中国科学院微生物研究所、中国微生物学会主办
文章信息
- 汪淑贤, 方嘉煜, 张延平, 李寅, 朱泰承
- WANG Shuxian, FANG Jiayu, ZHANG Yanping, LI Yin, ZHU Taicheng
- 天然甲醇化学品细胞工厂改造进展与展望
- Progress and perspectives of natural cell factories for chemical production from methanol
- 生物工程学报, 2024, 40(8): 2747-2760
- Chinese Journal of Biotechnology, 2024, 40(8): 2747-2760
- 10.13345/j.cjb.240163
-
文章历史
- Received: February 28, 2024
- Accepted: May 9, 2024
- Published: May 13, 2024
2. 中国科学院大学, 北京 100049
2. University of Chinese Academy of Sciences, Beijing 100049, China
随着人类社会的发展,石油、煤、天然气等传统化石能源日益枯竭,大气中二氧化碳等温室气体排放量持续增加,由此带来的资源、环境问题已成为21世纪人类社会发展面临的最严峻问题之一。一碳化合物,涵盖了一氧化碳、二氧化碳、甲烷等气体一碳化合物,以及甲醇、甲酸等可通过一碳气体转化得到的液态一碳化合物。这些一碳化合物来源广泛、价格低廉,有望成为绿色生物制造的重要原料来源[1]。对一碳化合物的高效利用,也可为碳达峰、碳中和目标的达成以及绿色可持续发展作出重要贡献。
甲醇是重要的大宗化工原料,全球产能超过1亿t。我国煤炭资源丰富,煤制甲醇工艺成熟,产能充沛。近些年,随着氢气还原二氧化碳以及电还原二氧化碳技术的日趋成熟,甲醇有望成为可持续绿色生物制造的重要原料之一,因此以甲醇为原料进行微生物转化,已经成为近几年代谢工程和合成生物学等领域的研究前沿和热点[2-5]。以甲醇为原料进行生物制造,核心之一是构建高效的甲醇细胞工厂。目前,开发甲醇细胞工厂主要采用两种技术路线:构建以模式微生物为底盘的非天然甲醇细胞工厂和构建以甲基营养型微生物为底盘的天然甲醇细胞工厂。目前对前者的报道相对较多,近年来也有较多文章对甲醇代谢的基本元件和途径以及该领域的前沿动态进行了综述[6-12]。本文综述了天然甲醇细胞工厂的研究进展,包括天然甲醇细胞工厂与非天然甲醇工厂的比较、天然甲醇细胞工厂仍存在的科学问题以及围绕这些科学问题近些年取得的进展和展望。
1 构建甲醇细胞工厂两种路线的比较构建非天然甲醇细胞工厂主要是在模式菌株中引入甲醇代谢途径,使其能够利用甲醇进行生长并生产目标化学品。这种方法的优势在于模式生物的遗传背景清晰,遗传操作方法成熟,生理机制研究深入,化学品生产案例丰富。理论上,甲醇同化途径并不复杂,只需引入几个关键酶即可实现完整途径的构建。如核酮糖单磷酸途径(ribulose monophosphate pathway, RuMP)与大多数微生物自身的磷酸戊糖途径(pentose phosphate pathway, PPP)有较大的重合,只需在大肠杆菌等模式宿主中引入甲醇脱氢酶(methanol dehydrogenase, MDH)、3-己酮糖-6-磷酸合成酶(3-hexulose-6-phosphate synthase, HPS)和3-己酮糖-6-磷酸异构酶(6-phospho-3-hexuloisomerase, PHI)即可完成RuMP途径的异源重构[13]。然而,实践中发现甲醇同化途径的改造并不如预期简单。虽然通过13C标记可以检测到重组菌对甲醇的同化利用,但异源宿主很难仅以甲醇作为唯一碳源高效生长和生产,通常需要添加辅助碳源或/和对工程菌进行长时间的适应性进化[14-17]。更重要的是,该路线产生的化学品产量相对较低,甲醇利用率通常仅是天然甲醇细胞工厂的10%甚至更低。
另一条技术路线是构建天然甲醇细胞工厂。在自然界中,甲醇营养型细菌(如甲醇芽孢杆菌、扭脱甲基杆菌)和酵母(如巴斯德毕赤酵母、多形汉逊酵母),能够将甲醇作为唯一碳源和能源进行代谢生长。通过在这些微生物中引入化学品合成途径,可以获得高效代谢甲醇的细胞工厂。例如,Zhu等[18]在扭脱甲基杆菌AM1中异源表达来自粪肠球菌的3-羟基-3-甲基戊二酸单酰辅酶A合成酶和3-羟基-3-甲基戊二酸单酰辅酶A还原酶,以及来自罗氏真养菌的乙酰乙酰辅酶A硫解酶,使得扭脱甲基杆菌AM1能合成2.2 g/L的甲羟戊酸。Miao等[19]通过在毕赤酵母中异源表达天冬氨酸脱羧酶以及磷酸烯醇式丙酮酸羧化酶,使得毕赤酵母最高可生产5.6 g/L的β-丙氨酸。此外,一些甲基营养型细菌本身就能合成一定的化学品。例如,甲醇芽孢杆菌是利用甲醇合成某些氨基酸的优良宿主。野生型的甲醇芽孢杆菌MGA3通过甲醇补料分批发酵可产生59.0 g/L的L-谷氨酸和少量的L-赖氨酸,经过诱变和筛选获得的突变体M168-20则能够生产69.0 g/L的L-谷氨酸和11.0 g/L的L-赖氨酸[20]。扭脱甲基杆菌则是聚羟基丁酸酯(polyhydroxybutyrate, PHB)的天然生产者。早在1986年,Suzuki等[21]通过对扭脱甲基杆菌的发酵工艺进行控制和优化,使该菌株PHB产量达149.0 g/L (表 1)。
| Strains | Products | Titer (g/L) | Carbon sources | Culture methods | References |
| Pichia pastoris | Malic acid | 2.8 | Methanol, yeast extract | Fermentor | [22] |
| Pichia pastoris | Fatty acid | 23.4 | Methanol | Fermentor | [23] |
| Pichia pastoris | Chondroitin sulfate | 2.1 | Methanol | Fermentor | [4] |
| Pichia pastoris | 6-methylsalicylic acid | 2.2 | Methanol | Fermentor | [24] |
| Pichia pastoris | β-alanine | 5.6 | Methanol, yeast extract, peptone | Fermentor | [19] |
| Ogataea polymorpha | Lactate | 3.8 | Methanol | Shake flask | [25] |
| Ogataea polymorpha | Fatty alcohol | 3.6 | Methanol | Fermentor | [26] |
| Ogataea polymorpha | Malic acid | 13.2 | Methanol, yeast extract | Fermentor | [27] |
| Bacillus methanolicus | l-glutamic acid | 69.0 | Methanol | Fermentor | [20] |
| Bacillus methanolicus | l-lysine | 65.0 | Methanol | Fermentor | [20] |
| Methylorubrum extorquens | Mevalonate | 2.7 | Methanol | Fermentor | [28] |
| Methylorubrum extorquens | α-humulene | 1.6 | Methanol | Fermentor | [29] |
| Methylorubrum extorquens | Polyhydroxybutyrate | 149.0 | Methanol | Fermentor | [21] |
