
中国科学院微生物研究所、中国微生物学会主办
文章信息
- 王莹, 王晓梅, 赵蕴琦, 吴盛辉, 张涌, 权富生
- WANG Ying, WANG Xiaomei, ZHAO Yunqi, WU Shenghui, ZHANG Yong, QUAN Fusheng
- 卵泡液细胞外囊泡携带的microRNA对卵泡闭锁影响的研究进展
- Progress in the effect of microRNA carried by extracellular vesicles in follicular fluid on follicular atresia
- 生物工程学报, 2022, 38(8): 2767-2783
- Chinese Journal of Biotechnology, 2022, 38(8): 2767-2783
- 10.13345/j.cjb.210901
-
文章历史
- Received: December 7, 2021
- Accepted: March 25, 2022
卵泡发育进程中,卵泡闭锁是一种重要的生理现象。哺乳动物性成熟期后,在每个性周期都会有大量卵泡发育,一般情况下,单胎动物只有1−2个优势卵泡能够发展至成熟卵泡并排卵,大部分卵泡会在不同的发育阶段通过细胞凋亡而发生闭锁。卵泡闭锁的发生受多种因素调节,已有的研究表明,颗粒细胞(granulosa cells, GCs)、卵母细胞(oocytes) 以及卵泡内膜细胞(theca cells, TCs) 凋亡均可导致卵泡闭锁的发生,其中,颗粒细胞凋亡起主要作用[1-3]。
卵泡液中含有各种营养物质、微量元素、代谢废物、激素、生长因子以及细胞外囊泡等成分,不同的卵泡液成分对颗粒细胞、卵母细胞以及卵泡内膜细胞代谢、增殖和凋亡等生理过程有不同的影响。这些成分包含有近年来研究的热点问题——细胞外囊泡(extracellular vesicles, EVs),EVs携带大量microRNA,这些microRNA在卵泡发育过程中发挥怎样的作用和如何发挥作用,是该领域研究的关键问题,结合本实验室卵泡液EVs的研究,本文现就有关卵泡液中EVs携带的microRNA对卵泡闭锁的研究进行了总结。
1 卵泡液细胞外囊泡简介 1.1 细胞外囊泡类型及其分离EVs主要分为3种类型[4-7]:起源于内体途径形成的外泌体(exosomes);从质膜上出芽形成的微泡(microvesicles);以及细胞在凋亡裂解时释放的凋亡小体(apoptotic bodies) (图 1)。三种EVs的胞内来源不同[8],这也预示着不同类型的EVs可能执行不同的生物学功能。从细胞膜释放后,EVs通过细胞表面膜蛋白发挥信息交换作用[9],并且在细胞之间发生物质交换、信号传导和信息传递等[10-11]。EVs被认为可以携带大量的生物学物质,包括蛋白质、细胞因子[7]、膜受体、受体配体、核酸(如DNA、mRNA、长短非编码RNA)、脂质[12]和聚糖等。现有的EVs分离纯化方法主要分为两大类:传统的超速离心方法和新方法,如微流体过滤法、免疫吸附法以及非接触法[13]。
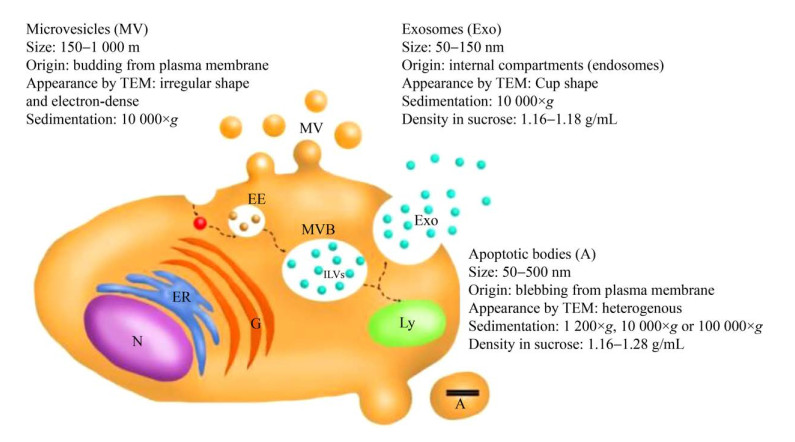
|
| 图 1 细胞外囊泡:生物发生与释放[7] Fig. 1 Extracellular vesicles (EVs): biogenesis and release[7]. EVs are mainly classified in three subgroups: a) microvesicles/microparticles that bud from the plasma membrane; b) exosomes that are generated as ILVs by inward invagination of endosomes membranes giving rise to MVBs and then released into the extracellular space upon fusion of MVBs with the plasma membrane; c) apoptotic bodies that blebs from cells undergoing apoptosis. N: nucleus; ER: endoplasmic reticulum; G: Golgi complex; MVB: multi-vesicular body; ILV: intraluminal vesicle; Ly: lysosome; EE: early endosome. |
