酵母则需要木糖还原酶(xylose reductase, XR)和木糖醇脱氢酶(xylitol dehydrogenase, XDH)两步催化[13]。在XR-XDH途径中,前两个反应通常伴随NADPH的消耗和NADH的产生。在细菌和酵母菌中,木酮糖分别在XYLB和XKS1基因编码的木酮糖激酶(xylulose kinase, XK)催化下形成木酮糖-5-磷酸后进入磷酸戊糖途径(pentose phosphate pathway, PPP)[14-16]。由于XR-XDH途径的NADPH和NADH的双辅因子偏好,这两种辅因子的不平衡是该途径的主要瓶颈。相比之下,XI途径不涉及辅因子,因此受到了更多的关注,但异构酶催化效率低,限制了其工业应用[15]。除了上述提到的2条已知的基于磷酸化的木糖代谢途径之外,天然存在的木糖降解途径还有非磷酸化途径,被称为木糖氧化途径(xylose oxidation pathways, XOPs)[17]。这些途径中,木糖首先通过由XYLB基因编码的木糖脱氢酶氧化为D-木糖内酯,然后D-木糖内酯在XYLC基因编码的D-木糖内酯酶(xylonolactonase, XLA)的催化下或自发水解为D-木糖酸中间体。接着D-木糖酸通过XYLD基因编码的D-木糖酸脱水酶(xylonate dehydratase, XD)催化脱水生成2-酮-3-脱氧-D-木糖酸。之后,XOPs分支出2条途径:产生丙酮酸和乙醇醛的Dahms途径[18]或产生α-酮戊二酸的Weimberg途径[19]。这些途径的整个或部分基因在大多数古细菌以及一些细菌和真菌中都有发现[17]。在上述途径中,对XI和XR-XDH途径的研究更加广泛[20],而XOPs是独立于其他碳水化合物的同化途径,设计同时利用木糖和葡萄糖或与XOPs正交利用的途径同样具有很大潜力[21]。
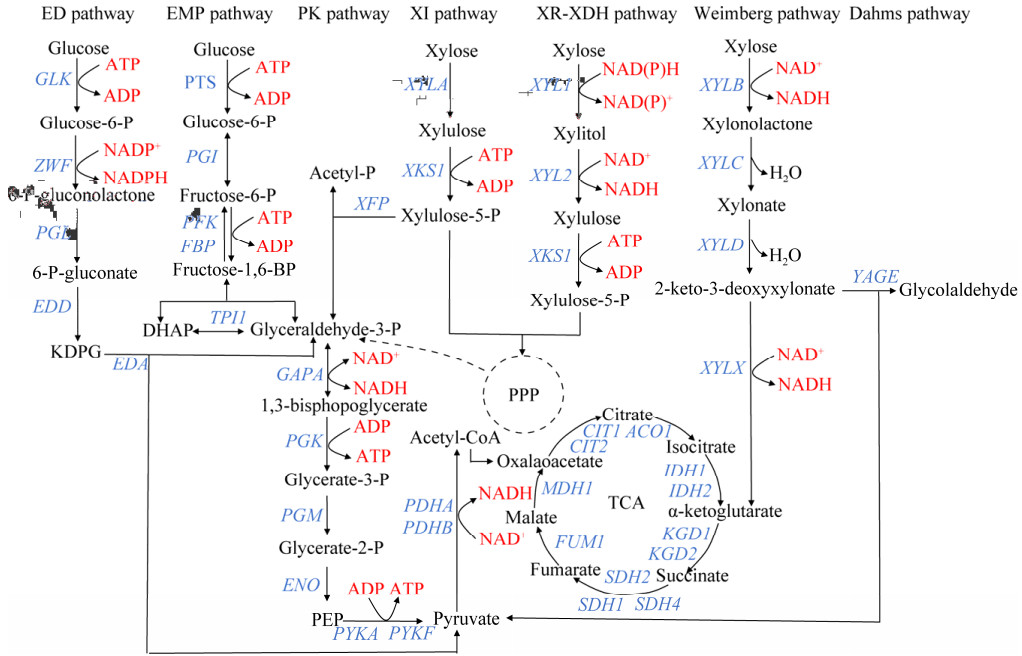
|
| 图 1 微生物中葡萄糖和木糖共利用代谢途径 Fig. 1 The metabolic pathway for co-utilizing glucose and xylose in microorganisms. ED pathway: Entner-doudoroff pathway; EMP pathway: Emden-meyerhof-parnas pathway; PK pathway: Phosphoketolase pathway; XI pathway: Xylose isomerase pathway; XR-XDH pathway: Xylose reductase-xylitol dehydrogenase pathway; PPP: Pentose phosphate pathway; TCA: Tricarboxylic acid cycle; DHAP: Dihydroxyacetone phosphate; KDPG: 2-keto-3-deoxy-6-phosphogluconate; PEP: Phosphoenolpyruvate; ATP: Aadenosine triphosphate; ADP: Adenosine diphosphate; NAD+: Nicotinamide adenine dinucleotide, oxidized form; NADH: Nicotinamide adenine dinucleotide, reduced form; NADP+: Nicotinamide adenine dinucleotide phosphate, oxidized form; NADPH: Nicotinamide adenine dinucleotide phosphate, reduced form; GLK: Glucokinase; ZWF: Glucose-6-phosphate dehydrogenase; PGL: 6-phosphogluconolactonase; EDD: Phosphogluconate dehydratase; EDA: Bifunctional 4-hydroxy-2-oxoglutarate aldolase; PTS: Phosphotransferase system; PGI: Phosphoglucose isomerase; PFK: Phosphofructokinase; FBP: Fructose-1, 6-bisphosphatase; TPI1: Triosephosphate isomerase 1; GAPA: Glyceraldehyde-3-phosphate dehydrogenase; PGK: Phosphoglycerate kinase; PGM: Phosphoglycerate mutase; ENO: Enolase; PYKA: Pyruvate kinase II; PYKF: Pyruvate kinase I; PDHA: Pyruvate dehydrogenase E1 component subunit alpha; PDHB: Pyruvate dehydrogenase E1 component subunit beta; XFP: Xylulose-5-phosphate phosphoketolase; XYLA: Xylose isomerase; XKS1: Xylulose kinase; XYL1: Xylose reductase, XYL2: Xylitol dehydrogenase; XYLB: Xylose dehydrogenase; XYLC: Xylono-1, 5-lactonase; XYLD: Xylonate dehydratase; YAGE: 2-keto-3-deoxyxylonate aldolase; XYLX: 2-keto-3-deoxyxylonate dehydratase; CIT: Citrate synthase; ACO1: Aconitate hydratase; IDH: Isocitrate dehydrogenase; KGD: α-ketoglutarate dehydrogenase; SDH: Succinate dehydrogenase; FUM: Fumarate hydratase; MDH: Fumarate hydratase. |
| |
葡萄糖在微生物胞内代谢通过糖酵解(Emden-Meyerhof-Parnas, EMP; Entner-Doudoroff, ED)途径,代谢产物丙酮酸被氧化脱羧为乙酰辅酶A后进入三羧酸循环(tricarboxylic acid cycle, TCA)。EMP或ED途径常被用于构建基因工程菌株的葡萄糖代谢模块,木糖XR、XI及Weimberg途径常与葡萄糖的EMP或ED途径组合,构建具有木糖-葡萄糖共利用能力的工程菌株(图 1)。
2 葡萄糖抑制效应及缓解CCR的策略木糖通常与葡萄糖共存于木质纤维素水解物中,CCR效应的存在极大制约了微生物利用木糖的效率。CCR通过全局或特定调节因子影响分解代谢酶的合成[22],在木质纤维素利用过程中,当葡萄糖存在时,其分解代谢物会抑制其他糖代谢基因的转录,微生物只合成用于葡萄糖代谢的酶,而不合成其他糖代谢酶。细菌中约有5%−10%的基因受CCR调控,环状腺苷酸单磷酸受体蛋白(cyclic amp receptor protein, CRP)、信号代谢产物环磷酸腺苷(cyclic adenosine monophosphate, cAMP)、腺苷酸环化酶(adenylate cyclase, AC)以及葡萄糖转运器的葡萄糖特异性酶(glucose-specific enzyme, EIIAGlc)参与了大肠杆菌(Escherichia coli)的CCR调控过程。酿酒酵母(Saccharomyces cerevisiae)主要通过Snf3/Rgt2介导的传感器/受体-抑制子调控途径(sensor/ receptor-repressor, SSR)、Mig1-Hxk2葡萄糖抑制途径以及环磷酸腺苷/蛋白激酶(protein kinase A, PKA)途径对葡萄糖代谢进行调控[23]。研究人员对涉及葡萄糖信号通路的基因或基因网络进行修改,从而缓解CCR效应,促进木糖利用。
大肠杆菌中葡萄糖特异性磷酸转移酶系统(glucose-specific phosphotransferase system, PTSGlc)是最有效的葡萄糖转运系统,在CCR中起关键作用;由ptsG编码的EIICBGlc膜蛋白能够介导糖的跨膜运输,通过该基因的敲除,削弱了大肠杆菌的CCR通路,实现对混合碳源葡萄糖和木糖的同步利用[24]。已有多篇文献报道了该策略的有效性,在大肠杆菌中敲除ptsG基因后,工程菌在10%混合糖(5%葡萄糖和5%木糖)培养基中发酵的乳酸产量为83.0 g/L,相比于对照菌株提高了25.9%,在秸秆水解液中发酵的乳酸产量为25.2 g/L,转化率为86.4%[25]。在另一项研究中,在大肠杆菌中通过失活ptsG基因并过表达发酵单胞菌(Zymomonas mobilis)的葡萄糖载体基因Zmglf,重组大肠杆菌在葡萄糖和木糖混合培养中产生了2.6 g/L的正丁醇,碳得率达到理论得率的32%;此外,研究人员还构建了一个包含葡萄糖选择性菌株和木糖选择性菌株的共培养系统,能够同步利用葡萄糖和木糖。在使用葡萄糖和木糖混合培养基时,正丁醇滴度达到5.2 g/L,产量为理论产量的63%[26]。然而,磷酸转移酶系统(phosphotransferase system, PTS)失活也会导致葡萄糖的摄取速率下降,从而降低细胞生长效率;由于cAMP-CRP会影响非PTS糖类的转运和摄取,因此调控或放大crp基因是一种可行方案。采用ΔptsG和crp*组合策略,构建的E. coli IS5-dI能够缓解葡萄糖抑制,同步利用葡萄糖和木糖。该菌株以脱毒玉米芯水解产物为底物发酵生产木糖醇的产量比出发菌株提高14.7%[27]。当S. cerevisiae在葡萄糖和木糖混合培养基中生长时,通过调控其Mig1-Hxk2葡萄糖抑制途径,实现了对葡萄糖相关基因转录的去阻遏。敲除MIG1基因可使木糖消耗率提高25%,此外SNF1的缺失加速了S. cerevisiae对木糖利用,但同时敲除MIG1和SNF1会加速葡萄糖利用,减慢木糖利用[28]。在天然利用木糖的非常规酵母的研究中,通过对葡萄糖感应信号途径的改造,同样成功构建了能够同时高效利用葡萄糖和木糖的工程菌株,敲除马克斯克鲁维酵母(K. marxianus)中的HXK1和SNF1以及过表达木糖特异性转运蛋白以缓解葡萄糖阻遏效应,能够使工程菌K. marxianus YHY013同步利用葡萄糖和木糖,并在木质纤维素生物质水解液中高产木糖醇[29]。在多形汉逊酵母(Ogataea polymorpha)中过表达参与碳水化合物感应的转录激活因子AZF1基因以及编码己糖转运蛋白的HXS1基因,能够同时提升工程菌株对葡萄糖和木糖的利用,AZF1和HXS1基因的双重过表达可进一步增强产乙醇的能力,增幅达20%−40%[30]。
然而,由于CCR效应的信号通路和快速葡萄糖发酵表型之间复杂且未知的相互作用机制,采用单一策略无法解决葡萄糖和木糖高效共利用的问题。如果以葡萄糖的寡聚体形式作为碳源(如纤维二糖),则不会引起CCR,因此另一种策略是将纤维素二糖与木糖共发酵[31]。起初通过在细胞表面展示β-葡萄糖苷酶,使其在细胞外将纤维素二糖水解成葡萄糖,产生的葡萄糖会立即被S. cerevisiae利用,从而避免了CCR,同时促进木糖的消耗[32],然而共发酵过程需要精确控制纤维二糖水解和葡萄糖消耗速率,以防止葡萄糖积累[33]。此后,纤维二糖的胞外水解被胞内水解所取代[34]。通过将纤维二糖转运蛋白、胞内β-葡萄糖苷酶和木糖代谢途径整合到S. cerevisiae中,使其能够共发酵纤维二糖和木糖,与以纤维二糖或木糖为唯一碳源的发酵结果相比,乙醇产量显著提高[35-36]。利用类似原理,研究者还构建了能够同时利用纤维二糖和木糖的E. coli[37]和凝结芽孢杆菌(Bacillus coagulans)等重组菌株[38]。微生物中纤维二糖和木糖发酵途径的成功整合是实现经济生物燃料生产的关键步骤。
3 葡萄糖和木糖共利用的代谢工程策略 3.1 木糖转运体工程跨膜运输是木糖利用的关键步骤,提高木糖跨膜转运的效率是增加其利用率并拓展微生物碳源的有效途径[39]。研究表明,野生型糖转运蛋白多倾向于首先转运葡萄糖而非木糖[40-41]。例如,S. cerevisiae中缺乏木糖特异性转运蛋白,因此天然己糖转运蛋白,包括Hxt1、Hxt2、Hxt4、Hxt5、Hxt7、HxtZ和Gal2,负责介导木糖转运[42-43]。然而,这些己糖转运体对葡萄糖的亲和力是其他糖的10−100倍,葡萄糖的存在会强烈抑制木糖吸收、限制木糖同化,由此延长了混合糖发酵时间[44-45]。野生型Z. mobilis是通过葡萄糖载体蛋白Glf转运木糖,Glf对葡萄糖的高亲和力导致其竞争性抑制木糖摄取[46-47]。因此,挖掘和设计对葡萄糖不敏感的木糖特异性转运体对生物质水解物混合糖的经济转化至关重要。