天然甲醇营养型微生物的主要优势在于其高效的甲醇代谢速率,通常能达到百毫克至克每升每小时,且具备对甲醇和甲醛的高效解毒机制,能够有效应对一碳底物的毒性。然而,与模式菌株相比,这些微生物的基因标注不够完善,生理研究积累较少,遗传操作相对困难,深入改造较为挑战。以甲基营养型酵母毕赤酵母为例,过去以毕赤酵母为细胞工厂进行化学品生产很少有超过g/L的案例。但随着CRISPR等基因编辑技术和大片段拼装技术的发展,近年来已能对毕赤酵母等非传统酵母进行较为深入的改造,进而可以以甲醇为原料合成超过克每升甚至几十克每升的化学品。表 2对各类甲醇细胞工厂的参数进行了比较。
| Natural/Synthetic | Natural methanol cell factories | Non-natural methanol cell factories | ||
| Bacteria/Yeast | Bacteria | Yeast | Bacteria | Yeast |
| Specific growth rate (h–1) |
0.090 0–0.408 0[30-31] | 0.150 0–0.190 0[32-33] | 0.082 0[14] | 0.000 4–0.051 0[34-35] |
| Cell yield (g/g) |
0.350[30] | 0.380–0.425[36-37] | 0.148[14] | − |
| Product titer (g/L) |
0.40–149.00[20, 38] | 0.75–48.20[22, 39] | 0.23–1.66[17, 40] | 0.26–0.92[34, 41] |
| Product yield (g/g) |
0.031–0.360[29, 42] | 0.025–0.230[39, 43] | − | 0.148–0.250[34, 41] |
| Product productivity (g/(L·h)) |
0.006 6–2.780 0[28, 42] | 0.007 8–0.156 0[22, 39] | 0.001 6–0.083 0[17, 40] | 0.003 6–0.009 6[34, 41] |
| Methanol uptake rate (g/(L·h)) |
0.078–9.790[28, 44] | 0.178–1.400[23, 45] | 0.036–0.053[14, 17] | 0.014–0.065[34, 41] |
| Methanol tolerance (mol/L) |
0.30–2.00[20, 46] | 1.56[47] | 0.47–1.20[14, 40] | 0.94[41] |
| Genetic manipulation | Homologous recombination, episomal vectors | Homologous recombination, CRISPR-Cas9 | Homologous recombination, episomal vectors, CRISPR-Cas9 | Homologous recombination, episomal vectors, CRISPR-Cas9 |
| −: No data. | ||||
天然甲醇细胞工厂相较于非天然甲醇细胞工厂展现出更高的甲醇利用效率,已达到数十克每升的产量,表明天然甲醇细胞工厂在短期内更有可能实现产业化。然而,为了真正实现利用甲醇的生物制造目标,天然甲醇细胞工厂仍面临若干关键挑战。
首先,甲醇同化效率低是甲醇细胞工厂利用甲醇作为生物转化原料时的主要瓶颈之一。这主要归因于甲醇氧化过程中产生的甲醛具有强烈的生理毒性。为了避免甲醛的积累,细胞通常通过异化途径将甲醛氧化为二氧化碳,导致碳资源的损失。例如,毕赤酵母在发酵过程中的甲醇转化效率通常仅为0.20−0.25 g DCW/g[48],远低于使用葡萄糖或甘油作为碳源时的转化效率(> 0.40 g DCW/g);而在甲醇芽孢杆菌中,高达38%的甲醇由于被氧化成CO2而损失[49]。
其次,甲醇利用速率需要进一步提升。与糖类原料相比,甲醇的利用速率存在显著差距,例如酿酒酵母的葡萄糖利用速率可以达到2.70 g/(g DCW·h)左右[50],而毕赤酵母利用甲醇的速率大概在0.23 g/(g DCW·h)左右[51]。此外,甲醇同化路径依赖于复杂的碳重排网络,例如在木酮糖单磷酸途径(xylulose monophosphate pathway, XuMP)或RuMP途径中,甲醇首先反应生成C6化合物或2分子C3化合物,这一过程需要通过磷酸戊糖途径持续再生磷酸戊糖,而该过程涉及多步催化和碳重排,限制了甲醇利用速率的提升。
再者,天然甲醇细胞工厂生产化学品的得率普遍较低,绝大多数不足10%[19, 28-29, 43, 52-53],仅有个别案例可达20%‒30%[42, 46],远低于使用糖类碳源时的得率。除上述甲醇异化导致碳源被大量浪费外,在多数甲醇细胞工厂采用的好氧发酵过程中,同化的碳源大多数被用于支持细胞生长而非转化为目标化学品,特别是毕赤酵母等甲基营养型酵母本身并不倾向于积累化学品。例如,Jin等[4]通过毕赤酵母利用甲醇合成硫酸软骨素,产量达到2.1 g/L,但仅为细胞干重的1.3%。因此,在以毕赤酵母等为底盘构建甲醇细胞工厂时,则需打破酵母自身代谢调控的刚性,进而使得同化的甲醇更多地流向化学品生产。
最后,在天然甲醇细胞工厂发酵过程中,容易积累过量的甲醇,进而产生底物抑制的现象,甚至还会使得细胞发生不可逆的中毒,最终导致发酵失败[54]。因此,在设计发酵工艺时,通常需要采取较为精确的流加策略,以控制甲醇浓度在合理的水平,才能保证在维持生产强度的同时避免发生底物抑制,这对工业生产操作提出了较高的要求[55-56]。因此,如果能进一步提高天然甲醇细胞工厂的甲醇耐受性,无疑会降低操作的复杂性,降低发酵失败风险,在工业生产上将具有较为重要的意义。
3 天然甲醇细胞工厂改造进展围绕天然甲醇细胞工厂存在的甲醇同化效率低、利用速率不够、产品得率低以及甲醇毒性等问题,研究者们进行了大量的探索与尝试,在天然甲醇细胞工厂改造方面取得了许多积极的进展(图 1)。
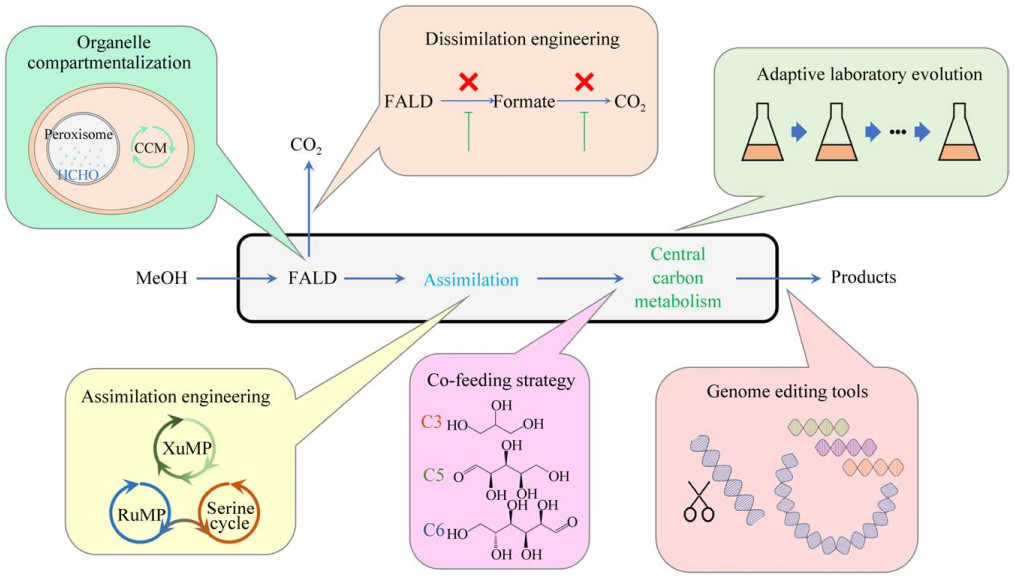
|
| 图 1 天然甲醇细胞工厂改造策略 Fig. 1 Strategies for engineering natural methanol cell factories. CCM: Central carbon metabolism; FALD: Formaldehyde. CCM:中心碳代谢;FALD:甲醛 |
| |
天然甲基营养型菌与模式生物相比,同源重组效率低,可使用的筛选标记和基因编辑工具较少,因此目前主要用于生产乳酸、3-羟基丙酸、脂肪酸等合成途径短的大宗化学品。然而,随着合成生物学的发展,应用于天然甲基营养微生物的基因编辑技术也取得了突破,改造非模式生物的难度正在逐渐降低。Vogl等[57]对毕赤酵母的一系列启动子进行了研究与表征,开发出一套高效且受严格调控的启动子文库。Gao等[58]对毕赤酵母的基因组序列进行了分析,基于高表达效率、高稳定性和低损伤性的标准,筛选出了一系列的异源基因整合位点。Gao等[59]在汉逊酵母中建立了成簇的规则间隔短回文重复序列/CRISPR相关蛋白9 (clustered regularly interspaced short palindromic repeat/CRISPR-associated protein 9, CRISPR/Cas9)系统,并增强了菌株的同源重组能力,实现了多个片段的无痕敲除和组装。Zhu等[60]在扭脱甲基杆菌中开发了小调节RNA (small regulatory RNA, sRNA)系统,可以用于抑制目的基因的表达。Gao等[61]在筛选出稳定的整合位点的基础上,将长春碱合成途径在毕赤酵母内分模块进行整合和优化,实现了毕赤酵母以甲醇为原料从头合成长春质碱。这些案例表明以天然甲基营养型菌株作为底盘生产合成途径较长的高附加值化学品具有巨大潜力。