| |
在哺乳动物中,几乎所有体液都含有EVs,包括卵泡液,其与卵泡发育息息相关。先前的研究已经成功从牛[14-16]、山羊[17]、猪[18-20]、人[21-22]、猫[23]、马[24]等哺乳动物卵泡液中分离出EVs。研究表明,从不同大小的卵泡中分离出的EVs具有不同的特性和功能[16]。在相同的时间内,牛颗粒细胞对小卵泡(3−5 mm) 来源EVs的摄取程度明显高于中等卵泡(6−9 mm) 和大卵泡(> 9 mm) 的EVs,其中,摄取时间为24 h的摄取效果明显优于2 h。来源于小卵泡、中等卵泡和大卵泡中的EVs对牛颗粒细胞均具有促进细胞增殖的作用,但是,小卵泡的EVs作用效果最显著[16]。来源于小卵泡和大卵泡的EVs可以促进卵丘扩张,促进小鼠和牛卵丘细胞成熟及Ptgs2、Ptx3和Tnfaip6等相关基因的表达,其中,小卵泡的EVs作用效果明显优于对照组和大卵泡组[14]。卵泡液中的EVs促进了卵母细胞、颗粒细胞和卵泡中其他细胞类型之间的通讯,其内容物miRNA作为调控卵母细胞的发育和卵巢成熟的重要成分之一,在EVs介导的卵泡细胞通讯中扮演着重要角色[22]。
2 卵泡液EVs中microRNA在卵泡闭锁中的作用健康卵泡外观是清澈透明的,具有完整的颗粒细胞层,卵丘卵母细胞复合体清晰可见;早期闭锁卵泡外观是浑浊的橙黄色,颗粒细胞层部分破损,卵丘卵母细胞复合体不易辨认;晚期闭锁卵泡外观是浑浊的灰色,颗粒细胞层和卵丘卵母细胞复合体严重脱落[25]。正常生长的卵泡通过卵泡存活因子起到促生长和抗凋亡的作用;通过凋亡因子调控卵泡颗粒细胞和膜细胞等凋亡,导致卵泡退化闭锁。这些因子包括促黄体素(LH) 和促卵泡素(FSH)[26-27]。如果缺少存活因子,凋亡相关信号通路被激活,发生卵泡闭锁,此时,颗粒细胞层减少,卵母细胞核固缩、透明带膨胀塌陷,卵泡膜细胞胞质内出现类脂质、黄素化,构成所谓“间质腺”,与基膜脱离后落入卵泡腔内,卵泡液被吸收,卵泡壁塌陷。然后卵母细胞退化,颗粒细胞、卵泡膜细胞逐渐形成纤维体,被卵泡间质吸收。与此同时,颗粒细胞合成的雌激素减少,孕酮增加,促性腺激素受体数目减少[25]。卵泡闭锁可以发生在卵泡生长和发育过程的任何阶段中,但是在排卵阶段,卵泡内细胞对凋亡信号不敏感,所以没有发生卵泡闭锁现象。卵泡闭锁受不同内分泌因子、旁分泌因子、细胞类型及其状态、信号通路、热应激信号、卵巢黄体的类型、激素、卵泡波以及微环境等调控。但是,大部分卵泡闭锁与颗粒细胞凋亡有主要联系[1-3]。
microRNA是一类由内源基因编码的长度约为22个核苷酸的非编码单链RNA分子,它们在动植物中参与转录后基因表达调控。每个microRNA可以有多个靶基因,而几个microRNA也可以调节同一个基因。这种复杂的调节网络既可以通过一个microRNA来调控多个基因的表达,也可以通过几个microRNA的组合来精细调控某个基因的表达。microRNA存在多种形式,最原始的是pri-miRNA,长度大约为300–1 000个碱基;pri-miRNA经过一次加工后,成为pre-miRNA即microRNA前体,长度大约为70–90个碱基;pre-miRNA再经过Dicer酶酶切后,成为长约20–24 nt的成熟microRNA。已经被鉴定的microRNAs据推测大都是由具有发夹结构,约70个碱基大小形成发夹结构的单链RNA前体经过Dicer酶加工后生成的,有5′端磷酸基和3′端羟基,大小约21−25 nt的小分子RNA片段,定位于RNA前体的3′端或者5′端。microRNA在细胞分化、生物发育及疾病发生发展过程中发挥巨大作用。随着分子生物学的发展,已经证实microRNA参与到多种生物学调控中,包括细胞增殖凋亡、自噬、分化、胚胎发育、干细胞的更新、应激反应和代谢等[28-33]。通过作用于翻译和转录后修饰,microRNA在基因表达调控中发挥关键作用[34]。