迄今为止,通过基于功能互补研究或基于与已知木糖转运蛋白相似序列的生物信息学检索,已经确认了来自9种不同微生物的约30个异源糖转运蛋白具有运输木糖的能力[31, 48-52]。为了促进木糖同化,引入了来自细菌、真菌以及植物的木糖转运体以提高酵母中木糖的转运[53]。第一个在S. cerevisiae中成功表达的非原生木糖转运体是来自假丝酵母(Candida intermedia)的Gxf1和Gxs1[50]。这2个转运蛋白在己糖转运体缺陷的S. cerevisiae中促进了细胞对木糖的利用。尽管动力学研究表明,Gxf1主要是一种己糖转运体,但它对木糖的亲和力是野生型S. cerevisiae己糖转运体的3倍。利用该特性,C. intermedia和P. stipitis的木糖共转运体Gxf1、Sut1和At5g59250被整合到S. cerevisiae中,重组菌株对木糖的转运和亲和力得到了提高[54]。此外,通过对S. cerevisiae中CiGxs1转运体定向进化,发现包括N326H、C-末端截短、I171F和M40V等多个突变能够减轻葡萄糖抑制。在高浓度葡萄糖(70 g/L)存在的情况下,突变体S. cerevisia EBY.VW4000中木糖的转运显著提高[55]。将枯草芽孢杆菌(Bacillus subtilis)中编码木糖转运蛋白的araE基因整合到S. cerevisiae D452-2中后,木糖的转运和木糖醇的产量都得到了显著提高[56]。从黑曲霉(Aspergillus niger)中引入XLTA和STR1基因到S. cerevisiae中,其木糖的利用也得到了提高[57]。近期的研究还聚焦于S. cerevisiae己糖转运蛋白家族,包括内源性Gal2以及外源引入的来自里氏木霉(Trichoderma reesei) 的Xltr1p[58-60]。通过对己糖转运蛋白的关键氨基酸残基定点突变,例如,在Gal2中引入N376Y/M435I双突变[58, 60],或在Xltr1p中进行N326F突变[59],成功解除了葡萄糖对木糖运输的抑制并提升了木糖的转运速率。尽管采用的是不同的突变策略,但这些研究均表明通过精准调控糖转运蛋白结构,可实现不受葡萄糖抑制的木糖特异性转运。除酵母外,研究者对细菌中的木糖转运机制也进行了广泛研究,并基于此改善木糖在细菌中的运输。细菌中存在2种特异的木糖转运系统,分别是三磷酸腺苷结合盒转运体(adenosine triphosphate binding cassette, ABC)转运系统和质子/阳离子耦联的转运系统。大肠杆菌是利用ABC转运系统对木糖进行跨膜转运,在该系统中木糖通过特异性的木糖结合蛋白由三磷酸腺苷(adenosine triphosphate, ATP)水解产生的能量驱动进行转运。芽孢杆菌、沙门氏菌和乳酸杆菌对木糖的转运则是通过质子/阳离子耦联的转运系统中的跨膜转运蛋白完成,并由Na+浓度梯度或质子电位推动。采用耦联细胞生长筛选策略来鉴定大肠杆菌中不受葡萄糖抑制的木糖转运体,通过基因挖掘,鉴定出了AraE和木糖ABC转运蛋白(AraFGH),在没有阿拉伯糖诱导的情况下,转录调节因子araC (L156I)的点突变导致araE和araFGH基因的表达上调,而araE (D223Y)的点突变进一步增强了木糖的转运,这一发现促进了葡萄糖和木糖的同步消耗[61]。结合代谢工程和生长耦联的自适应进化,对敲除xylFGH的E. coli工程菌进行进化培养后获得能够高效发酵木糖产D-乳酸的菌株;通过基因组测序和定量蛋白质组学分析,发现新型木糖转运蛋白GatC,显著提高了该进化菌株对木糖的生长和消耗速率[62]。引入了ABC型木糖转运系统的Z. mobilis重组菌株在葡萄糖存在的情况下木糖的利用率和乙醇产率均有所提高[63]。
3.2 理性路径工程 3.2.1 基于氧化还原途径的微生物代谢工程优化XR-XDH途径是强化微生物木糖代谢的一种有效策略。多项研究聚焦于将来自树干毕赤酵母的XYL1和XYL2基因在S. cerevisiae中表达[64-65]。通过表达来源于S. stipites的XK、XR和XDH,重组菌株S. cerevisiae HX57D的木糖消耗和乙醇产量显著提高[66]。已有文献报道K. marxianus IIPE453可以将葡萄糖和木糖转化为木糖醇和乙醇[67],为了进一步提高木糖醇产量,在出发菌中过表达内源XR基因,优化后的菌株K. marxianus IIPE453t的D-木糖还原酶转录水平提高了2.1倍,木糖醇产量提高了1.6倍,并且不影响其乙醇发酵能力[68]。在出芽短梗霉(Aureobasidium pullulan)中过表达XR和XDH基因,木糖消耗比出发菌株增加了17.8%,在模拟生物质水解液中也表现出良好的发酵性能,普鲁兰多糖的产量比出发菌株提高了97.7%[69]。在热带假丝酵母(Candida tropicalis)中,通过异源表达来源于粗糙脉孢菌(Neurospora crassa)的NcXR基因,使重组菌株克服了葡萄糖对XR-XDH途径的抑制作用,并且木糖醇产量从出发菌株的0.83 g/(L·h)提升至1.44 g/(L·h)[70]。
然而,XR和XDH具有不同的辅因子偏好性,XR倾向于使用NADPH作为辅因子,而XDH只使用NAD+产生NADH,因此在木糖利用时导致了辅因子不平衡和副产物(例如木糖醇和甘油)的积累。研究者已经通过多种策略,改善了细胞中XR-XDH途径的辅酶平衡,包括改变XR和XDH辅酶的偏好、调整XR和XDH的表达水平、操纵内源氧化还原途径或引入异源NADH依赖的反应[71-72]。研究表明,辅因子结合口袋的定点诱变成功地改变了S. cerevisiae工程菌中XR和XDH的辅因子偏好性。表达NADH偏好的XR突变体(K270M, K270R, K270R/N272D, N272D/P275Q, R276H)与野生型NAD+特异性XDH相结合提高了乙醇产量,并降低了木糖醇积累[73]。类似地,表达野生型XR和NADP+特异性XDH突变体(D207A/I208R/F209S/N211R)将S. cerevisiae的乙醇产量增加了41%[74]。在S. cerevisiae D452-2中过表达P. stipitis来源的NADH偏好的木糖还原酶突变体(XRMUT)和NAD+依赖的XDH以及内源性XK,使工程菌SX2MUT消耗22.9 g/L的木糖仅产生了0.24 g/L的木糖醇,经过辅酶改造的工程菌显著减少发酵副产物的积累[75]。利用蛋白工程技术中的定点突变,在P. stipitis中构建了一个严格依赖NADPH的XR,NADPH能够在XR和XDH之间有效循环,改造后的木糖还原酶对NADPH的催化活性与野生型XR相比增加了1.3倍[76]。上述研究结果表明,蛋白质工程在改造辅酶专一性、降低副产物积累等方面具有巨大潜力,有助于提高木糖利用效率。此外,对胞内辅酶浓度的测定结果表明维持NADPH/NADP+和NADH/NAD+的比例对重组酿酒酵母的木糖高效发酵制造乙醇至关重要。在S. cerevisiae中异源表达P. tipitis的XR、XDH和XK,敲除ZWF1基因(编码葡萄糖-6-磷酸脱氢酶),并过表达来源于乳酸克鲁维酵母(Kluyveromyces lactis)的NADP+依赖型D-甘油醛-3-磷酸脱氢酶(glyceraldehyde-3-phosphate dehydrogenase, GAPDH),构建了将木糖转化为乙醇的S. cerevisiae工程菌,与出发菌株相比,其乙醇产量增加了50%[77]。该策略通过在具有D-木糖代谢途径的S. cerevisiae工程菌中引入辅酶相关的代谢途径,减少NADPH的旁路消耗,增加NADH利用率,实现了辅酶平衡,提高了木糖代谢效率。将来源于乳酸乳球菌(Lactococcus lactis)的noxE基因引入到木糖利用S. cerevisiae KAM-3X中;在XDH利用NAD+的同时,通过水形成的NADH氧化酶产生NAD+,建立了辅酶微循环,使过表达noxE的工程菌乙醇产量增加39.33%[78]。
Weimberg途径从霉实假单胞菌(Pseudomonas fragi)、新月柄杆菌(Caulobacter crescentus)和嗜盐古菌(Halophilic archaea)等菌株中被鉴定出来[20],在C. crescentus中的研究最为深入[19]。Weimberg途径是指木糖代谢为α-酮戊二酸的氧化非磷酸化过程,相比于XR-XDH途径,该途径终产物为α-酮戊二酸,可绕过磷酸戊糖途径直接将戊糖同化到三羧酸循环中,并且该途径不产生二氧化碳,碳得率更高。研究人员在S. cerevisiae中表达C. crescentus的xylD、xylX、xylA和谷氨酸棒状杆菌(Corynebacterium glutamicum)的ksaD,并敲除FRA2基因,构建了酿酒酵母的Weimberg通路,用于木质纤维素水解产物利用[79]。通过在B. subtilis中引入异源Weimberg途径,在葡萄糖耗尽后诱导γ-聚谷氨酸(γ-polyglutamic acid, γ-PGA)合成酶的表达,所构建的木糖利用工程菌B. subtilis PX43与天然木糖异构酶菌株相比,γ-PGA滴度提高了6倍[80]。此外,利用木糖诱导启动子将Weimberg途径引入恶臭假单胞菌(Pseudomonas putida)中,使其具备了利用木糖的能力,通过定向进化、基因组重测序和反向代谢工程,构建了能够同步利用葡萄糖和木糖的菌株P. putida 8EM42,该菌株生产3-羟基丙酸的能力得到显著改善[81]。
Dahms途径与Weimberg途径相似,不同之处是2-酮-3-脱氧木糖酸(2-keto-3-deoxyxylonate,Kdx)在醛缩酶催化下生成丙酮酸酯和乙醇醛。通过在S. cerevisiae中表达Dahms途径,包括过表达C. crescentus中的xylB和xylD以及E. coli中的yagE或yjhH和aldA,使S. cerevisiae工程菌能够利用木糖生产乙醇酸(150 mg/L)和乙二醇(14 mg/L)[82]。将E. coli中的EMP和PPP完全失活,导致葡萄糖流向芳香族途径,构建黏糠酸的平行代谢途径的同时引入木糖Dahms途径到宿主菌株,使木糖转化为丙酮酸和乙醛酸,进入TCA循环,这一过程不仅实现了高产黏糠酸和L-酪氨酸,还促进了微生物细胞生长[11]。以不同的合成启动子调节E. coli中Dahms途径的代谢通量,获得了能够利用木糖合成聚乳酸-聚羟基乙酸共聚物的工程菌[83]。
3.2.2 基于非氧化还原途径的微生物代谢工程除了上述氧化还原途径,大多数原核生物和一些真菌通过XI途径直接将木糖转化为木酮糖,不受氧化还原平衡的影响。筛选具有高体内活性的木糖异构酶一直是通过XI途径工程改造木糖发酵的研究重点[84]。研究者从各种真菌和细菌中探索新型XI酶,并通过外源引入XI途径构建重组菌株,用于代谢木糖和生物合成[85]。从牛瘤胃宏基因组文库中筛选出的Ru-xylA在S. cerevisiae中功能性表达,并通过诱变和生长筛选,获得了含有2个突变的Ru-xylA (K11T/D220V)突变体,使酶活性增加68%[86]。研究人员利用功能宏基因组学工具鉴定出山羊瘤胃微生物组中一种新的木糖异构酶,编码该酶的基因XYSC1在S. cerevisiae中成功表达后,重组菌株能够以木糖为唯一碳源生长[87]。近年来,随着人工智能的发展,在系统发育指导下,通过对大数据的挖掘,结合合理修饰和祖先序列重建策略,探索出了适用于S. cerevisiae的新型木糖异构酶,利用所挖掘的木糖异构酶XIs构建的S. cerevisiae CRD5HS在无须解毒或洗涤处理的玉米秸秆和玉米芯中分别实现了85.95 g/L和94.76 g/L的乙醇产量[88]。本实验室将来源于Piromyces sp.和Lachnoclostridium phytofermentans的PspXI和LpXI在多形汉逊酵母(Ogataea polymorpha)工程菌中过表达,并强化了内源XYL3的表达,提高了木糖的分解代谢,通过系统改造O. polymorpha,强化了木糖同化与转运效率,实现葡萄糖与木糖同步利用,并通过增强乙酰辅酶A和NADPH的供应,建立了高产乙酰辅酶A衍生物脂肪酸和3-羟基丙酸(3-hydroxypropionic acid, 3-HP)的木质纤维素生物炼制平台;其中,过表达XYL3在提高木糖利用率方面发挥最重要的作用[89]。此外,在卷枝毛霉(Mucor circinelloides)基因组中也发现了XI途径,通过在M. circinelloides中过表达编码XI的XYLA和编码XK的XKS1基因,增强了木糖利用和脂质积累[90]。