3.2 异化途径改造在甲醇代谢过程中,异化途径发挥着解毒和为细胞提供能量及还原力的重要作用,但同时也造成了严重的碳损失,这种碳资源的浪费是造成甲醇生物转化合成化学品产量及得率低的一个关键因素。因此,通过改造甲醛异化途径以减少甲醛的异化比例,成为提高甲醇利用效率的重要策略。Liu等[62]通过降低醇氧化酶的活性,从而降低胞内甲醛的合成通量,最终将毕赤酵母的细胞得率提高了14%。然而,在毕赤酵母、博伊丁假丝酵母等甲基营养型酵母中敲除甲醛脱氢酶基因或甲酸脱氢酶基因,会导致这些酵母在甲醇中的生长受到强烈抑制,这可能是甲醛或甲酸等有毒物质的积累导致的[22, 63-64],表明异化途径不能通过简单地敲除单个或多个基因来阻断。Wu等[39]通过使用弱启动子降低了甲醛脱氢酶的表达,最终将3-羟基丙酸的产量提高了14%,而弱化甲酸脱氢酶的表达则没有效果。Yu等[65]通过比较转录组学的方法揭示了当异化途径削弱时,甲醛同化途径和氧化磷酸戊糖途径会同时下调,从而使得固定的碳和获取的能量都减少,最终导致细胞生长受限。这些研究表明异化途径需要更精准的代谢调控,尤其要考虑代谢通量的平衡以及还原力提供途径的补充。
3.3 同化途径改造为了提高天然甲醇细胞工厂的生物转化效率,并增加甲醇向细胞组分或目标化合物转化的通量,强化单碳(C1)同化途径成为了关键的改进策略。这种方法不仅可以提升底物的利用效率,还能通过减少异化途径的竞争,加强目标化合物的生产能力。
在改造非天然甲醇细胞工厂的过程中,通过增强非氧化磷酸戊糖途径关键基因的表达来加强中间代谢物的碳重排,已经在一定程度上取得了成功,提高了甲醇的同化速率。如Woolston等[66]激活了1, 7-二磷酸景天庚酮糖磷酸酶重排途径,将大肠杆菌的甲醇利用效率提高了2倍。Bennett等[67]在大肠杆菌中表达来自甲醇芽孢杆菌非氧化磷酸戊糖途径的5个酶,13C标记结果表明改造菌株的胞内中间代谢物以及氨基酸标记程度均显著高于对照菌株,表明改造后的大肠杆菌甲醇同化效率得到了提高。然而,对天然甲基营养菌进行类似的甲醇代谢途径改造的尝试还相对较少,成功案例更是少见。例如,Krainer等[68]研究者在毕赤酵母中过表达XuMP同化途径的关键酶,如二羟丙酮合成酶(dihydroxyacetone synthase, DAS)和转酮醇酶(transketolase, TKL),结果发现这些改造反而导致细胞生长速度和甲醇消耗速率显著降低。Cai等[23]过表达毕赤酵母同化途径中的多个关键酶,例如DAS、5-磷酸木酮糖异构酶(ribulose-5-phosphate epimerase, RPE)、1, 6-二磷酸果糖磷酸酶(fructose-1, 6-bisphosphatase, FBP)、6-磷酸葡萄糖脱氢酶(glucose-6-phosphate dehydrogenase, ZWF)和6-磷酸葡萄糖酸脱氢酶(6-phosphogluconate dehydrogenase, GND),仅DAS过表达菌株的细胞得率在一定程度上得到了提高,这暗示了仅增强单一途径可能不足以显著提升细胞得率和化学品产量。
另一方面,Yuan等[69]通过将外源的RuMP循环融合到扭脱甲基杆菌AM1菌株的丝氨酸循环代谢中,构建了新型的协同同化途径。这种策略不仅提高了细胞生长速率和甲醇消耗速率,还使3-羟基丙酸的产量增加了3.1倍,表明合理构建协同甲醇同化途径可以有效提高甲醇细胞工厂的性能。
综上所述,虽然通过改造模式微生物以增强甲醇同化和生物制造效率的研究还处于早期阶段,但已有的研究成果表明,与非天然甲醇细胞工厂相比,天然甲醇细胞工厂的甲醇利用效率已经相对较高。因此,那些在非天然系统中成功应用的策略可能并不直接适用于天然系统,这意味着改造天然甲醇细胞工厂需要探索新的思路和策略。
3.4 适应性实验室进化在微生物细胞工厂的开发中,尤其是非天然系统中,适配底盘细胞与C1代谢途径的整合较为困难。这主要因为模式微生物天然更偏向于分解代谢糖类原料进行能量和生物质的转化,引入用于合成代谢的C1途径则需要在细胞内进行复杂的代谢调节。
与理性改造相比,适应性实验室进化技术不需要深入了解复杂的遗传背景,具有广泛的适用性和实用性。目前适应性进化在开发非天然甲醇细胞工厂方面应用较多,在天然甲醇细胞工厂(如毕赤酵母)中的应用还相对较少。例如,Moser等[70]通过适应性进化显著提升了毕赤酵母在甲醇培养基中的生长速率和重组蛋白的产量。Gassler等[71]则通过将毕赤酵母的甲醇代谢途径替换为CO2固定途径,并利用适应性进化实现了毕赤酵母的自养生长。Meng等[72]结合适应性进化、加强氮代谢以及细胞壁工程等技术对毕赤酵母进行改造,获得了一株甲醇利用效率高、耐受33 ℃和蛋白含量高的适合工业化生产单细胞蛋白的毕赤酵母工程菌株。
适应性进化技术在构建甲醇细胞工厂的应用中具有巨大潜力。首先,考虑到甲醇作为底物对细胞具有毒性,通过适应性进化技术提高细胞对甲醇的耐受性,能够使得甲醇作为碳源的发酵过程更为高效。其次,异化途径的改造可能会导致细胞生长受到抑制,这可能是由于能量供应不足或有害中间代谢物(如甲醛、甲酸)的积累所导致。因此,在削弱异化途径的同时,进一步的适应性进化可能是提升细胞产率的关键。
总而言之,适应性实验室进化作为一种强有力的工具,为提升甲醇细胞工厂的性能开辟了新途径。未来的研究应探索适应性进化与理性改造相结合的策略,以应对C1代谢改造过程中遇到的复杂挑战,从而推进甲醇及其他单碳底物在生物制造中的有效利用。这一方法不仅有助于提高细胞对甲醇的耐受性和利用效率,还能通过精确的代谢调控,优化细胞工厂的整体性能,为实现单碳化合物在工业生产中的广泛应用奠定坚实基础。
3.5 C1/Cn共底物策略如前所述,为了获得高效的甲醇细胞工厂,需要对C1同化网络进行系统而深入地改造甚至重构。与之相对地,采用C1与Cn共底物策略是一个相对简单却行之有效的方法,其中Cn代表其他类型的碳源,如C3 (如甘油)、C5 (如木糖、阿拉伯糖)或C6 (如葡萄糖、果糖、山梨醇)。这类C1/Cn共底物策略在提升非天然和天然甲醇细胞工厂的性能方面已得到广泛应用[73]。
在非天然甲醇细胞工厂构建中,通过引入戊糖(木糖或阿拉伯糖)作为共底物,有效提升了RuMP途径所需的磷酸戊糖供应,进而显著提高了工程化大肠杆菌或谷氨酸棒杆菌等微生物的甲醇利用效率[74-76]。尽管如此,受限于非天然甲醇细胞工厂的低甲醇利用率,这个策略距离达到工业应用尚有较大差距。
对于天然甲醇营养型微生物,尤其是如毕赤酵母这样的甲醇营养型酵母,研究者很早就在高密度培养中采用甘油、葡萄糖或山梨醇等作为甲醇的共底物[77-80],在摇瓶或发酵罐中采用混合补料策略,以提升酵母的蛋白表达水平。然而,在分批发酵模式下,共底物特别是甘油和葡萄糖对毕赤酵母甲醇代谢途径以及醇氧化酶启动子有强烈的阻遏作用,使得甲醇和这些碳源无法在分批发酵中实现共利用,从而需要复杂的补料策略,这给工业放大和应用造成了很大障碍。
另外,在毕赤酵母中,共底物策略虽然被广泛应用于提升发酵性能,包括:减少氧气需求、减少热产生、增强能量供应、改善酵母活性、增加生物量产量及降低代谢负担等,但不同的研究对于共底物的功效认识并不相同。因此,对C1/Cn共底物策略的潜在机制尚未完全解析。未来需深入研究这些策略的机制,以开发出更有效的改造策略来克服现有的限制,实现甲醇和其他底物在工业生产中的高效共利用。
3.6 化学品合成区室化过氧化物酶体是甲基营养型酵母的一种重要细胞器,细胞内的重要代谢包括甲醇代谢、脂肪酸β-氧化和乙醛酸代谢等都发生在过氧化物酶体中[81]。目前甲醇细胞工厂化学品主要在胞质中合成,然而通过区室化工程在过氧化物酶体中合成化学品也具有多种优势,包括提高局部底物和酶浓度、与胞质中的竞争反应分隔开等[82]。在毕赤酵母中,番茄红素合成的前体物质法尼焦磷酸在过氧化物酶体中大量合成,Bhataya等[83]将合成番茄红素的基因定位到过氧化物酶体中,番茄红素合成量达到73.9 mg/L。Liu等[84]将α-法尼烯合成途径同时定位在毕赤酵母的过氧化物酶体和细胞质,α-法尼烯的产量可达2.18 g/L,比单独定位在过氧化物酶体或细胞质中分别提高了1.3倍和2.1倍。Zhai等[26]将脂肪醇合成途径定位到多形汉逊酵母的过氧化物酶体中,避免了胞质中脂肪酸代谢途径的干扰,并通过甲醇利用途径的优化将脂肪醇的产量提高了3.9倍。