卵泡内的microRNA参与了原始卵泡形成、卵泡募集和选择、卵泡闭锁、卵母细胞交流、颗粒细胞功能和黄体化等多种卵泡内的生物进程。在卵泡生长发育各阶段几乎都有可能出现卵泡闭锁现象,卵母细胞的凋亡主要会导致初级卵泡的闭锁,有时候也会伴随着卵泡细胞的凋亡;而颗粒细胞凋亡最有可能导致次级卵泡和三级卵泡发生闭锁[35]。目前研究最多的是颗粒细胞凋亡能导致卵泡闭锁,颗粒细胞凋亡也是导致卵泡闭锁现象最重要的原因。但是,细胞之间的相互作用对卵泡闭锁也有很重要的影响。同时,在卵泡液微环境中,颗粒细胞、卵母细胞以及卵泡内膜细胞这3种细胞研究最多、联系最密切。所以考虑从颗粒细胞、卵母细胞以及卵泡内膜细胞携带的microRNA着手,按照不同的细胞类型,同时结合从卵巢卵泡液分离得到的EVs中的microRNA表达谱,介绍这些microRNA与卵泡闭锁发生的必然联系。
EVs是存在于生物流体中的膜结合囊泡,其携带和转移调节分子,例如microRNA和蛋白质,能介导细胞间通讯。卵泡液EVs对卵泡生长、卵母细胞发育和成熟均有重要作用[22, 36-53]。通过鉴定人类卵泡液EVs中microRNA,发现它们在人类卵泡液中具有很高的代表性,并参与卵泡成熟。它们可能代表辅助生殖技术中卵母细胞质量的非侵入性生物标志物[28],并且在不同发情周期的卵泡中,EVs中microRNA含量不同[29]。总之,卵泡液EVs中microRNA参与卵泡发育,对卵泡生长具有重要意义。
来自于不同卵泡大小EVs中的microRNA对卵泡的作用会随卵泡尺寸的大小改变而变化,对细胞状态也有不同程度的影响。例如:小卵泡EVs中含量丰富的microRNA与细胞增殖通路相关,大卵泡EVs中含量丰富的microRNA与炎症反应通路相关[30]。除此之外,卵泡闭锁期间microRNA表达量会发生明显变化[31]。microRNA在卵巢中的表达随细胞类型、功能和发情周期的不同而改变。已有研究证明,EVs携带的microRNA在牛卵泡液中的存在,在卵泡液微环境中,卵母细胞生长依赖于EVs携带的microRNA特征而变化[32]。目前,已经有很多关于卵泡液EVs携带的microRNA的研究,从卵巢卵泡液中分离EVs的方法也较成熟。早前已经有研究者从卵巢卵泡液中分离得到EVs,进而鉴定了EVs携带的microRNA,通过对EVs进行标记,证明卵泡内细胞能够摄取EVs,行使相应的功能[33]。总之,卵泡液EVs中microRNA通过作用于卵泡内的细胞来调控卵泡闭锁。
3 卵泡液EVs中microRNA通过卵泡内细胞调控卵泡闭锁根据文献报道,本文对卵泡液EVs中microRNA参与调控卵泡闭锁主要的细胞类型进行了总结,见图 2和表 1。在卵泡中,颗粒细胞是数量最多的细胞类型,在卵泡生长、发育过程中起着至关重要的作用。颗粒细胞作为卵母细胞营养物质的供给者,调控卵母细胞发育和成熟。颗粒细胞凋亡是诱导卵泡闭锁的关键原因,同时还影响卵泡数量和质量[3]。颗粒细胞凋亡过程受多种因素的调控,microRNA为其中之一的调控因素[3]。在卵泡发育成熟过程中,颗粒细胞、卵母细胞和卵泡内膜细胞之间是互相作用并共同调控卵泡功能,发生卵泡闭锁。内膜是一种特殊的基质层,它包含毛细血管,卵泡膜细胞可合成雄激素,由临近的颗粒细胞将雄激素转化成雌二醇,为卵泡发育提供营养物质,介导卵母细胞与颗粒细胞之间的交互作用进而参与调控细胞凋亡和卵泡发育过程。
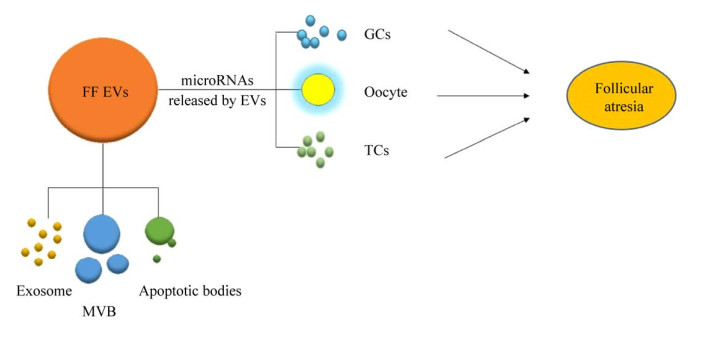
|
| 图 2 卵泡液细胞外囊泡中的microRNA对卵泡闭锁的调节 Fig. 2 Regulation of follicular atresia by microRNAs in extracellular vesicles of follicular fluid. FF EVs: extracellular vesicles in follicular fluid; exosome: exosomes; MVB: multi-vesicular body; apoptotic bodies: apoptotic bodies; GCs: granulosa cells; oocyte: oocyte; TCs: theca cells. |
| |