在以木糖为碳源的生物合成中,通常以乙酰辅酶A为中间体生成产物。丙酮酸脱羧生成乙酰辅酶A的过程中会产生一分子CO2,丙酮酸不能完全转化为生物制品。在木糖代谢的磷酸转酮酶(phosphoketolase, PK)途径中,乙酰辅酶A是由PK催化产生,避免了经过丙酮酸脱羧反应生成乙酸辅酶A产生的碳损失,因此通过PK途径从木糖进行生物合成的碳得率较高[91]。S. cerevisiae将木糖从木质纤维素转化为乙醇的过程中,NADH的再氧化限制了木糖的利用,因此异源表达磷酸转酰基酶和乙醛脱氢酶基因,结合天然磷酸转酮酶,在S. cerevisiae TMB3001c中构建功能性PK途径,能够提高S. cerevisiae工程菌的乙醇产量和木糖消耗[92]。通过灭活E. coli中的核糖-3-磷酸差向异构酶,并引入外源PK途径,引导碳通量至木糖高效利用方向,阻断乙酸生成途径,同时加强乙酰辅酶A的合成,促进了下游产物聚3-羟基丁酸脂积累[93]。
3.2.3 基于磷酸戊糖途径的微生物代谢工程木糖通过XI或XR/XDH途径转化为木酮糖-5-磷酸后,大部分木酮糖-5-磷酸进入磷酸戊糖途径进行进一步代谢。磷酸戊糖途径是将D-木酮糖整合到中心碳代谢的关键切入点,因此该途径中相关酶的活性对木糖的有效同化至关重要[94]。研究表明,磷酸戊糖途径中的关键限制节点主要包括核糖-5-磷酸异构酶(ribose-5-phosphate isomerase, Rki)、核酮糖-5-磷酸差向异构酶(ribulose-5-phosphate epimerase, Rpe)、转酮醇酶(transketolase, Tkl)、转醛醇酶(transaldolase, Tal)和木酮糖激酶[8]。PPP非氧化部分基因(RKI1、RPE1、TKL1和TAL1)在表达XR-XDH或XI的S. cerevisiae菌株基础上共表达,均可改善重组菌株在木糖中的生长情况。具体而言,在木糖利用工程菌S. cerevisiae TMB3050中将XI与PPP途径结合,过表达编码整个非氧化磷酸戊糖途径的基因,发现TAL、TKL、RKI和RPE的表达促进了木糖的利用,重组菌株的生长速率比出发菌株高30倍[95]。此外,在表达XI的S. cerevisiae中,异源过表达来源于K. marxianus的RKI1、TAL1和TKL1基因后,发现木糖利用是对照菌株的1.87倍,并且高温(38 ℃)条件下,工程菌S. cerevisiae YK246的乙醇产量能够达到1.03 g/(L·h)[96]。与葡萄糖代谢相比,PPP具有更高的代谢通量,该特性在生产源于PPP中间体的化学品方面是有益的。景天庚酮糖-7-磷酸(sedoheptulose-7-phosphate, S7P)的积累是微生物高效利用木糖发酵的一个显著瓶颈。但最近的一项研究表明通过强化S. cerevisiae的磷酸戊糖途径,重组菌株利用了S7P的积累,从木糖和葡萄糖混合物中产生了31.0 mg/L的shinorine,它是一种小分子的类菌胞素氨基酸(mycosporine-like amino acid, MAA),具有防晒功能,需要以S7P作为生物合成的前体[97]。除了S. cerevisiae以外,调控磷酸戊糖途径的代谢工程策略,也被成功应用于改善大肠杆菌对木糖的利用中。在E. coli中过表达磷酸戊糖途径基因和敲除糖酵解途径基因,促进NADPH的供应,从而显著提升了木糖醇产量;与对照菌株相比,NADPH增强菌株从玉米芯水解物中生产的木糖醇增加了13.3%[97](表 1)。
| Strategy |
Modified strains | Gene/protein modification | Substrates | References | |
| Eliminate CCR effects | E. coli JH15 | ptsG | D-glucose and D-xylose | [25] | |
| E. coli BuT-8-PDH | ptsG, glf (Z. mobilis) | D-glucose and D-xylose | [26] | ||
| E. coli IS5-dI | ptsG, crp | Corncob hydrolysates | [98] | ||
| S. cerevisiae CBP.CR2 | MIG1 | D-glucose and D-xylose | [99] | ||
| K. marxianus YZB194 | PGI1, XYL2, KU70, GPD1 | D-glucose and D-xylose | [100] | ||
| S. cerevisiae DA24-16-BT3 | CDT1, GH1-1 (N. crassa) | D-glucose, cellobiose and D-xylose | [35] | ||
| E. coli CLGA8 | cep94A | Cellobiose and D-xylose | [37] | ||
| B. coagulans NL01 | celA, celB, celC, celR, celX, pbgl | Cellobiose and D-xylose | [38] | ||
| Transmembrane transport of xylose | S. cerevisiae TMB 3201 | Gxf1 and Gxs1 | D-glucose and D-xylose | [50] | |
| S. cerevisiae TMB3403 | Gxf1, Sut1 and At5g59250 | D-glucose and D-xylose | [54] | ||
| S. cerevisiae EBY.VW4000 | CiGXS1 transporter | D-xylose | [55] | ||
| S. cerevisiae D452-2 | araE, XYL1 | D-xylose | [56] | ||
| S. cerevisiae EBY.VW4000 | XlaA | D-xylose | [57] | ||
| E. coli HQ304 | AraE, GalABC, AraFGH (ABC transporter) | D-glucose and D-xylose | [61] | ||
| E. coli JU15 | fgh, gatC | D-xylose | [62] | ||
| Z. mobilis | XylE (H+ symporter), XylFGH (ABC transporter) | D-glucose and D-xylose | [63] | ||
| Xylose metabolism pathway |
Redox pathways |
S. cerevisiae HX57D | XR, XDH and XK (S. stipitis) |
D-glucose and D-xylose | [66] |
| K. marxianus IIPE453t | XR (native strain) | D-glucose and D-xylose | [68] | ||
| A. pullulan CBS 110374 |
XR, XDH (Spathaspora passalidarum) | D-glucose and D-xylose | [69] | ||
| C. tropicalis LNG2 | XR (N. crassa) | D-glucose and D-xylose | [70] | ||
| S. cerevisiae SX2MUT | XRMUT and XDH (P. stipitis) XK (native strain) |
D-glucose and D-xylose | [75] | ||
| P. stipitis | XR (NADPH-dependent) | D-xylose | [76] | ||
| S. cerevisiae H2684 | XR, XDH and XK (P. tipitis), ZWF1, GAPDH (NADP dependent) |
D-glucose and D-xylose | [77] | ||
| S. cerevisiae KAM-3X | noxE (L. lactis) | D-xylose | [78] | ||
| S. cerevisiae TMB4590 | xylD, xylX and xylA (C. crescentu), kasD (C. glutamicum) |
D-xylose | [79] | ||
| B. subtilis PX43 | Pgs | D-glucose and D-xylose | [80] | ||
| P. putida 8EM42 | xylX, xylA, xylB, xylC, xylD | D-glucose and D-xylose | [81] | ||
| S. cerevisiae H4311 | xylB and xylD (C. crescentus), agE/yjhH and aldA (E. coli) |
D-xylose | [82] | ||
| E. coli GX1xMA | xdh, xyl, yjhH and yjhG | D-glucose and D-xylose | [11] | ||
| E. coli X17ld | xlyB and xlyC (C. crescentu) | D-glucose and D-xylose | [83] | ||
| Non-redox pathways | S. cerevisiae CRD5HS | Xis | corncob hydrolysates | [88] | |
| O. polymorpha | PspXI (Piromyces sp.), LpXI (L. phytofermentans), XYL3 | D-glucose and D-xylose | [89] | ||
| M. circinelloides | XI and XK | D-xylose | [90] | ||
| S. cerevisiae TMB3001c | Acdh (E. histolytica), xfp (B. lactis) and pta (B. subtilis) |
D-xylose | [92] | ||
| E. coli | xfp, pta | D-xylose | [93] | ||
| Pentose phosphate pathways |
S. cerevisiae TMB3050 | XK, TAL, TKL, RKI, RPE | D-xylose | [95] | |
| S. cerevisiae YK246 | RKI1, TAL1 and TKL1 (K. marxianus) |
D-glucose and D-xylose | [96] | ||
| S. cerevisiae | TAL1, STB5 and TKL1 | D-glucose and D-xylose | [97] | ||
| E.coli W3110 | zwf, gnd, pfkA, pfkB, pgi | corncob hydrolysates | [98] | ||
尽管通过代谢工程获得的重组菌株能够利用木糖,但复杂的多基因特征较难改造,因此高效共利用葡萄糖和木糖仍然是一个关键挑战。为了克服理性代谢工程的局限性,一种自下而上的替代策略被广泛使用,称为反向代谢工程[101]。在此背景下,基于随机突变和系统选择的进化工程,也被称为适应性实验室进化(adaptive laboratory evolution, ALE),是提高木糖利用的一种常见而有效的策略。代谢和进化工程策略是相互交织的,许多葡萄糖与木糖共利用的代谢工程菌株可以通过ALE策略进一步改进[102]。
研究人员采用多种诱变策略和组学技术相结合的方法,包括基因组测序、转录组学、蛋白质组学、代谢组学以及通量组学等,寻找潜在基因靶标,从而提高微生物在同步利用葡萄糖和木糖方面的发酵性能,并揭示增强木糖同化的遗传分子机制。组学辅助的进化工程被证明是了解木糖代谢生理状态和发现基因扰动靶标以增强木糖发酵的有效方法。