4 结论与展望甲醇因其广泛的来源、低廉的价格、便捷的运输存储方式以及较低的环境污染潜力,展现出成为未来生物制造重要替代原料的巨大潜力。在此背景下,开发高效的甲醇细胞工厂是实现甲醇生物制造的关键。当前,非天然甲醇细胞工厂在甲醇的利用速率和化学品的产量方面普遍落后于天然甲醇细胞工厂,使得在短期内天然细胞工厂路线更为接近实现甲醇细胞工厂生产化学品的产业化目标。然而,尽管天然路线目前更具应用前景,要实现其产业化仍面临许多挑战,特别是在提高甲醇的同化效率和化学品转化率方面。
目前,关于成功改造这些微生物以提升甲醇利用效率的研究仍然较为稀缺。虽然一些改造策略在非天然甲醇工厂的构建过程中成功应用,但由于人工甲基营养菌和天然甲基营养菌在甲醇利用能力、面临的限制性因素以及调控机制方面存在显著差异,这些改造策略并不能简单地套用于天然甲醇细胞工厂。
在这一背景下,短期内采用C1+Cn的策略成为一个重要的过渡方案,它不仅能够发挥甲醇的优势,还能利用包括废弃物在内的廉价共底物,有望显著提升甲醇的利用效率。此外,对甲醇细胞工厂的深入研究至关重要:(1) 开发高效的改造方法,实现对细胞工厂十几个乃至数十个基因的精准改造,同时要保证这些改造基因的稳定性;(2) 改造或设计全新甲醇同化途径,尤其是高效的线性途径。这些同化途径不一定是要通用的,也可以是针对某类重要化学品的途径;(3) 深入了解细胞生理,除了常用的组学分析外,还包括如13C代谢流分析等手段进行定量化分析。这些分析不仅包括代谢和能量分析,还包括它们如何有效耦合,以满足能量需求同时避免过度氧化导致的能量浪费;(4) 提高适应性实验室进化效率,从目前的研究来看,采用适应性实验室进化似乎是一条解决C1途径与细胞底盘适配的必要途径。因此,如何确保通过分子层面引入高效进化机制和高通量筛选实现目的表型如甲醇的高利用、高耐受将成为未来改造的重要方向。
| [1] |
CLOMBURG JM, CRUMBLEY AM, GONZALEZ R. Industrial biomanufacturing: the future of chemical production[J]. Science, 2017, 355(6320): aag0804. DOI:10.1126/science.aag0804
|
| [2] |
ÀVILA-CABRÉ S, PÉREZ-TRUJILLO M, ALBIOL J, FERRER P. Engineering the synthetic β-alanine pathway in Komagataella phaffii for conversion of methanol into 3-hydroxypropionic acid[J]. Microbial Cell Factories, 2023, 22(1): 237. DOI:10.1186/s12934-023-02241-9
|
| [3] |
GAO JQ, LI YX, YU W, ZHOU YJ. Rescuing yeast from cell death enables overproduction of fatty acids from sole methanol[J]. Nature Metabolism, 2022, 4(7): 932-943. DOI:10.1038/s42255-022-00601-0
|
| [4] |
JIN XR, ZHANG WJ, WANG Y, SHENG JY, XU RR, LI JH, DU GC, KANG Z. Biosynthesis of non-animal chondroitin sulfate from methanol using genetically engineered Pichia pastoris[J]. Green Chemistry, 2021, 23(12): 4365-4374. DOI:10.1039/D1GC00260K
|
| [5] |
ORITA I, NISHIKAWA K, NAKAMURA S, FUKUI T. Biosynthesis of polyhydroxyalkanoate copolymers from methanol by Methylobacterium extorquens AM1 and the engineered strains under cobalt-deficient conditions[J]. Applied Microbiology and Biotechnology, 2014, 98(8): 3715-3725. DOI:10.1007/s00253-013-5490-9
|
| [6] |
CHEN AY, LAN EI. Chemical production from methanol using natural and synthetic methylotrophs[J]. Biotechnology Journal, 2020, 15(6): e1900356. DOI:10.1002/biot.201900356
|
| [7] |
郭姝媛, 吴良焕, 刘香健, 王博, 于涛. 微生物中一碳代谢网络构建的进展与挑战[J]. 合成生物学, 2022, 3(1): 116-137. GUO SY, WU LH, LIU XJ, WANG B, YU T. Developing C1-based metabolic network in methylotrophy for biotransformation[J]. Synthetic Biology Journal, 2022, 3(1): 116-137 (in Chinese). |
| [8] |
刘康, 乔杨怡, 张尚杰, 郭峰, 马江锋, 信丰学, 章文明, 姜岷. 甲醇生物转化合成化学品的研究进展[J]. 生物工程学报, 2023, 39(6): 2430-2448. LIU K, QIAO YY, ZHANG SJ, GUO F, MA JF, XIN FX, ZHANG WM, JIANG M. Advances in biotransformation of methanol into chemicals[J]. Chinese Journal of Biotechnology, 2023, 39(6): 2430-2448 (in Chinese). |
| [9] |
RUßMAYER H, BUCHETICS M, GRUBER C, VALLI M, GRILLITSCH K, MODARRES G, GUERRASIO R, KLAVINS K, NEUBAUER S, DREXLER H, STEIGER M, TROYER C, AL CHALABI A, KREBIEHL G, SONNTAG D, ZELLNIG G, DAUM G, GRAF AB, ALTMANN F, KOELLENSPERGER G, et al. Systems-level organization of yeast methylotrophic lifestyle[J]. BMC Biology, 2015, 13(1): 80. DOI:10.1186/s12915-015-0186-5
|
| [10] |
WANG Y, FAN LW, TUYISHIME P, ZHENG P, SUN JB. Synthetic methylotrophy: a practical solution for methanol-based biomanufacturing[J]. Trends in Biotechnology, 2020, 38(6): 650-666. DOI:10.1016/j.tibtech.2019.12.013