| Cell type affected | microRNA in follicular fluid EVs | References |
| GCs | miR-155-HIFIA; miR-199a-5p; miR-222; miR-150; miR-378-VEGFA; miR-372; miR-382; miR-29; miR-375; miR-146b; miR-181b; miR-10a-5p; miR-26b; miR-144-5p miR-146a; miR-10a; miR-644-5p |
[24, 31, 34-50] |
| Oocytes | miR-204; miR-197; miR-146b; miR-30; miR-383; miR-2285; miR-451; miR-132; miR-486; miR-874; let-7c; miR-375; miR-24; miR-19a; miR-125b; miR-106b; miR-374a; miR-15b |
[15, 51] |
| TCs | miR-199a-5p; miR-150; miR-378; miR-155; miR-222 |
[24, 31] |
关于卵泡液EVs携带的microRNA通过颗粒细胞调控卵泡闭锁的研究总结见表 2。目前已经有大量的研究表明,microRNA可以调控颗粒细胞参与卵泡闭锁。其中,miR-181b能通过抑制SMAD7基因的表达来激活TGF-β通路来抑制颗粒细胞凋亡[41]。卵泡闭锁均发生在排卵前的阶段,而在排卵前卵泡颗粒细胞中未检测到miR-382,但是与排卵前相比,卵泡颗粒细胞中miR-372和miR-382水平升高[40]。这说明miR-372和miR-382可能参与了卵泡闭锁。除此之外,miR-375和miR-222参与调控颗粒细胞凋亡[44-45]。
| microRNA | Targets | Function | References |
| miR-26b | HAS2 | Promote GCs apoptosis | [38] |
| miR-181b | SMAD7 | Can inhibit SMAD7 by activating TGF-β pathway inhibition, thereby inhibiting GCs apoptosis | [41] |
| miR-146b | CYP19A1 | Reduce the proliferation ability of bovine cumulus cells and significantly increase the rate of apoptosis of cumulus cells | [42] |
| miR-29 | PTX3 | Involved in the PI3K/AKT/mTOR and Erk1/2 signaling pathways, and plays an important role in GCs proliferation, apoptosis and steroid production | [43] |
| miR-222 | THBS1 | Inhibit GCs apoptosis | [44] |
| miR-375 | BMPR2 | Inhibit GCs apoptosis | [45] |
| miR-10a-5p | CTGF | Promote GCs apoptosis | [46] |
内源性非编码RNA研究的出现,为探索卵泡闭锁的调控机制提供了新的视角。miR-26b通过HAS2-HA-CD44-caspase-3途径直接靶向诱导颗粒细胞凋亡和卵泡闭锁[38]。HAS2表达的降低是miR-26b促进GC凋亡的机制之一。而miR-146b是一种调控颗粒细胞功能、卵泡发育和雌性生殖的新型表观遗传因子。通过降低CYP19A1表达来上调miR-146b,从而促进猪卵巢颗粒细胞凋亡[43]。miR-29及其靶基因参与了卵泡生长过程中的PI3K/AKT/mTOR和Erk1/2信号通路[43],其在颗粒细胞增殖、凋亡和类固醇生成中发挥重要作用。这些结果为研究卵泡生长发育增添了新的视角,丰富了EVs中microRNA与卵泡闭锁相关研究。