研究人员利用基因组尺度模型和适应性实验室进化,开发了同时利用木质纤维素生物质水解物中的葡萄糖和木糖的各类工程菌。将E. coli中的乳酸脱氢酶基因ldhA、富马酸还原酶基因frdA和丙酮酸甲酸裂解酶基因pflB敲除,同时将丙酮酸脱氢酶基因pdh的启动子替换为甘油醛-3-磷酸脱氢酶基因gapA的启动子,并对E. coli工程菌进行适应性实验室进化;在生物反应器中以0.01/h的稀释率进行葡萄糖和木糖的恒化培养7 d,然后在含有木糖的琼脂平板上传代10 d,并在木糖浓度梯度培养基中传代41 d,进化后菌株E. coli SCD78能够在36 h内同时消耗10 g/L的葡萄糖和木糖,且实现了高乙醇产量;蛋白质组学数据表明,TCA循环蛋白、呼吸相关蛋白和部分转运蛋白显著上调,这可能在增加糖的总消耗和糖的共利用中发挥作用[103]。利用Z. mobilis作为宿主菌,将代谢工程与ALE相结合,成功开发了能够在高浓度下高效利用葡萄糖和木糖的工程菌Z. mobilis 8b-S38,该菌株携带来自黄胸散白蚁(Reticulitermes flaviceps)的木糖异构酶基因RsXI和来自E. coli的xylB,在含有50 g/L木糖的培养基中连续进行了38次的传代培养;结果表明8b-S38在不同木糖浓度(50–150 g/L)下的木糖消耗速率均快于出发菌,并且乙醇产量提高了60%–140%;对进化菌株进行基于下一代测序技术(next generation sequencing, NGS)的基因组重测序和RNA-Seq转录组学研究表明,在木糖培养基中,Z. mobilis 8b-S38中编码分子伴侣蛋白、ATP依赖性蛋白酶、噬菌体冲击蛋白、核糖体蛋白、鞭毛操纵子和转录调节因子的基因表达水平显著增加;这些基因的上调表达可能有助于维持正常的细胞代谢和生长,修复细胞损伤,并重新平衡细胞能量,帮助细胞抵御压力环境[104]。ALE也被用来提高S. cerevisiae对木糖的利用能力,以2% D-木糖为唯一碳源,经过12次传代,重组菌株在含D-木糖的培养基中生长显著增强。对进化菌株进行全基因组重测序,然后进行反向代谢工程,发现了2个单核苷酸突变,分别位于CCR4和TIF1基因上,使酵母的特定生长速率提高了23%和14%;值得关注的是,这些基因在之前的文献报道中并未被确认与D-木糖的利用相关。随后对异丁醇途径中关键酶的表达水平进行微调,使进化菌株异丁醇产量提高了110%[105]。相关进展汇总于表 2。
| Organism/Strain | ALE method | Results/Outcome | References |
| E. coli B with deletion of ldhA, frdA and pflB and replacement of pdh with gapA, followed by deletion of of ptsG | At a dilution rate of 0.01/h, constant cultivation of glucose and xylose was conducted for 7 days, followed by subculturing on xylose-containing agar plates for 10 days, and subsequent serial passaging for 41 days in a xylose concentration gradient medium | Adapted strain E. coli SCD78 exhibited simultaneous consumption of 10 g/L glucose and xylose within 36 h, coupled with high ethanol yield | [103] |
| E. coli MG1655 with deletion of ptsH and ldhA and replacement of pflB with pdc and adhB from Z. mobilis | Culture was serially transferred 5 times on 10 g/L glucose, then 5 times on a mixture of 6 g/L glucose and 4 g/L xylose, before finally being transferred to a medium containing 10 g/L glucose and 5% ethanol | Adapted strain E. coli JK32E demonstrated enhanced co-utilization of glucose and xylose, ultimately yielding high concentrations of ethanol following the introduction of plasmid carrying Z. mobilis pdc and adhB | [106] |
| Z. mobilis with the RsXI from R. flaviceps and xylB from E. coli |
Culture was transferred 38 times in a medium containing 50 g/L xylose | Adapted strain Z. mobilis 8b-S38 exhibited significantly increased rates of xylose consumption and ethanol production | [104] |
| Z. mobilis ZW658 carries genes associated with xylose metabolism from E. coli within its genome | Serial transfers of culture with increasing concentrations of xylose from 3% to 10% (50 transfers), followed by single colony isolations, for over 200 d in total | The evolved strain AD50 demonstrated a 1.65-fold increase in xylose uptake rate, enabling efficient co-utilization of glucose and xylose and leading to high ethanol production | [107] |
| S. cerevisiae PWY2353 with integrated xylose transport and metabolism, with GRE3 overexpression and PHO13 deletion |
12 times transfers with 2% D-xylose as the sole carbon source | The evolved strain S. cerevisiae PWY2353ev34b exhibits significantly enhanced growth in a culture medium containing D-xylose, with a 110% increase in isobutanol production | [105] |
合成生物学和代谢工程等新兴领域的快速发展拓展了单菌体系的功能,为大宗或高附加值化学品的高效生物合成提供了广阔前景。然而重组菌株中异源途径的过表达会增加底盘细胞的代谢压力,导致胞内能量分配失衡,进而影响细胞的正常生长和目标产物的合成[108]。混菌发酵是一种微生物混合共培养的发酵技术,其通过在同一培养体系中同时培养两种或两种以上微生物,模拟自然共生环境,实现不同物种之间物质、能量和信号交流,从而达到代谢分区的目的。混合发酵通过共培养微生物之间的协同代谢、诱导效应、基因转移以及种群间的感应等使途径的全局调控更灵活,代谢途径和功能被合理分割并分配给不同宿主,使复杂底物和中间产物更高效转化为终产物,能够维持发酵系统的持续稳定运行,在减轻宿主代谢负担、提供适宜的酶催化环境以及底物共同利用等方面具有显著优势[109]。
混菌共培养具有高效的底物转化能力,通过结合不同菌株优势,利用种间相互作用,解决部分微生物不能同步利用葡萄糖和木糖的限制[110]。在人工多菌体系中,通过在不同菌株中构建葡萄糖和木糖的平行利用途径,各菌株基于专有的代谢途径缓解底物共利用中的CCR,从而实现葡萄糖和木糖的同时利用。具体而言,合成及分解代谢正交的E. coli-E. coli共培养体系中包含了只利用葡萄糖的野生型E. coli和只利用木糖的工程菌E. coli LY180,通过简单调整两种菌之间的接种比例,有效平衡了净分解代谢活性,在批式发酵中实现了对葡萄糖-木糖混合物(质量比2:1)的共同利用(100 g/L总糖的98%),并生产46 g/L乙醇,与LY180单菌培养获得的乙醇产量(36 g/L)相比有显著提高[111]。嗜鞣管囊酵母(Pachysolen tannophilus)是能够利用木糖产乙醇的天然酵母,将Z. mobilis CCT 4494和P. tannophilus CCT 1891进行混菌发酵,以香蕉皮的水解产物为底物,并首先接种Z. mobilis CCT 4494,加速了葡萄糖的利用,从而缓解CCR。与单菌株培养相比,共培养体系的乙醇产量更高(11.32 g/L),达到最高理论产率的77.59%[112]。
经过代谢工程改造的微生物都包含了最适合承载的代谢途径,将功能性代谢途径分配到不同宿主菌中,利用混菌发酵同步利用葡萄糖和木糖可以避开复杂的微生物代谢调控过程[113]。通过模块化构建策略建立的E. coli-P. putida共培养体系包含了用于合成中长链脂肪酸(medium chain length polyhydroxyalkanoates, mcl-PHAs)的P. putida KT2440和敲除了ptsG、manZ、envR和atpFH基因的E. coli重组菌株。P. putida KT2440利用葡萄糖以及E. coli工程菌分泌的乙酸和脂肪酸来合成mcl-PHAs,而E. coli工程菌更倾向于利用木糖而缓慢利用葡萄糖,因此降低了两个菌株之间的生长竞争。在葡萄糖-木糖混合碳源中,共培养体系的mcl-PHAs产量显著增加,从实际木质纤维素水解产物中获得了0.469 g/L的mcl-PHAs[114]。利用类似原理的混菌共培养体系从葡萄糖和木糖的混合物中已经实现了其他高价值产品的生物合成,例如乙醇[2]、乳酸[115]、黏糠酸、4-羟基苯甲酸[116]和正丁醇[26, 117]。
此外,混菌共培养技术还通过降低生物质水解物的毒性来促进微生物对葡萄糖和木糖的转化效率[118-120]。在S. cerevisiae Y5与P. stipitis CBS6054共培养体系中,以未经脱毒处理的木质纤维素水解物为碳源,至木糖全部耗尽共发酵96 h,最终乙醇浓度为27.4 g/L,产率达到理论值的85.1%;该工艺的实施使高浓度糠醛和羟甲基糠醛环境下的木质纤维素材料的预处理与利用成为可能,对于木质纤维素的高效利用而言,是一项重大进展[118]。
4 总结与展望微生物高效共利用葡萄糖和木糖的代谢工程研究飞速发展并取得重要成果。利用多种代谢工程策略,包括基因编辑、代谢通路优化和宿主工程等手段,已成功提高了微生物对葡萄糖和木糖的利用效率,从而实现了对这两种碳源的高效利用和协同代谢。此外,一些新型微生物代谢工程平台的建立,如非常规酵母、细菌和真菌等,为深入探究葡萄糖和木糖代谢途径的工程化提供了新的思路和技术支持。
然而,当前研究还存在一些挑战和待解决的问题。首先,目前构建的微生物菌株在代谢途径稳定性、产物选择性以及耐受性等方面仍然存在改进的空间。其次,由于生物质的组成复杂性和多样性,微生物在利用复杂底物时可能面临着代谢途径工程和调控的困难。此外,微生物在工业生产过程中可能受到废物积累、抑制物质以及操作条件等因素的影响,因此需要进一步优化工艺条件和提高菌株的稳定性和耐受性。
未来的研究可以更多关注以下几个方面:(1) 深入解析微生物在复杂碳源混合条件下的代谢调控机制,通过系统生物学和代谢组学手段揭示微生物的适应性机制,为菌株改造和优化提供理论指导。(2) 探索新的基因编辑和代谢工程技术,例如CRISPR-Cas9系统的应用、合成生物学工具的开发等,以加速菌株构建和优化的速度和效率。(3) 加强微生物对纤维素水解液的耐受性,开发新的生物质预处理和转化技术,提高生物质的利用效率和降低生产成本。综上所述,对微生物在葡萄糖和木糖的高效同步利用方面的研究将为可持续能源和生物化工领域的发展提供新的思路和技术支持。
| [1] |