|
| [11] |
ZHAN CJ, LI XW, YANG YK, NIELSEN J, BAI ZH, CHEN Y. Strategies and challenges with the microbial conversion of methanol to high-value chemicals[J]. Biotechnology and Bioengineering, 2021, 118(10): 3655-3668. DOI:10.1002/bit.27862
|
| [12] |
ZHU TC, ZHAO TX, BANKEFA OE, LI Y. Engineering unnatural methylotrophic cell factories for methanol-based biomanufacturing: challenges and opportunities[J]. Biotechnology Advances, 2020, 39: 107467. DOI:10.1016/j.biotechadv.2019.107467
|
| [13] |
MÜLLER JEN, MEYER F, LITSANOV B, KIEFER P, POTTHOFF E, HEUX S, QUAX WJ, WENDISCH VF, BRAUTASET T, PORTAIS JC, VORHOLT JA. Engineering Escherichia coli for methanol conversion[J]. Metabolic Engineering, 2015, 28: 190-201. DOI:10.1016/j.ymben.2014.12.008
|
| [14] |
CHEN FYH, JUNG HW, TSUEI CY, LIAO JC. Converting Escherichia coli to a synthetic methylotroph growing solely on methanol[J]. Cell, 2020, 182(4): 933-946.e14. DOI:10.1016/j.cell.2020.07.010
|
| [15] |
WANG X, WANG XL, LU XL, MA C, CHEN KQ, OUYANG PK. Methanol fermentation increases the production of NAD(P)H-dependent chemicals in synthetic methylotrophic Escherichia coli[J]. Biotechnology for Biofuels, 2019, 12: 17. DOI:10.1186/s13068-019-1356-4
|
| [16] |
WHITAKER WB, JONES JA, BENNETT RK, GONZALEZ JE, VERNACCHIO VR, COLLINS SM, PALMER MA, SCHMIDT S, ANTONIEWICZ MR, KOFFAS MA, PAPOUTSAKIS ET. Engineering the biological conversion of methanol to specialty chemicals in Escherichia coli[J]. Metabolic Engineering, 2017, 39: 49-59. DOI:10.1016/j.ymben.2016.10.015
|
| [17] |
YU H, LIAO JC. A modified serine cycle in Escherichia coli coverts methanol and CO2 to two-carbon compounds[J]. Nature Communications, 2018, 9(1): 3992. DOI:10.1038/s41467-018-06496-4
|
| [18] |
ZHU WL, CUI JY, CUI LY, LIANG WF, YANG S, ZHANG C, XING XH. Bioconversion of methanol to value-added mevalonate by engineered Methylobacterium extorquens AM1 containing an optimized mevalonate pathway[J]. Applied Microbiology and Biotechnology, 2016, 100(5): 2171-2182. DOI:10.1007/s00253-015-7078-z
|
| [19] |
MIAO LT, LI Y, ZHU TC. Metabolic engineering of methylotrophic Pichia pastoris for the production of β-alanine[J]. Bioresources and Bioprocessing, 2021, 8(1): 89. DOI:10.1186/s40643-021-00444-9
|
| [20] |
BRAUTASET T, JAKOBSEN ØM, DEGNES KF, NETZER R, NÆRDAL I, KROG A, DILLINGHAM R, FLICKINGER MC, ELLINGSEN TE. Bacillus methanolicus pyruvate carboxylase and homoserine dehydrogenase Ⅰ and Ⅱ and their roles for L-lysine production from methanol at 50 ℃[J]. Applied Microbiology and Biotechnology, 2010, 87(3): 951-964. DOI:10.1007/s00253-010-2559-6
|
| [21] |
SUZUKI T, YAMANE T, SHIMIZU S. Mass production of poly-β-hydroxybutyric acid by fed-batch culture with controlled carbon/nitrogen feeding[J]. Applied Microbiology and Biotechnology, 1986, 24(5): 370-374. DOI:10.1007/BF00294592
|
| [22] |
GUO F, DAI ZX, PENG WF, ZHANG SJ, ZHOU J, MA JF, DONG WL, XIN FX, ZHANG WM, JIANG M. Metabolic engineering of Pichia pastoris for malic acid production from methanol[J]. Biotechnology and Bioengineering, 2021, 118(1): 357-371. DOI:10.1002/bit.27575
|
| [23] |
CAI P, WU XY, DENG J, GAO LH, SHEN YW, YAO L, ZHOU YJ. Methanol biotransformation toward high-level production of fatty acid derivatives by engineering the industrial yeast Pichia pastoris[J]. Proceedings of the National Academy of Sciences of the United States of America, 2022, 119(29): e2201711119.