卵泡闭锁是结缔组织生长因子(CTGF) 参与的复杂精细调控的生物学过程[46-47]。在体外上调闭锁卵泡中的miR-10a-5p,促进颗粒细胞凋亡。同时,在猪颗粒细胞凋亡和卵泡闭锁过程中,还有circRNA调节CTGF通路,即circIeRNA/miR-10a-5p/CTGF颗粒细胞凋亡途径,为circRNAs在卵巢生理功能调节中的作用提供了新的见解。除此之外,更多更深入的研究已经应用全基因组深度环状RNA测序来筛选健康和早期闭锁的猪卵巢腔卵泡中的环状RNA[46],为卵泡闭锁起始阶段的转录组谱提供了有价值的参考。
3.2 卵泡液EVs携带的microRNA通过卵母细胞调控卵泡闭锁卵泡液EVs参与卵母细胞生长发育的信号通路[28, 53],这与EVs携带的生物分子有关。最新研究表明,卵泡液EVs改善了玻璃化卵母细胞的减数分裂恢复,并可作为一种工具来改善配子低温保存。卵泡液EVs能够调节卵泡生长、卵母细胞能量代谢、卵母细胞成熟、应激反应和细胞间的通讯[23]。这证明卵泡液EVs可能在卵母细胞发育和卵泡发育状态之间发挥重要作用,可能参加了卵泡闭锁的相关调控。
近年来,研究人员开启了在EVs中携带microRNA、siRNA、CRISPR-cas9复合物和蛋白质等特定分子的可能性的研究,为EVs存在更多应用与功能开辟了新的前景。ANCs与APCs的实验对比分析显示[15],从ANC卵泡液中分离的EVs中miR-2285、miR-451、miR-132、miR-486和miR-874下调,这些microRNA参与了多种途径,包括TGF-β信号通路,这与卵母细胞和胚胎发育有关。miR-204、miR-197、miR-146b、miR-30d和miR-383在卵泡液中的水平较低,会下调PI3K-Akt信号通路中的几个基因,该信号通路与卵泡功能相关,可以控制卵泡和卵母细胞的生长和存活[51]。因此,通过下调miR-204、miR-197、miR-146b、miR-30和miR-383,直接影响了卵泡闭锁。在不同的发情周期阶段,EVs携带的microRNA含量是不同的[54]。生物毒性物质对卵泡闭锁有重要的影响。最近研究发现,两种生物毒性物质邻苯二甲酸盐和苯酚的浓度与卵巢卵泡液中EVs携带的8种microRNA表达量相关,即let-7c、miR-375、miR-24、miR-19a、miR-125b、miR-106b、miR-374a和miR-15b[48]。因此,这些microRNA表达量的改变也会调控卵泡闭锁,但是,其中的具体机制还有待研究。
3.3 卵泡液EVs携带的microRNA通过卵泡内膜细胞调控卵泡闭锁有研究已经证实,microRNA可以通过卵泡内膜细胞调控卵泡功能,进而影响卵泡闭锁。目前相关的文献很少,仅有的文献中报道的microRNA如下[24, 34]:miR-199a-5p、miR-150、miR-378、miR-155和miR-222。目前还尚未有更多更深入的研究去发现其中的具体作用机制,还有待进一步探究。
牛卵泡液健康组和卵泡闭锁组的转录组分析发现大部分卵泡闭锁与细胞周期和DNA复制有关[55]。在卵泡闭锁时,细胞周期和DNA复制会停止,而不是像颗粒细胞会表现出凋亡相关基因上调。在卵泡闭锁过程中,内膜上几乎没有发现与细胞凋亡相关的指标。但也有研究发现,在马卵泡内膜细胞中有两个显著上调的转录本,分别是与排卵期间甾体样基因的转录抑制因子有关的NR4A2基因和介导卵母细胞成熟的EREG基因[56]。卵泡内膜细胞可以通过与卵母细胞间的相互作用来调控卵泡闭锁。
4 卵泡液EVs中microRNA调控卵泡闭锁的关键途径 4.1 卵泡液EVs携带的microRNA诱导卵泡闭锁卵泡液EVs携带的microRNA诱导卵泡闭锁作用的途径通过细胞生长因子、激素以及细胞凋亡调节系统发挥作用,详细总结见表 3和图 3。
| Impact factors | Targets | miRNA | Function | References |
| EGF/TGF | TGFBR1 | let-7a; let-7g | Promote GCs apoptosis | [57-60] |
| FoxO1 | miR-183-96-182 cluster | Promote GCs apoptosis | [61-62] | |