ZHENG XJ, XIAN XL, HU L, TAO SH, ZHANG XD, LIU Y, LIN XQ. Efficient short-time hydrothermal depolymerization of sugarcane bagasse in one-pot for cellulosic ethanol production without solid-liquid separation, water washing, and detoxification[J]. Bioresource Technology, 2021, 339: 125575. DOI:10.1016/j.biortech.2021.125575
|
| [2] |
WANG L, YORK SW, INGRAM LO, SHANMUGAM KT. Simultaneous fermentation of biomass-derived sugars to ethanol by a co-culture of an engineered Escherichia coli and Saccharomyces cerevisiae[J]. Bioresource Technology, 2019, 273: 269-276. DOI:10.1016/j.biortech.2018.11.016
|
| [3] |
ASEMOLOYE MD, BELLO TS, OLADOYE PO, REMILEKUN GBADAMOSI M, BABARINDE SO, EBENEZER ADEBAMI G, OLOWE OM, TEMPORITI MEE, WANEK W, MARCHISIO MA. Engineered yeasts and lignocellulosic biomaterials: shaping a new dimension for biorefinery and global bioeconomy[J]. Bioengineered, 2023, 14(1): 2269328. DOI:10.1080/21655979.2023.2269328
|
| [4] |
MOYSÉS DN, REIS VCB, de ALMEIDA JRM, de MORAES LMP, TORRES FAG. Xylose fermentation by Saccharomyces cerevisiae: challenges and prospects[J]. International Journal of Molecular Sciences, 2016, 17(3): 207. DOI:10.3390/ijms17030207
|
| [5] |
HOU J, QIU CX, SHEN Y, LI HX, BAO XM. Engineering of Saccharomyces cerevisiae for the efficient co-utilization of glucose and xylose[J]. FEMS Yeast Research, 2017, 17(4): fox034.
|
| [6] |
BUIJS NA, SIEWERS V, NIELSEN J. Advanced biofuel production by the yeast Saccharomyces cerevisiae[J]. Current Opinion in Chemical Biology, 2013, 17(3): 480-488. DOI:10.1016/j.cbpa.2013.03.036
|
| [7] |
PARK JM, VINUSELVI P, LEE SK. The mechanism of sugar-mediated catabolite repression of the propionate catabolic genes in Escherichia coli[J]. Gene, 2012, 504(1): 116-121. DOI:10.1016/j.gene.2012.04.074
|
| [8] |
CHANDUKISHORE T, DAS S, DAS P, VEERANKI VD, PRABHU AA. Re-routing the hemicellulosic fraction of lignocellulosic biomass toward value added products: a pragmatic bio refinery approach[J]. Journal of Environmental Chemical Engineering, 2024, 12(2): 111971. DOI:10.1016/j.jece.2024.111971
|
| [9] |
DONZELLA L, VARELA JA, SOUSA MJ, MORRISSEY JP. Identification of novel pentose transporters in Kluyveromyces marxianus using a new screening platform[J]. FEMS Yeast Research, 2021, 21(4): foab026. DOI:10.1093/femsyr/foab026
|
| [10] |
CHUPAZA MH, PARK YR, KIM SH, YANG JW, JEONG GT, KIM SK. Bioethanol production from Azolla filiculoides by Saccharomyces cerevisiae, Pichia stipitis, Candida lusitaniae, and Kluyveromyces marxianus[J]. Applied Biochemistry and Biotechnology, 2021, 193(2): 502-514. DOI:10.1007/s12010-020-03437-0
|
| [11] |
FUJIWARA R, NODA S, TANAKA T, KONDO A. Metabolic engineering of Escherichia coli for shikimate pathway derivative production from glucose-xylose co-substrate[J]. Nature Communications, 2020, 11: 279. DOI:10.1038/s41467-019-14024-1
|
| [12] |
BRAT D, BOLES E, WIEDEMANN B. Functional expression of a bacterial xylose isomerase in Saccharomyces cerevisiae[J]. Applied and Environmental Microbiology, 2009, 75(8): 2304-2311. DOI:10.1128/AEM.02522-08
|
| [13] |
HO NWY, CHEN ZD, BRAINARD AP. Genetically engineered Saccharomyces yeast capable of effective cofermentation of glucose and xylose[J]. Applied and Environmental Microbiology, 1998, 64(5): 1852-1859. DOI:10.1128/AEM.64.5.1852-1859.1998
|
| [14] |
SUN LL, WU B, ZHANG ZQ, YAN J, LIU PT, SONG C, SHABBIR S, ZHU QL, YANG SH, PENG N, HE MX, TAN FR. Cellulosic ethanol production by consortia of Scheffersomyces stipitis and engineered Zymomonas mobilis[J]. Biotechnology for Biofuels, 2021, 14(1): 221. DOI:10.1186/s13068-021-02069-8
|
| [15] |
LI XW, CHEN Y, NIELSEN J. Harnessing xylose pathways for biofuels production[J]. Current Opinion in Biotechnology, 2019, 57: 56-65. DOI:10.1016/j.copbio.2019.01.006
|
| [16] |
HOSSAIN SA, ŠVEC D, MRŠA V, TEPARIĆ R. Overview of catalytic properties of fungal xylose reductases and molecular engineering approaches for improved xylose utilisation in yeast[J]. Applied Food Biotechnology, 2018, 5(2): 47-58.
|
| [17] |
VALDEHUESA KNG, RAMOS KRM, NISOLA GM, BAÑARES AB, CABULONG RB, LEE WK, LIU HW, CHUNG WJ. Everyone loves an underdog: metabolic engineering of the xylose oxidative pathway in recombinant microorganisms[J]. Applied Microbiology and Biotechnology, 2018, 102(18): 7703-7716. DOI:10.1007/s00253-018-9186-z
|
| [18] |
de PAULA RG, ANTONIÊTO ACC, RIBEIRO LFC, SRIVASTAVA N, O'DONOVAN A, MISHRA PK, GUPTA VK, SILVA RN. Engineered microbial host selection for value-added bioproducts from lignocellulose[J]. Biotechnology Advances, 2019, 37(6): 107347. DOI:10.1016/j.biotechadv.2019.02.003
|
| [19] |
STEPHENS C, CHRISTEN B, FUCHS T, SUNDARAM V, WATANABE K, JENAL U. Genetic analysis of a novel pathway for D-xylose metabolism in Caulobacter crescentus[J]. Journal of Bacteriology, 2007, 189(5): 2181-2185. DOI:10.1128/JB.01438-06
|
| [20] |
ZHAO Z, XIAN M, LIU M, ZHAO G. Biochemical routes for uptake and conversion of xylose by microorganisms[J]. Biotechnology for Biofuels, 2020, 13: 21. DOI:10.1186/s13068-020-1662-x
|
| [21] |
LIU DF, ZHANG YL, LI JG, SUN WL, YAO YH, TIAN CG. The Weimberg pathway: an alternative for Myceliophthora thermophila to utilize D-xylose[J]. Biotechnology for Biofuels and Bioproducts, 2023, 16(1): 13. DOI:10.1186/s13068-023-02266-7
|
| [22] |
GÖRKE B, STÜLKE J. Carbon catabolite repression in bacteria: many ways to make the most out of nutrients[J]. Nature Reviews Microbiology, 2008, 6: 613-624. DOI:10.1038/nrmicro1932
|
| [23] |
STASYK OG, STASYK OV. Glucose sensing and regulation in yeasts[M]//Non-conventional Yeasts: from Basic Research to Application. Cham: Springer, 2019: 477-519.