|
| [24] |
GAO LM, CAI MH, SHEN W, XIAO SW, ZHOU XS, ZHANG YX. Engineered fungal polyketide biosynthesis in Pichia pastoris: a potential excellent host for polyketide production[J]. Microbial Cell Factories, 2013, 12: 77. DOI:10.1186/1475-2859-12-77
|
| [25] |
WEFELMEIER K, SCHMITZ S, HAUT AM, OTTEN J, JÜLICH T, BLANK LM. Engineering the methylotrophic yeast Ogataea polymorpha for lactate production from methanol[J]. Frontiers in Bioengineering and Biotechnology, 2023, 11: 1223726. DOI:10.3389/fbioe.2023.1223726
|
| [26] |
ZHAI XX, GAO JQ, LI YX, GRININGER M, ZHOU YJ. Peroxisomal metabolic coupling improves fatty alcohol production from sole methanol in yeast[J]. Proceedings of the National Academy of Sciences of the United States of America, 2023, 120(12): e2220816120.
|
| [27] |
WEFELMEIER K, SCHMITZ S, KÖSTERS BJ, LIEBAL UW, BLANK LM. Methanol bioconversion into C3, C4, and C5 platform chemicals by the yeast Ogataea polymorpha[J]. Microbial Cell Factories, 2024, 23(1): 8. DOI:10.1186/s12934-023-02283-z
|
| [28] |
LIANG WF, CUI LY, CUI JY, YU KW, YANG S, WANG TM, GUAN CG, ZHANG C, XING XH. Biosensor-assisted transcriptional regulator engineering for Methylobacterium extorquens AM1 to improve mevalonate synthesis by increasing the acetyl-CoA supply[J]. Metabolic Engineering, 2017, 39: 159-168. DOI:10.1016/j.ymben.2016.11.010
|
| [29] |
SONNTAG F, KRONER C, LUBUTA P, PEYRAUD R, HORST A, BUCHHAUPT M, SCHRADER J. Engineering Methylobacterium extorquens for de novo synthesis of the sesquiterpenoid α-humulene from methanol[J]. Metabolic Engineering, 2015, 32: 82-94. DOI:10.1016/j.ymben.2015.09.004
|
| [30] |
MÜLLER JEN, LITSANOV B, BORTFELD-MILLER M, TRACHSEL C, GROSSMANN J, BRAUTASET T, VORHOLT JA. Proteomic analysis of the thermophilic methylotroph Bacillus methanolicus MGA3[J]. Proteomics, 2014, 14(6): 725-737. DOI:10.1002/pmic.201300515
|
| [31] |
SANFORD PA, WOOLSTON BM. Synthetic or natural? Metabolic engineering for assimilation and valorization of methanol[J]. Current Opinion in Biotechnology, 2022, 74: 171-179. DOI:10.1016/j.copbio.2021.12.001
|
| [32] |
EGLI T, BOSSHARD C, HAMER G. Simultaneous utilization of methanol-glucose mixtures by Hansenula polymorpha in chemostat: influence of dilution rate and mixture composition on utilization pattern[J]. Biotechnology and Bioengineering, 1986, 28(11): 1735-1741. DOI:10.1002/bit.260281118
|
| [33] |
GUO F, QIAO YY, XIN FX, ZHANG WM, JIANG M. Bioconversion of C1 feedstocks for chemical production using Pichia pastoris[J]. Trends in Biotechnology, 2023, 41(8): 1066-1079. DOI:10.1016/j.tibtech.2023.03.006
|
| [34] |
DAI ZX, GU HL, ZHANG SJ, XIN FX, ZHANG WM, DONG WL, MA JF, JIA HH, JIANG M. Metabolic construction strategies for direct methanol utilization in Saccharomyces cerevisiae[J]. Bioresource Technology, 2017, 245(Pt B): 1407-1412.
|
| [35] |
ZHAN CJ, LI XW, LAN GX, BAIDOO EEK, YANG YK, LIU YZ, SUN Y, WANG SJ, WANG YY, WANG GK, NIELSEN J, KEASLING JD, CHEN Y, BAI ZH. Reprogramming methanol utilization pathways to convert Saccharomyces cerevisiae to a synthetic methylotroph[J]. Nature Catalysis, 2023, 6(5): 435-450. DOI:10.1038/s41929-023-00957-w
|
| [36] |
KUVSHINNIKOV VD, VOROB'EV AV, EROSHIN VK, MINKEVICH IG. Growth efficiency and the specific rate of the yeast Hansenula polymorpha during continuous cultivation on methanol[J]. Prikladnaia Biokhimiia i Mikrobiologiia, 1978, 14(3): 366-372.
|
| [37] |
van DIJKEN JP, OTTO R, HARDER W. Growth of Hansenula polymorpha in a methanol-limited chemostat. Physiological responses due to the involvement of methanol oxidase as a key enzyme in methanol metabolism[J]. Archives of Microbiology, 1976, 111(1/2): 137-144.
|
| [38] |
SUZUKI T, YAMANE T, SHIMIZU S. Mass production of poly-β-hydroxybutyric acid by fully automatic fed-batch culture of methylotroph[J]. Applied Microbiology and Biotechnology, 1986, 23(5): 322-329.
|
| [39] |
WU XY, CAI P, GAO LH, LI YX, YAO L, ZHOU YJ. Efficient bioproduction of 3-hydroxypropionic acid from methanol by a synthetic yeast cell factory[J]. ACS Sustainable Chemistry & Engineering, 2023, 11(16): 6445-6453.
|
| [40] |
WANG Y, FAN LW, TUYISHIME P, LIU J, ZHANG K, GAO N, ZHANG ZH, NI XM, FENG JH, YUAN QQ, MA HW, ZHENG P, SUN JB, MA YH. Adaptive laboratory evolution enhances methanol tolerance and conversion in engineered Corynebacterium glutamicum[J]. Communications Biology, 2020, 3(1): 217. DOI:10.1038/s42003-020-0954-9
|
| [41] |
ZHANG SJ, GUO F, YANG Q, JIANG YJ, YANG SH, MA JF, XIN FX, HASUNUMA T, KONDO A, ZHANG WM, JIANG M. Improving methanol assimilation in Yarrowia lipolytica via systematic metabolic engineering combined with compartmentalization[J]. Green Chemistry, 2023, 25(1): 183-195. DOI:10.1039/D2GC02783F
|
| [42] |
BRAUTASET T, WILLIAMS MD, DILLINGHAM RD, KAUFMANN C, BENNAARS A, CRABBE E, FLICKINGER MC. Role of the Bacillus methanolicus citrate synthase Ⅱ gene, citY, in regulating the secretion of glutamate in L-lysine-secreting mutants[J]. Applied and Environmental Microbiology, 2003, 69(7): 3986-3995. DOI:10.1128/AEM.69.7.3986-3995.2003
|
| [43] |
MELO NTM, MULDER KCL, NICOLA AM, CARVALHO LS, MENINO GS, MULINARI E, PARACHIN NS. Effect of pyruvate decarboxylase knockout on product distribution using Pichia pastoris (Komagataella phaffii) engineered for lactic acid production[J]. Bioengineering, 2018, 5(1): 17. DOI:10.3390/bioengineering5010017
|
| [44] |
KIM SW, KIM P, LEE HS, KIM JH. High production of poly-β-hydroxybutyrate (PHB) from Methylobacterium organophilum under potassium limitation[J]. Biotechnology Letters, 1996, 18(1): 25-30. DOI:10.1007/BF00137805
|
| [45] |
YAMADA R, OGURA K, KIMOTO Y, OGINO H. Toward the construction of a technology platform for chemicals production from methanol: D-lactic acid production from methanol by an engineered yeast Pichia pastoris[J]. World Journal of Microbiology and Biotechnology, 2019, 35(2): 37. DOI:10.1007/s11274-019-2610-4
|
| [46] |
BOZDAG A, KOMIVES C, FLICKINGER MC. Growth of Bacillus methanolicus in 2 mol/L methanol at 50 ℃: the effect of high methanol concentration on gene regulation of enzymes involved in formaldehyde detoxification by the ribulose monophosphate pathway[J]. Journal of Industrial Microbiology & Biotechnology, 2015, 42(7): 1027-1038.