| VEGFA | miR-361-5p | Regulate follicular atresia and GCs apoptosis | [63] | |
| SMAD7 | miR-181b | Promote GC apoptosis | [64] | |
| TGFBR2 | miR-29c | Regulate follicular atresia and GCs apoptosis | [65] | |
| FSHR | miR-143 | Promote GC apoptosis and induce FSHR expression | [66] | |
| SMAD4 | miR-26b | Promote GCs apoptosis | [67] | |
| Androgen | H3K27me3 | miR-143 | Negatively regulate estrogen and androgen, induce GCs apoptosis | [68] |
| AR | miR-125b | Promote the expression of pro-apoptotic proteins and enhance FSHR expression | [69] | |
| Fas /FasL | SMAD5 | miR-23a; miR-27a | Promote GCs apoptosis | [70] |
| GnRH | Unknown | miR-26a | Regulate GnRH expression | [71] |
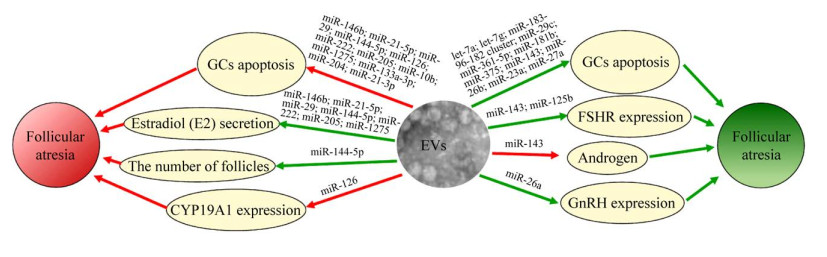
|
| 图 3 卵泡液EVs中microRNA调控卵泡闭锁 Fig. 3 microRNA carried by extracellular vesicles (EVs) in follicular fluid regulates follicular atresia. The green arrow and red arrow represent positive regulation and negative regulation, respectively. |
| |
表皮生长因子EGF是一种活性物质,主要作用是促进皮肤细胞的增殖、分化,加速新陈代谢。EGF能显著促进卵泡颗粒细胞增殖和卵母细胞成熟。EGF能对大、中、小卵泡产生明显作用,而其他细胞因子没有这么强的激发作用。EGF可以抑制大鼠颗粒细胞的卵泡刺激素(FSH) 和雌激素的合成,使卵泡内的雌激素和雄激素比值下降,从而调控卵泡的闭锁[57-59]。随着卵泡的发育与成熟,转化生长因子α (TGF-α) 表达逐渐减弱,协同调控卵母细胞生长、卵泡细胞增殖分化和黄体细胞凋亡[72]。转化生长因子β (TGF-β) 诱导垂体分泌FSH,它和FSH共同促进卵泡发育[73]。诸多研究表明,很多miRNA能调控转化生长因子表达。其中,let-7a[57]和let-7g[58]通过靶向TGFBR1和下调TGF-β信号通路来调节猪卵巢颗粒细胞凋亡,诱导卵泡闭锁[60]。miR-183-96-182簇是哺乳动物卵巢功能所必需的多顺反子miRNA簇,SMAD4通过抑制FoxO1,诱导miR-183-96-182簇抑制颗粒细胞凋亡,由TGF-β/SMAD信号通路调节卵泡闭锁和雌性生殖[61-62]。miR-361-5p在闭锁过程中显著上调,受SMAD4调控后下调。SMAD4是TGF-β信号传导的中心细胞内介质。TGF-β信号已被认为是颗粒细胞凋亡的中心触发因素。SMAD4/miR-361-5p/VEGFA调控网络在颗粒细胞凋亡和卵泡闭锁中发挥作用[63]。miR-181b是一种在猪卵泡闭锁期间下调的microRNA,下调的miR-181b可以抑制SMAD7和TGF-β信号通路来促进颗粒细胞凋亡[64]。据报道,miR-29c、miR-143和miR-26b也能通过EGF和TGF来调节卵泡闭锁[65-67]。骨形态发生蛋白15 (BMP15) 和生长分化因子9 (GDF9) 是TGF-β超家族的成员。通过自分泌和旁分泌机制,这两种因子可以局部调节卵巢中的细胞分化、增殖和其他功能,过表达miR-375后显著增加颗粒细胞凋亡率和BMP15/GDF9受体的表达,因此miR-375在卵泡生长、闭锁、排卵、受精、繁殖和维持中起着至关重要的作用[45]。