|
| [24] |
KIM SM, CHOI BY, RYU YS, JUNG SH, PARK JM, KIM GH, LEE SK. Simultaneous utilization of glucose and xylose via novel mechanisms in engineered Escherichia coli[J]. Metabolic Engineering, 2015, 30: 141-148. DOI:10.1016/j.ymben.2015.05.002
|
| [25] |
丁小云, 顾健健, 王永泽, 赵锦芳, 王金华, 赵筱. 产D-乳酸重组大肠杆菌ptsG基因的敲除及其混合糖同步发酵[J]. 生物技术通报, 2015, 31(12): 221-226. DING XY, GU JJ, WANG YZ, ZHAO JF, WANG JH, ZHAO X. The knockout of gene ptsG of recombinant Escherichia coli producing d-lactic acid and the simultaneous fermentation of mixed sugars[J]. Biotechnology Bulletin, 2015, 31(12): 221-226 (in Chinese). |
| [26] |
SAINI M, LIN LJ, CHIANG CJ, CHAO YP. Synthetic consortium of Escherichia coli for n-butanol production by fermentation of the glucose-xylose mixture[J]. Journal of Agricultural and Food Chemistry, 2017, 65(46): 10040-10047. DOI:10.1021/acs.jafc.7b04275
|
| [27] |
YUAN XS, TU S, LIN JP, YANG LR, SHEN HH, WU MB. Combination of the CRP mutation and ptsG deletion in Escherichia coli to efficiently synthesize xylitol from corncob hydrolysates[J]. Applied Microbiology and Biotechnology, 2020, 104(5): 2039-2050. DOI:10.1007/s00253-019-10324-0
|
| [28] |
蔡艳青, 齐显尼, 齐奇, 蔺玉萍, 王正祥, 王钦宏. 敲除MIG1和SNF1基因对酿酒酵母共利用葡萄糖和木糖的影响[J]. 生物工程学报, 2018, 34(1): 54-67. CAI YQ, QI XN, QI Q, LIN YP, WANG ZX, WANG QH. Effect of MIG1 and SNF1 deletion on simultaneous utilization of glucose and xylose by Saccharomyces cerevisiae[J]. Chinese Journal of Biotechnology, 2018, 34(1): 54-67 (in Chinese). |
| [29] |
HUA Y, WANG JC, ZHU YL, ZHANG B, KONG X, LI WJ, WANG DM, HONG J. Release of glucose repression on xylose utilization in Kluyveromyces marxianus to enhance glucose-xylose co-utilization and xylitol production from corncob hydrolysate[J]. Microbial Cell Factories, 2019, 18(1): 24. DOI:10.1186/s12934-019-1068-2
|
| [30] |
SEMKIV MV, RUCHALA J, TSARUK AY, ZAZULYA AZ, VASYLYSHYN RV, DMYTRUK OV, ZUO MX, KANG YQ, DMYTRUK KV, SIBIRNY AA. The role of hexose transporter-like sensor hxs1 and transcription activator involved in carbohydrate sensing azf1 in xylose and glucose fermentation in the thermotolerant yeast Ogataea polymorpha[J]. Microbial Cell Factories, 2022, 21(1): 162. DOI:10.1186/s12934-022-01889-z
|
| [31] |
KIM SR, HA SJ, WEI N, OH EJ, JIN YS. Simultaneous co-fermentation of mixed sugars: a promising strategy for producing cellulosic ethanol[J]. Trends in Biotechnology, 2012, 30(5): 274-282. DOI:10.1016/j.tibtech.2012.01.005
|
| [32] |
KATAHIRA S, MIZUIKE A, FUKUDA H, KONDO A. Ethanol fermentation from lignocellulosic hydrolysate by a recombinant xylose- and cellooligosaccharide-assimilating yeast strain[J]. Applied Microbiology and Biotechnology, 2006, 72(6): 1136-1143. DOI:10.1007/s00253-006-0402-x
|
| [33] |
NAKAMURA N, YAMADA R, KATAHIRA S, TANAKA T, FUKUDA H, KONDO A. Effective xylose/cellobiose co-fermentation and ethanol production by xylose-assimilating S. cerevisiae via expression of β-glucosidase on its cell surface[J]. Enzyme and Microbial Technology, 2008, 43(3): 233-236. DOI:10.1016/j.enzmictec.2008.04.003
|
| [34] |
PARISUTHAM V, CHANDRAN SP, MUKHOPADHYAY A, LEE SK, KEASLING JD. Intracellular cellobiose metabolism and its applications in lignocellulose-based biorefineries[J]. Bioresource Technology, 2017, 239: 496-506. DOI:10.1016/j.biortech.2017.05.001
|
| [35] |
HA SJ, GALAZKA JM, KIM SR, CHOI JH, YANG XM, SEO JH, GLASS NL, CATE JHD, JIN YS. Engineered Saccharomyces cerevisiae capable of simultaneous cellobiose and xylose fermentation[J]. Proceedings of the National Academy of Sciences of the United States of America, 2011, 108(2): 504-509.
|
| [36] |
OH EJ, HA SJ, KIM SR, LEE WH, GALAZKA JM, CATE JHD, JIN YS. Enhanced xylitol production through simultaneous co-utilization of cellobiose and xylose by engineered Saccharomyces cerevisiae[J]. Metabolic Engineering, 2013, 15: 226-234. DOI:10.1016/j.ymben.2012.09.003
|
| [37] |
CABULONG RB, BAÑARES AB, NISOLA GM, LEE WK, CHUNG WJ. Enhanced glycolic acid yield through xylose and cellobiose utilization by metabolically engineered Escherichia coli[J]. Bioprocess and Biosystems Engineering, 2021, 44(6): 1081-1091. DOI:10.1007/s00449-020-02502-6
|
| [38] |
ZHENG ZJ, JIANG T, ZOU LH, OUYANG SP, ZHOU J, LIN X, HE Q, WANG LM, YU B, XU HJ, OUYANG J. Simultaneous consumption of cellobiose and xylose by Bacillus coagulans to circumvent glucose repression and identification of its cellobiose-assimilating operons[J]. Biotechnology for Biofuels, 2018, 11: 320. DOI:10.1186/s13068-018-1323-5
|
| [39] |
DIEN BS, NICHOLS NN, BOTHAST RJ. Fermentation of sugar mixtures using Escherichia coli catabolite repression mutants engineered for production of l-lactic acid[J]. Journal of Industrial Microbiology and Biotechnology, 2002, 29(5): 221-227. DOI:10.1038/sj.jim.7000299
|
| [40] |
YOUNG EM, TONG A, BUI H, SPOFFORD C, ALPER HS. Rewiring yeast sugar transporter preference through modifying a conserved protein motif[J]. Proceedings of the National Academy of Sciences of the United States of America, 2014, 111(1): 131-136.
|
| [41] |
LEANDRO MJ, FONSECA C, GONÇALVES P. Hexose and pentose transport in ascomycetous yeasts: an overview[J]. FEMS Yeast Research, 2009, 9(4): 511-525. DOI:10.1111/j.1567-1364.2009.00509.x
|
| [42] |
HAMACHER T, BECKER J, GÁRDONYI M, HAHN-HÄGERDAL B, BOLES E. Characterization of the xylose-transporting properties of yeast hexose transporters and their influence on xylose utilization[J]. Microbiology, 2002, 148(Pt 9): 2783-2788.
|
| [43] |
JEFFRIES TW, JIN YS. Metabolic engineering for improved fermentation of pentoses by yeasts[J]. Applied Microbiology and Biotechnology, 2004, 63(5): 495-509. DOI:10.1007/s00253-003-1450-0
|
| [44] |
YOUNG E, LEE SM, ALPER H. Optimizing pentose utilization in yeast: the need for novel tools and approaches[J]. Biotechnology for Biofuels, 2010, 3: 24. DOI:10.1186/1754-6834-3-24
|
| [45] |
JOJIMA T, OMUMASABA CA, INUI M, YUKAWA H. Sugar transporters in efficient utilization of mixed sugar substrates: current knowledge and outlook[J]. Applied Microbiology and Biotechnology, 2010, 85(3): 471-480. DOI:10.1007/s00253-009-2292-1
|
| [46] |
WEISSER P, KRÄMER R, SPRENGER GA. Expression of the Escherichia coli pmi gene, encoding phosphomannose-isomerase in Zymomonas mobilis, leads to utilization of mannose as a novel growth substrate, which can be used as a selective marker[J]. Applied and Environmental Microbiology, 1996, 62(11): 4155-4161. DOI:10.1128/aem.62.11.4155-4161.1996
|
| [47] |
DUNN KL, RAO CV. Expression of a xylose-specific transporter improves ethanol production by metabolically engineered Zymomonas mobilis[J]. Applied Microbiology and Biotechnology, 2014, 98(15): 6897-6905. DOI:10.1007/s00253-014-5812-6
|
| [48] |
YOUNG E, POUCHER A, COMER A, BAILEY A, ALPER H. Functional survey for heterologous sugar transport proteins, using Saccharomyces cerevisiae as a host[J]. Applied and Environmental Microbiology, 2011, 77(10): 3311-3319. DOI:10.1128/AEM.02651-10
|
| [49] |
SALOHEIMO A, RAUTA J, STASYK OV, SIBIRNY AA, PENTTILÄ M, RUOHONEN L. Xylose transport studies with xylose-utilizing Saccharomyces cerevisiae strains expressing heterologous and homologous permeases[J]. Applied Microbiology and Biotechnology, 2007, 74(5): 1041-1052. DOI:10.1007/s00253-006-0747-1
|
| [50] |
LEANDRO MJ, GONÇALVES P, SPENCER-MARTINS I. Two glucose/xylose transporter genes from the yeast Candida intermedia: first molecular characterization of a yeast xylose-H+ symporter[J]. The Biochemical Journal, 2006, 395(3): 543-549. DOI:10.1042/BJ20051465
|
| [51] |
WEIERSTALL T, HOLLENBERG CP, BOLES E. Cloning and characterization of three genes (SUT1-3) encoding glucose transporters of the yeast Pichia stipitis[J]. Molecular Microbiology, 1999, 31(3): 871-883. DOI:10.1046/j.1365-2958.1999.01224.x
|
| [52] |
DU J, LI SJ, ZHAO HM. Discovery and characterization of novel D-xylose-specific transporters from Neurospora crassa and Pichia stipitis[J]. Molecular BioSystems, 2010, 6(11): 2150-2156. DOI:10.1039/c0mb00007h
|
| [53] |
HECTOR RE, QURESHI N, HUGHES SR, COTTA MA. Expression of a heterologous xylose transporter in a Saccharomyces cerevisiae strain engineered to utilize xylose improves aerobic xylose consumption[J]. Applied Microbiology and Biotechnology, 2008, 80(4): 675-684. DOI:10.1007/s00253-008-1583-2
|
| [54] |
RUNQUIST D, HAHN-HÄGERDAL B, RÅDSTRÖM P. Comparison of heterologous xylose transporters in recombinant Saccharomyces cerevisiae[J]. Biotechnology for Biofuels, 2010, 3: 5. DOI:10.1186/1754-6834-3-5
|
| [55] |
LI HB, SCHMITZ O, ALPER HS. Enabling glucose/xylose co-transport in yeast through the directed evolution of a sugar transporter[J]. Applied Microbiology and Biotechnology, 2016, 100(23): 10215-10223. DOI:10.1007/s00253-016-7879-8
|
| [56] |
KIM H, LEE HS, PARK H, LEE DH, BOLES E, CHUNG D, PARK YC. Enhanced production of xylitol from xylose by expression of Bacillus subtilis arabinose: H+ symporter and Scheffersomyces stipitis xylose reductase in recombinant Saccharomyces cerevisiae[J]. Enzyme and Microbial Technology, 2017, 107: 7-14. DOI:10.1016/j.enzmictec.2017.07.014
|
| [57] |
SLOOTHAAK J, TAMAYO-RAMOS JA, ODONI DI, LAOTHANACHAREON T, DERNTL C, MACH-AIGNER AR, MARTINS dos SANTOS VAP, SCHAAP PJ. Identification and functional characterization of novel xylose transporters from the cell factories Aspergillus niger and Trichoderma reesei[J]. Biotechnology for Biofuels, 2016, 9: 148. DOI:10.1186/s13068-016-0564-4
|
| [58] |
ROJAS SAT, SCHADEWEG V, KIRCHNER F, BOLES E, OREB M. Identification of a glucose-insensitive variant of Gal2 from Saccharomyces cerevisiae exhibiting a high pentose transport capacity[J]. Scientific Reports, 2021, 11: 24404. DOI:10.1038/s41598-021-03822-7
|
| [59] |
JIANG Y, SHEN Y, GU LC, WANG ZZ, SU N, NIU KL, GUO W, HOU SL, BAO XM, TIAN CG, FANG X. Identification and characterization of an efficient D-xylose transporter in Saccharomyces cerevisiae[J]. Journal of Agricultural and Food Chemistry, 2020, 68(9): 2702-2710. DOI:10.1021/acs.jafc.9b07113
|
| [60] |
FARWICK A, BRUDER S, SCHADEWEG V, OREB M, BOLES E. Engineering of yeast hexose transporters to transport D-xylose without inhibition by D-glucose[J]. Proceedings of the National Academy of Sciences of the United States of America, 2014, 111(14): 5159-5164.