|
| [47] |
SANTOSO A, HERAWATI N, RUBIANA Y. Effect of methanol induction and incubation time on expression of human erythropoietin in methylotropic yeast Pichia pastoris[J]. MAKARA of Technology Series, 2012, 16(1): 29-34.
|
| [48] |
COS O, SERRANO A, MONTESINOS JL, FERRER P, CREGG JM, VALERO F. Combined effect of the methanol utilization (Mut) phenotype and gene dosage on recombinant protein production in Pichia pastoris fed-batch cultures[J]. Journal of Biotechnology, 2005, 116(4): 321-335. DOI:10.1016/j.jbiotec.2004.12.010
|
| [49] |
SCHRADER J, SCHILLING M, HOLTMANN D, SELL D, FILHO MV, MARX A, VORHOLT JA. Methanol-based industrial biotechnology: current status and future perspectives of methylotrophic bacteria[J]. Trends in Biotechnology, 2009, 27(2): 107-115. DOI:10.1016/j.tibtech.2008.10.009
|
| [50] |
HEYLAND J, FU JN, BLANK LM. Correlation between TCA cycle flux and glucose uptake rate during respiro-fermentative growth of Saccharomyces cerevisiae[J]. Microbiology, 2009, 155(Pt 12): 3827-3837.
|
| [51] |
CASPETA L, SHOAIE S, AGREN R, NOOKAEW I, NIELSEN J. Genome-scale metabolic reconstructions of Pichia stipitis and Pichia pastoris and in silico evaluation of their potentials[J]. BMC Systems Biology, 2012, 6: 24. DOI:10.1186/1752-0509-6-24
|
| [52] |
CUI LY, WANG SS, GUAN CG, LIANG WF, XUE ZL, ZHANG C, XING XH. Breeding of methanol-tolerant Methylobacterium extorquens AM1 by atmospheric and room temperature plasma mutagenesis combined with adaptive laboratory evolution[J]. Biotechnology Journal, 2018, 13(6): e1700679.
|
| [53] |
HÖLSCHER T, BREUER U, ADRIAN L, HARMS H, MASKOW T. Production of the chiral compound (R)-3-hydroxybutyrate by a genetically engineered methylotrophic bacterium[J]. Applied and Environmental Microbiology, 2010, 76(16): 5585-5591. DOI:10.1128/AEM.01065-10
|
| [54] |
KARBALAEI M, REZAEE SA, FARSIANI H. Pichia pastoris: a highly successful expression system for optimal synthesis of heterologous proteins[J]. Journal of Cellular Physiology, 2020, 235(9): 5867-5881. DOI:10.1002/jcp.29583
|
| [55] |
BARRIGÓN JM, MONTESINOS JL, VALERO F. Searching the best operational strategies for Rhizopus oryzae lipase production in Pichia pastoris Mut+ phenotype: methanol limited or methanol non-limited fed-batch cultures?[J]. Biochemical Engineering Journal, 2013, 75: 47-54. DOI:10.1016/j.bej.2013.03.018
|
| [56] |
GURRAMKONDA C, POLEZ S, SKOKO N, ADNAN A, GÄBEL T, CHUGH D, SWAMINATHAN S, KHANNA N, TISMINETZKY S, RINAS U. Application of simple fed-batch technique to high-level secretory production of insulin precursor using Pichia pastoris with subsequent purification and conversion to human insulin[J]. Microbial Cell Factories, 2010, 9: 31. DOI:10.1186/1475-2859-9-31
|
| [57] |
VOGL T, STURMBERGER L, KICKENWEIZ T, WASMAYER R, SCHMID C, HATZL AM, GERSTMANN MA, PITZER J, WAGNER M, THALLINGER GG, GEIER M, GLIEDER A. A toolbox of diverse promoters related to methanol utilization: functionally verified parts for heterologous pathway expression in Pichia pastoris[J]. ACS Synthetic Biology, 2016, 5(2): 172-186. DOI:10.1021/acssynbio.5b00199
|
| [58] |
GAO JC, XU JH, ZUO YM, YE CF, JIANG LJ, FENG LJ, HUANG L, XU ZN, LIAN JZ. Synthetic biology toolkit for marker-less integration of multigene pathways into Pichia pastoris via CRISPR/Cas9[J]. ACS Synthetic Biology, 2022, 11(2): 623-633. DOI:10.1021/acssynbio.1c00307
|
| [59] |
GAO JQ, GAO N, ZHAI XX, ZHOU YJ. Recombination machinery engineering for precise genome editing in methylotrophic yeast Ogataea polymorpha[J]. iScience, 2021, 24(3): 102168. DOI:10.1016/j.isci.2021.102168
|
| [60] |
ZHU LP, SONG SZ, YANG S. Gene repression using synthetic small regulatory RNA in Methylorubrum extorquens[J]. Journal of Applied Microbiology, 2021, 131(6): 2861-2875. DOI:10.1111/jam.15159
|
| [61] |
GAO JC, ZUO YM, XIAO F, WANG YL, LI DF, XU JH, YE CF, FENG LJ, JIANG LJ, LIU TF, GAO D, MA B, HUANG L, XU ZN, LIAN JZ. Biosynthesis of catharanthine in engineered Pichia pastoris[J]. Nature Synthesis, 2023, 2(3): 231-242. DOI:10.1038/s44160-022-00205-2
|
| [62] |
LIU SF, DONG HF, HONG K, MENG J, LIN LC, WU X. Improving methanol utilization by reducing alcohol oxidase activity and adding co-substrate of sodium citrate in Pichia pastoris[J]. Journal of Fungi, 2023, 9(4): 422. DOI:10.3390/jof9040422
|
| [63] |
SAKAI Y, MURDANOTO AP, KONISHI T, IWAMATSU A, KATO N. Regulation of the formate dehydrogenase gene, FDH1, in the methylotrophic yeast Candida boidinii and growth characteristics of an FDH1-disrupted strain on methanol, methylamine, and choline[J]. Journal of Bacteriology, 1997, 179(14): 4480-4485. DOI:10.1128/jb.179.14.4480-4485.1997
|
| [64] |
SUNGA AJ, CREGG JM. The Pichia pastoris formaldehyde dehydrogenase gene (FLD1) as a marker for selection of multicopy expression strains of P. pastoris[J]. Gene, 2004, 330: 39-47. DOI:10.1016/j.gene.2003.12.015
|
| [65] |
YU YF, YANG JS, ZHAO FG, LIN Y, HAN SY. Comparative transcriptome and metabolome analyses reveal the methanol dissimilation pathway of Pichia pastoris[J]. BMC Genomics, 2022, 23(1): 366. DOI:10.1186/s12864-022-08592-8
|
| [66] |