4.1.2 雄激素在卵泡的生长及发育过程中,雄激素具有双重作用。适量的雄激素有利于卵泡的募集、生长及发育,并且减少卵泡闭锁;过量的雄激素会抑制卵泡生长,诱导卵泡凋亡和闭锁,最终发生排卵障碍。卵巢产生雄激素是雌二醇正常周期性分泌的必要条件,对卵泡发育具有重要作用,还能促进颗粒细胞增殖。雄激素仅在成熟卵泡中促进细胞凋亡和随后的卵泡闭锁,众多研究表明雄激素与卵泡闭锁之间的机制也可以由microRNA调控。其中,miR-143通过抑制HSD17β4、ER1和PTGS2的类固醇生成相关基因,负向调节雌激素和雄激素,诱导细胞凋亡和卵泡闭锁[68]。雄激素不足会降低miR-125b的表达,通过核和核外信号通路来诱导卵泡闭锁,进而增强促凋亡蛋白的表达。并且雄激素增强FSH受体表达,从而促进FSH介导的卵泡生长和发育[69]。除此之外,miR-23a/ miR-27a介导的细胞凋亡是通过FasL-Fas途径发生的,抑制miR-23a/miR-27a的表达促进颗粒细胞凋亡,诱导卵泡闭锁[70]。miR-26a还能通过调节促性腺激素释放激素的分泌来诱导卵泡闭锁,但是具体机制不清楚[71]。
4.2 卵泡液EVs携带的microRNA抑制卵泡闭锁卵泡液EVs携带的microRNA抑制卵泡闭锁的途径同样是通过激素、细胞生长因子发挥作用,详细总结结果见表 4和图 3。
| Impact factor | Target | miRNA | Function | References |
| Estrogen | CYP19A1 | miR-1275 | Inhibit GCs apoptosis and increase serum estradiol | [74] |
| PTEN | miR-144-5p | Promote the number of follicles, increase estradiol secretion and reduce GCs apoptosis | [75] | |
| Smad7 | miR-21-5p | Inhibit GCs apoptosis and increase serum estradiol | [76] | |
| FSHR | miR-126 | Induce CYP19A1 expression | [77] | |
| CREB1 | miR-205 | Inhibit GCs apoptosis and regulate estradiol secretion | [78] | |
| CYP19A1 | miR-10b | Inhibit follicular atresia | [79] | |
| IGF | IGF1 | miR-133a-3p; miR-10b | Inhibit GCs apoptosis | [79-81] |
| FOXK2 | miR-204 | Inhibit GCs apoptosis | [82] | |
| FGF | FGF2 | miR-21-3p | Inhibit AKT/mTOR signaling and inhibit GCs apoptosis | [83] |
雌激素由雌性动物卵巢和胎盘分泌的,天然雌激素主要是雌二醇(E2)、雌酮、雌三醇,其中,雌二醇生物活性最强,研究最多。目前已经发现很多miRNA能调控雌激素,从而调控卵泡闭锁。miR-1275是猪卵巢卵泡闭锁期间差异表达的microRNA之一[74]。miR-1275通过直接与3′UTR结合降低LRH-1的表达,阻止了LRH-1蛋白与CYP19A1启动子的相互作用,抑制雌二醇合成,促进颗粒细胞凋亡,并导致猪卵巢卵泡闭锁。骨髓间充质干细胞(bone marrow mesenchymal stem cells, BMSCs) 衍生的外泌体通过传递miR-144-5p来治疗大鼠的卵泡闭锁,通过添加miR-144-5p促进大鼠卵泡数量,增加E2分泌,减少颗粒细胞凋亡[75]。全基因组分析和定量实时PCR显示,miR-146b在卵泡闭锁期间显著上调[42]。