|
| [61] |
ZHU XN, FAN FY, QIU HN, SHAO MY, LI D, YU Y, BI CH, ZHANG XL. New xylose transporters support the simultaneous consumption of glucose and xylose in Escherichia coli[J]. mLife, 2022, 1(2): 156-170. DOI:10.1002/mlf2.12021
|
| [62] |
UTRILLA J, LICONA-CASSANI C, MARCELLIN E, GOSSET G, NIELSEN LK, MARTINEZ A. Engineering and adaptive evolution of Escherichia coli for D-lactate fermentation reveals GatC as a xylose transporter[J]. Metabolic Engineering, 2012, 14(5): 469-476. DOI:10.1016/j.ymben.2012.07.007
|
| [63] |
SARKAR P, GOSWAMI G, MUKHERJEE M, DAS D. Heterologous expression of xylose specific transporter improves xylose utilization by recombinant Zymomonas mobilis strain in presence of glucose[J]. Process Biochemistry, 2021, 102: 190-198. DOI:10.1016/j.procbio.2021.01.006
|
| [64] |
ELIASSON A, CHRISTENSSON C, WAHLBOM CF, HAHN-HÄGERDAL B. Anaerobic xylose fermentation by recombinant Saccharomyces cerevisiae carrying XYL1, XYL2, and XKS1 in mineral medium chemostat cultures[J]. Applied and Environmental Microbiology, 2000, 66(8): 3381-3386. DOI:10.1128/AEM.66.8.3381-3386.2000
|
| [65] |
HE BY, HAO B, YU HZ, TU F, WEI XY, XIONG K, ZENG YJ, ZENG H, LIU P, TU YY, WANG YT, KANG H, PENG LC, XIA T. Double integrating XYL2 into engineered Saccharomyces cerevisiae strains for consistently enhanced bioethanol production by effective xylose and hexose co-consumption of steam-exploded lignocellulose in bioenergy crops[J]. Renewable Energy, 2022, 186: 341-349. DOI:10.1016/j.renene.2021.12.103
|
| [66] |
XIE CY, YANG BX, WU YJ, XIA ZY, GOU M, SUN ZY, TANG YQ. Construction of industrial xylose-fermenting Saccharomyces cerevisiae strains through combined approaches[J]. Process Biochemistry, 2020, 96: 80-89. DOI:10.1016/j.procbio.2020.05.022
|
| [67] |
DASGUPTA D, GHOSH D, BANDHU S, ADHIKARI DK. Lignocellulosic sugar management for xylitol and ethanol fermentation with multiple cell recycling by Kluyveromyces marxianus IIPE453[J]. Microbiological Research, 2017, 200: 64-72. DOI:10.1016/j.micres.2017.04.002
|
| [68] |
DASGUPTA D, JUNGHARE V, NAUTIYAL AK, JANA A, HAZRA S, GHOSH D. Xylitol production from lignocellulosic pentosans: a rational strain engineering approach toward a multiproduct biorefinery[J]. Journal of Agricultural and Food Chemistry, 2019, 67(4): 1173-1186. DOI:10.1021/acs.jafc.8b05509
|
| [69] |
GUO J, HUANG SY, CHEN YF, GUO XW, XIAO DG. Heterologous expression of Spathaspora passalidarum xylose reductase and xylitol dehydrogenase genes improved xylose fermentation ability of Aureobasidium pullulans[J]. Microbial Cell Factories, 2018, 17(1): 64. DOI:10.1186/s12934-018-0911-1
|
| [70] |
JEON WY, YOON BH, KO BS, SHIM WY, KIM JH. Xylitol production is increased by expression of codon-optimized Neurospora crassa xylose reductase gene in Candida tropicalis[J]. Bioprocess and Biosystems Engineering, 2012, 35(1): 191-198.
|
| [71] |
GROTKJAER T, CHRISTAKOPOULOS P, NIELSEN J, OLSSON L. Comparative metabolic network analysis of two xylose fermenting recombinant Saccharomyces cerevisiae strains[J]. Metabolic Engineering, 2005, 7(5/6): 437-444.
|
| [72] |
ZHOU H, CHENG JS, WANG BL, FINK GR, STEPHANOPOULOS G. Xylose isomerase overexpression along with engineering of the pentose phosphate pathway and evolutionary engineering enable rapid xylose utilization and ethanol production by Saccharomyces cerevisiae[J]. Metabolic Engineering, 2012, 14(6): 611-622. DOI:10.1016/j.ymben.2012.07.011
|
| [73] |
WATANABE S, ABU SALEH A, PACK SP, ANNALURU N, KODAKI T, MAKINO K. Ethanol production from xylose by recombinant Saccharomyces cerevisiae expressing protein-engineered NADH-preferring xylose reductase from Pichia stipitis[J]. Microbiology, 2007, 153(Pt 9): 3044-3054.
|
| [74] |
WATANABE S, SALEH AA, PACK SP, ANNALURU N, KODAKI T, MAKINO K. Ethanol production from xylose by recombinant Saccharomyces cerevisiae expressing protein engineered NADP+-dependent xylitol dehydrogenase[J]. Journal of Biotechnology, 2007, 130(3): 316-319. DOI:10.1016/j.jbiotec.2007.04.019
|
| [75] |
LEE SH, KODAKI T, PARK YC, SEO JH. Effects of NADH-preferring xylose reductase expression on ethanol production from xylose in xylose-metabolizing recombinant Saccharomyces cerevisiae[J]. Journal of Biotechnology, 2012, 158(4): 184-191. DOI:10.1016/j.jbiotec.2011.06.005
|
| [76] |
KHATTAB SMR, WATANABE S, SAIMURA M, KODAKI T. A novel strictly NADPH-dependent Pichia stipitis xylose reductase constructed by site-directed mutagenesis[J]. Biochemical and Biophysical Research Communications, 2011, 404(2): 634-637. DOI:10.1016/j.bbrc.2010.12.028
|
| [77] |
VERHO R, LONDESBOROUGH J, PENTTILÄ M, RICHARD P. Engineering redox cofactor regeneration for improved pentose fermentation in Saccharomyces cerevisiae[J]. Applied and Environmental Microbiology, 2003, 69(10): 5892-5897. DOI:10.1128/AEM.69.10.5892-5897.2003
|
| [78] |
ZHANG GC, LIU JJ, DING WT. Decreased xylitol formation during xylose fermentation in Saccharomyces cerevisiae due to overexpression of water-forming NADH oxidase[J]. Applied and Environmental Microbiology, 2012, 78(4): 1081-1086. DOI:10.1128/AEM.06635-11
|
| [79] |
BORGSTRÖM C, WASSERSTROM L, ALMQVIST H, BROBERG K, KLEIN B, NOACK S, LIDÉN G, GORWA-GRAUSLUND MF. Identification of modifications procuring growth on xylose in recombinant Saccharomyces cerevisiae strains carrying the Weimberg pathway[J]. Metabolic Engineering, 2019, 55: 1-11. DOI:10.1016/j.ymben.2019.05.010
|
| [80] |
HALMSCHLAG B, HOFFMANN K, HANKE R, PUTRI SP, FUKUSAKI E, BÜCHS J, BLANK LM. Comparison of isomerase and weimberg pathway for γ-PGA production from xylose by engineered Bacillus subtilis[J]. Frontiers in Bioengineering and Biotechnology, 2020, 7: 476. DOI:10.3389/fbioe.2019.00476
|
| [81] |
TIWARI R, SATHESH-PRABU C, KIM Y, LEE SK. Simultaneous utilization of glucose and xylose by metabolically engineered Pseudomonas putida for the production of 3-hydroxypropionic acid[J]. Bioresource Technology, 2024, 395: 130389. DOI:10.1016/j.biortech.2024.130389
|
| [82] |
SALUSJÄRVI L, TOIVARI M, VEHKOMÄKI ML, KOIVISTOINEN O, MOJZITA D, NIEMELÄ K, PENTTILÄ M, RUOHONEN L. Production of ethylene glycol or glycolic acid from D-xylose in Saccharomyces cerevisiae[J]. Applied Microbiology and Biotechnology, 2017, 101(22): 8151-8163. DOI:10.1007/s00253-017-8547-3
|
| [83] |
CHOI SY, KIM WJ, YU SJ, PARK SJ, IM SG, LEE SY. Engineering the xylose-catabolizing Dahms pathway for production of poly(D-lactate-co-glycolate) and poly(D-lactate-co-glycolate-co-D-2-hydroxybutyrate) in Escherichia coli[J]. Microbial Biotechnology, 2017, 10(6): 1353-1364. DOI:10.1111/1751-7915.12721
|
| [84] |
KWAK S, JIN YS. Production of fuels and chemicals from xylose by engineered Saccharomyces cerevisiae: a review and perspective[J]. Microbial Cell Factories, 2017, 16(1): 82. DOI:10.1186/s12934-017-0694-9
|
| [85] |
KATAHIRA S, MURAMOTO N, MORIYA S, NAGURA R, TADA N, YASUTANI N, OHKUMA M, ONISHI T, TOKUHIRO K. Screening and evolution of a novel protist xylose isomerase from the termite Reticulitermes speratus for efficient xylose fermentation in Saccharomyces cerevisiae[J]. Biotechnology for Biofuels, 2017, 10: 203. DOI:10.1186/s13068-017-0890-1
|
| [86] |
HOU J, SHEN Y, JIAO CL, GE RL, ZHANG XJ, BAO XM. Characterization and evolution of xylose isomerase screened from the bovine rumen metagenome in Saccharomyces cerevisiae[J]. Journal of Bioscience and Bioengineering, 2016, 121(2): 160-165. DOI:10.1016/j.jbiosc.2015.05.014
|
| [87] |
de SOUZA COLOMBO G, VIANA MENDES I, de MORAIS SOUTO B, CHAVES BARRETO C, ASSIS SERRA L, FERREIRA NORONHA E, SKORUPA PARACHIN N, MOREIRA de ALMEIDA JR, FERRAZ QUIRINO B. Identification and functional expression of a new xylose isomerase from the goat rumen microbiome in Saccharomyces cerevisiae[J]. Letters in Applied Microbiology, 2022, 74(6): 941-948. DOI:10.1111/lam.13689
|
| [88] |
CHEN ST, XU ZX, DING BN, ZHANG YW, LIU SM, CAI CG, LI MZ, DALE BE, JIN MJ. Big data mining, rational modification, and ancestral sequence reconstruction inferred multiple xylose isomerases for biorefinery[J]. Science Advances, 2023, 9(5): eadd8835. DOI:10.1126/sciadv.add8835
|
| [89] |
GAO JQ, YU W, LI YX, JIN MJ, YAO L, ZHOU YJ. Engineering co-utilization of glucose and xylose for chemical overproduction from lignocellulose[J]. Nature Chemical Biology, 2023, 19: 1524-1531. DOI:10.1038/s41589-023-01402-6