WOOLSTON BM, KING JR, REITER M, van HOVE B, STEPHANOPOULOS G. Improving formaldehyde consumption drives methanol assimilation in engineered E. coli[J]. Nature Communications, 2018, 9(1): 2387. DOI:10.1038/s41467-018-04795-4
|
| [67] |
BENNETT RK, GONZALEZ JE, WHITAKER WB, ANTONIEWICZ MR, PAPOUTSAKIS ET. Expression of heterologous non-oxidative pentose phosphate pathway from Bacillus methanolicus and phosphoglucose isomerase deletion improves methanol assimilation and metabolite production by a synthetic Escherichia coli methylotroph[J]. Metabolic Engineering, 2018, 45: 75-85. DOI:10.1016/j.ymben.2017.11.016
|
| [68] |
KRAINER FW, DIETZSCH C, HAJEK T, HERWIG C, SPADIUT O, GLIEDER A. Recombinant protein expression in Pichia pastoris strains with an engineered methanol utilization pathway[J]. Microbial Cell Factories, 2012, 11: 22. DOI:10.1186/1475-2859-11-22
|
| [69] |
YUAN XJ, CHEN WJ, MA ZX, YUAN QQ, ZHANG M, HE L, MO XH, ZHANG C, ZHANG CT, WANG MY, XING XH, YANG S. Rewiring the native methanol assimilation metabolism by incorporating the heterologous ribulose monophosphate cycle into Methylorubrum extorquens[J]. Metabolic Engineering, 2021, 64: 95-110. DOI:10.1016/j.ymben.2021.01.009
|
| [70] |
MOSER JW, PRIELHOFER R, GERNER SM, GRAF AB, WILSON IBH, MATTANOVICH D, DRAGOSITS M. Implications of evolutionary engineering for growth and recombinant protein production in methanol-based growth media in the yeast Pichia pastoris[J]. Microbial Cell Factories, 2017, 16(1): 49. DOI:10.1186/s12934-017-0661-5
|
| [71] |
GASSLER T, BAUMSCHABL M, SALLABERGER J, EGERMEIER M, MATTANOVICH D. Adaptive laboratory evolution and reverse engineering enhances autotrophic growth in Pichia pastoris[J]. Metabolic Engineering, 2022, 69: 112-121. DOI:10.1016/j.ymben.2021.11.007
|
| [72] |
MENG J, LIU SF, GAO L, HONG K, LIU SG, WU X. Economical production of Pichia pastoris single cell protein from methanol at industrial pilot scale[J]. Microbial Cell Factories, 2023, 22(1): 198. DOI:10.1186/s12934-023-02198-9
|
| [73] |
刘永飞, 刘建明, 聂晶磊, 曾安平. 基于CO2等碳一底物的化学品生物合成技术进展及挑战[J]. 科学通报, 2023, 68(19): 2470-2488. LIU YF, LIU JM, NIE JL, ZENG AP. Advances and perspectives of biosynthesis of chemicals based on CO2 and other one-carbon feedstocks[J]. Chinese Science Bulletin, 2023, 68(19): 2470-2488 (in Chinese). |
| [74] |
CHEN CT, CHEN FYH, BOGORAD IW, WU TY, ZHANG RX, LEE AS, LIAO JC. Synthetic methanol auxotrophy of Escherichia coli for methanol-dependent growth and production[J]. Metabolic Engineering, 2018, 49: 257-266. DOI:10.1016/j.ymben.2018.08.010
|
| [75] |
GUO Q, LIU MM, ZHENG SH, ZHENG LJ, MA Q, CHENG YK, ZHAO SY, FAN LH, ZHENG HD. Methanol-dependent carbon fixation for irreversible synthesis of D-allulose from D-xylose by engineered Escherichia coli[J]. Journal of Agricultural and Food Chemistry, 2022, 70(44): 14255-14263. DOI:10.1021/acs.jafc.2c06616
|
| [76] |
TUYISHIME P, WANG Y, FAN LW, ZHANG QQ, LI QG, ZHENG P, SUN JB, MA YH. Engineering Corynebacterium glutamicum for methanol-dependent growth and glutamate production[J]. Metabolic Engineering, 2018, 49: 220-231. DOI:10.1016/j.ymben.2018.07.011
|
| [77] |
BERRIOS J, FLORES MO, DÍAZ-BARRERA A, ALTAMIRANO C, MARTÍNEZ I, CABRERA Z. A comparative study of glycerol and sorbitol as co-substrates in methanol-induced cultures of Pichia pastoris: temperature effect and scale-up simulation[J]. Journal of Industrial Microbiology & Biotechnology, 2017, 44(3): 407-411.
|
| [78] |
CANALES C, ALTAMIRANO C, BERRIOS J. The growth of Pichia pastoris Mut+ on methanol-glycerol mixtures fits to interactive dual-limited kinetics: model development and application to optimised fed-batch operation for heterologous protein production[J]. Bioprocess and Biosystems Engineering, 2018, 41(12): 1827-1838. DOI:10.1007/s00449-018-2005-1
|
| [79] |
JORDÀ J, JOUHTEN P, CÁMARA E, MAAHEIMO H, ALBIOL J, FERRER P. Metabolic flux profiling of recombinant protein secreting Pichia pastoris growing on glucose: methanol mixtures[J]. Microbial Cell Factories, 2012, 11: 57. DOI:10.1186/1475-2859-11-57
|
| [80] |
WANG BL, NESBETH D, KESHAVARZ-MOORE E. Sorbitol/methanol mixed induction reduces process impurities and improves centrifugal dewatering in Pichia pastoris culture[J]. Enzyme and Microbial Technology, 2019, 130: 109366. DOI:10.1016/j.enzmictec.2019.109366
|
| [81] |
SIBIRNY AA. Yeast peroxisomes: structure, functions and biotechnological opportunities[J]. FEMS Yeast Research, 2016, 16(4): fow038. DOI:10.1093/femsyr/fow038
|
| [82] |
HUTTANUS HM, FENG XY. Compartmentalized metabolic engineering for biochemical and biofuel production[J]. Biotechnology Journal, 2017, 12(6).
|
| [83] |
BHATAYA A, SCHMIDT-DANNERT C, LEE PC. Metabolic engineering of Pichia pastoris X-33 for lycopene production[J]. Process Biochemistry, 2009, 44(10): 1095-1102. DOI:10.1016/j.procbio.2009.05.012
|
| [84] |
LIU H, CHEN SL, XU JZ, ZHANG WG. Dual regulation of cytoplasm and peroxisomes for improved α-farnesene production in recombinant Pichia pastoris[J]. ACS Synthetic Biology, 2021, 10(6): 1563-1573. DOI:10.1021/acssynbio.1c00186
|
 2024, Vol. 40
2024, Vol. 40