|
| [90] |
CHU LF, ZAN XY, TANG X, ZHAO LN, CHEN HQ, CHEN YQ, CHEN W, SONG YD. The role of a xylose isomerase pathway in the conversion of xylose to lipid in Mucor circinelloides[J]. RSC Advances, 2016, 6(81): 77944-77952. DOI:10.1039/C6RA12379A
|
| [91] |
HENARD CA, FREED EF, GUARNIERI MT. Phosphoketolase pathway engineering for carbon-efficient biocatalysis[J]. Current Opinion in Biotechnology, 2015, 36: 183-188. DOI:10.1016/j.copbio.2015.08.018
|
| [92] |
SONDEREGGER M, SCHÜMPERLI M, SAUER U. Metabolic engineering of a phosphoketolase pathway for pentose catabolism in Saccharomyces cerevisiae[J]. Applied and Environmental Microbiology, 2004, 70(5): 2892-2897. DOI:10.1128/AEM.70.5.2892-2897.2004
|
| [93] |
SHI LL, ZHENG YM, TAN BW, LI ZJ. Establishment of a carbon-efficient xylulose cleavage pathway in Escherichia coli to metabolize xylose[J]. Biochemical Engineering Journal, 2022, 179: 108331. DOI:10.1016/j.bej.2021.108331
|
| [94] |
ALMEIDA JRM, RUNQUIST D, NOGUÉ VSI, LIDÉN G, GORWA-GRAUSLUND MF. Stress-related challenges in pentose fermentation to ethanol by the yeast Saccharomyces cerevisiae[J]. Biotechnology Journal, 2011, 6(3): 286-299. DOI:10.1002/biot.201000301
|
| [95] |
KARHUMAA K, HAHN-HÄGERDAL B, GORWA-GRAUSLUND MF. Investigation of limiting metabolic steps in the utilization of xylose by recombinant Saccharomyces cerevisiae using metabolic engineering[J]. Yeast, 2005, 22(5): 359-368. DOI:10.1002/yea.1216
|
| [96] |
KOBAYASHI Y, SAHARA T, OHGIYA S, KAMAGATA Y, FUJIMORI KE. Systematic optimization of gene expression of pentose phosphate pathway enhances ethanol production from a glucose/xylose mixed medium in a recombinant Saccharomyces cerevisiae[J]. AMB Express, 2018, 8(1): 139. DOI:10.1186/s13568-018-0670-8
|
| [97] |
PARK SH, LEE K, JANG JW, HAHN JS. Metabolic engineering of Saccharomyces cerevisiae for production of shinorine, a sunscreen material, from xylose[J]. ACS Synthetic Biology, 2019, 8(2): 346-357. DOI:10.1021/acssynbio.8b00388
|
| [98] |
YUAN XS, MAO YD, TU S, LIN JP, SHEN HH, YANG LR, WU MB. Increasing NADPH availability for xylitol production via pentose-phosphate-pathway gene overexpression and embden-meyerhof-parnas-pathway gene deletion in Escherichia coli[J]. Journal of Agricultural and Food Chemistry, 2021, 69(33): 9625-9631. DOI:10.1021/acs.jafc.1c03283
|
| [99] |
ROCA C, HAACK MB, OLSSON L. Engineering of carbon catabolite repression in recombinant xylose fermenting Saccharomyces cerevisiae[J]. Applied Microbiology and Biotechnology, 2004, 63(5): 578-583. DOI:10.1007/s00253-003-1408-2
|
| [100] |
ZHANG B, REN LL, ZHAO ZP, ZHANG SY, XU DY, ZENG X, LI F. High temperature xylitol production through simultaneous co-utilization of glucose and xylose by engineered Kluyveromyces marxianus[J]. Biochemical Engineering Journal, 2021, 165: 107820. DOI:10.1016/j.bej.2020.107820
|
| [101] |
TOPALOĞLU A, ESEN Ö, TURANLı-YıLDıZ B, ARSLAN M, ÇAKAR ZP. From Saccharomyces cerevisiae to ethanol: unlocking the power of evolutionary engineering in metabolic engineering applications[J]. Journal of Fungi, 2023, 9(10): 984. DOI:10.3390/jof9100984
|
| [102] |
HIRASAWA T, MAEDA T. Adaptive laboratory evolution of microorganisms: methodology and application for bioproduction[J]. Microorganisms, 2022, 11(1): 92. DOI:10.3390/microorganisms11010092
|
| [103] |
DEV C, JILANI SB, YAZDANI SS. Adaptation on xylose improves glucose-xylose co-utilization and ethanol production in a carbon catabolite repression (CCR) compromised ethanologenic strain[J]. Microbial Cell Factories, 2022, 21(1): 154. DOI:10.1186/s12934-022-01879-1
|
| [104] |
LOU JY, WANG JW, YANG YF, YANG Q, LI RX, HU MM, HE QN, DU J, WANG X, LI M, YANG SH. Development and characterization of efficient xylose utilization strains of Zymomonas mobilis[J]. Biotechnology for Biofuels, 2021, 14(1): 231. DOI:10.1186/s13068-021-02082-x
|
| [105] |
PROMDONKOY P, MHUANTONG W, CHAMPREDA V, TANAPONGPIPAT S, RUNGUPHAN W. Improvement in D-xylose utilization and isobutanol production in S. cerevisiae by adaptive laboratory evolution and rational engineering[J]. Journal of Industrial Microbiology & Biotechnology, 2020, 47(6/7): 497-510.
|
| [106] |
KIM J, TREMAINE M, GRASS JA, PURDY HM, LANDICK R, KILEY PJ, REED JL. Systems metabolic engineering of Escherichia coli improves coconversion of lignocellulose-derived sugars[J]. Biotechnology Journal, 2019, 14(9): e1800441. DOI:10.1002/biot.201800441
|
| [107] |
SARKAR P, MUKHERJEE M, GOSWAMI G, DAS D. Adaptive laboratory evolution induced novel mutations in Zymomonas mobilis ATCC ZW658: a potential platform for co-utilization of glucose and xylose[J]. Journal of Industrial Microbiology & Biotechnology, 2020, 47(3): 329-341.
|
| [108] |
BEN SAID S, OR D. Synthetic microbial ecology: engineering habitats for modular consortia[J]. Frontiers in Microbiology, 2017, 8: 1125. DOI:10.3389/fmicb.2017.01125
|
| [109] |
NOSRATI-GHODS N, HARRISON STL, ISAFIADE AJ, TAI SL. Ethanol from biomass hydrolysates by efficient fermentation of glucose and xylose: a review[J]. ChemBioEng Reviews, 2018, 5(5): 294-311. DOI:10.1002/cben.201800009
|
| [110] |
SHIN J, LIAO SQ, KUANYSHEV N, XIN YP, KIM C, LU T, JIN YS. Compositional and temporal division of labor modulates mixed sugar fermentation by an engineered yeast consortium[J]. Nature Communications, 2024, 15: 781. DOI:10.1038/s41467-024-45011-w
|
| [111] |
FLORES AD, AYLA EZ, NIELSEN DR, WANG X. Engineering a synthetic, catabolically orthogonal coculture system for enhanced conversion of lignocellulose-derived sugars to ethanol[J]. ACS Synthetic Biology, 2019, 8(5): 1089-1099. DOI:10.1021/acssynbio.9b00007
|
| [112] |
FERREIRA J, SANTOS VAQ, CRUZ CHG. Ethanol production by co-culture of Zymomonas mobilis and Pachysolen tannophilus using banana peels hydrolysate as substrate[J]. Acta Scientiarum Technology, 2018, 40(1): 35169. DOI:10.4025/actascitechnol.v40i1.35169
|
| [113] |
ZOU LH, JIN XZ, TAO YM, ZHENG ZJ, OUYANG J. Recent advances in the exploitation and application of coculture systems for the bioconversion of lignocellulosic feedstocks[J]. Industrial Crops and Products, 2023, 203: 117117. DOI:10.1016/j.indcrop.2023.117117
|
| [114] |
LIU YR, YANG SY, JIA XQ. Construction of a "nutrition supply-detoxification" coculture consortium for medium-chain-length polyhydroxyalkanoate production with a glucose-xylose mixture[J]. Journal of Industrial Microbiology & Biotechnology, 2020, 47(3): 343-354.
|
| [115] |
EITEMAN MA, LEE SA, ALTMAN R, ALTMAN E. A substrate-selective co-fermentation strategy with Escherichia coli produces lactate by simultaneously consuming xylose and glucose[J]. Biotechnology and Bioengineering, 2009, 102(3): 822-827. DOI:10.1002/bit.22103
|
| [116] |
ZHANG HR, PEREIRA B, LI ZJ, STEPHANOPOULOS G. Engineering Escherichia coli coculture systems for the production of biochemical products[J]. Proceedings of the National Academy of Sciences of the United States of America, 2015, 112(27): 8266-8271.
|
| [117] |
ZHAO CH, SINUMVAYO JP, ZHANG YP, LI Y. Design and development of a "Y-shaped" microbial consortium capable of simultaneously utilizing biomass sugars for efficient production of butanol[J]. Metabolic Engineering, 2019, 55: 111-119. DOI:10.1016/j.ymben.2019.06.012
|
| [118] |
WAN P, ZHAI DM, WANG Z, YANG XS, TIAN S. Ethanol production from nondetoxified dilute-acid lignocellulosic hydrolysate by cocultures of Saccharomyces cerevisiae Y5 and Pichia stipitis CBS6054[J]. Biotechnology Research International, 2012, 2012, 656371.
|
| [119] |
MILLÁN ACOSTA A, COSOVANU D, CABAÑEROS LÓPEZ P, THOMSEN ST, GERNAEY KV, CANELA-GARAYOA R. Co-cultivation of a novel Fusarium striatum strain and a xylose consuming Saccharomyces cerevisiae yields an efficient process for simultaneous detoxification and fermentation of lignocellulosic hydrolysates[J]. Chemical Engineering Journal, 2021, 426: 131575. DOI:10.1016/j.cej.2021.131575
|
| [120] |
JAWAD M, WANG H, WU YD, REHMAN O, SONG YX, XU R, ZHANG Q, GAO HP, XUE C. Lignocellulosic ethanol and butanol production by Saccharomyces cerevisiae and Clostridium beijerinckii co-culture using non-detoxified corn stover hydrolysate[J]. Journal of Biotechnology, 2024, 379: 1-5. DOI:10.1016/j.jbiotec.2023.11.002
|
 2024, Vol. 40
2024, Vol. 40