miR-146b是一种保守且富含卵巢的miRNA,miR-146b直接靶向E2合成信号的关键酶CYP19A1,调节猪的E2分泌、颗粒细胞凋亡和卵泡闭锁。此外,miR-146b与CYP19A1的3′非翻译区相互作用以阻止翻译,从而调节CYP19A1介导的E2分泌和颗粒细胞凋亡[42]。miR-21-5p的增加能抑制颗粒细胞凋亡、增加血清E2[76]。miR-29通过激活PI3K/AKT/ mTOR信号通路靶向PTX3,抑制E2生成、抑制CYP19A1、CYP11A1、StAR和HSD3B的表达和抑制细胞凋亡[43]。SMAD4诱导CYP19A1表达(编码芳香酶,雌激素生物合成的关键酶),并且SMAD4通过miR-126/FSHR轴抑制颗粒细胞凋亡和卵泡闭锁[77]。miR-222模拟物促进雌激素水平,抑制颗粒细胞凋亡[44]。降低miR-205表达能显著抑制小鼠颗粒细胞凋亡,并促进雌激素分泌[78]。
4.2.2 胰岛素样生长因子(insulin-like growth factor, IGF)胰岛素样生长因子IGF是一种神经营养因子,IGF-Ⅰ、IGF-Ⅱ及其各自的受体和胰岛素生长因子结合蛋白构成了IGF系统。IGF 45%的分子结构与胰岛素相似,在受体和功能方面与胰岛素类似。胰岛素样生长因子结合蛋白(insulin-like growth factor binding proteins, IGFBP) 调节IGF与受体结合,调节IGF-Ⅰ的生物活性,能抑制过多的雄激素产生,抑制卵泡闭锁[84]。IGFBP是一种闭锁因子,能抑制颗粒细胞凋亡来抑制卵泡闭锁[85]。miR-10b通过PI3K/AKT通路和IGFⅠ调控牛颗粒细胞凋亡[79-80]。猪卵泡miR-133a-3p下调会抑制G1和G2/M期的运转,以及类固醇激素代谢,有助于颗粒细胞周期运转,减少颗粒细胞凋亡,抑制卵泡闭锁[81]。通过磷酸肌醇3-激酶(PI3K) 和蛋白激酶B (PKB/Akt) 途径,IGF1抑制颗粒细胞凋亡[86]。鸡萎缩的卵巢中下调miR-204能通过IGF调控PI3K/AKT/mTOR通路,增强FOXK2表达,抑制颗粒细胞凋亡,抑制卵泡闭锁[82]。IGF不是单一发挥作用的,研究发现IGF-I、IGFBP-1、IGFBP-2、IGFBP-4、IGFBP-5和IGFBP-6能与促性腺激素互作,IGF-I、IGFBP-1、IGFBP-2、IGFBP-3和IGFBP-4能与雌激素/孕激素互作,最终调控卵泡发育和闭锁[87]。
4.2.3 成纤维细胞生长因子(fibroblast growth factor, FGF)成纤维细胞生长因子FGF是一种多肽,由垂体和下丘脑分泌,包括7个成员:hst/ks3、bFGF、aFGF、int-2、FGF-5、FGF-6以及FGF-7,它们之间的同源性达到35%–55%,不同种属间成纤维细胞生长因子也有很高的同源性。其中,bFGF主要来源于黄体细胞和颗粒细胞,能促进颗粒细胞增殖,减少颗粒细胞凋亡,参与卵泡发育和生长。也有研究表明,FGF能调控颗粒细胞状态来控制卵泡闭锁,miR-21-3p直接靶向FGF2,通过抑制AKT/mTOR信号传导来抑制牛颗粒细胞自噬,抑制卵泡闭锁[83]。
5 卵泡液EVs中的microRNA对于生殖调控及其生殖疾病诊断的研究展望EVs能够从其他细胞中携带和转移不同的大分子,并通过这种方式影响受体细胞的功能。在不同的生物大分子中,研究最多的是microRNA。microRNA是一类参与基因表达调控的非编码RNA大家族。笔者所在实验室使用碘克沙醇密度梯度离心法将sEVs进一步区分亚型,得到两层密度不同sEVs亚型,分别命名为高密度细胞外囊泡(HD-sEVs或sEVs_F8) 和低密度细胞外囊泡(LD-sEVs或sEVs_F6)。笔者实验室对卵泡液中HD-sEVs和LD-sEVs进行microRNA测序分析,其富集的microRNA见表 5,可以看出不同EVs亚型之间microRNA差异极大。目前,实验室正着力于研究特定microRNA对卵泡发育、卵泡闭锁、颗粒细胞增殖和凋亡的影响,部分microRNA的功能得到验证,为卵泡发育机制研究提供了新的方向和认识[88]。深入理解microRNA网络的作用,不仅有助于理解颗粒细胞凋亡与卵泡发育、闭锁之间的机制,也为不孕症和其他卵巢功能障碍的诊断和治疗提供新的策略。卵母细胞-颗粒细胞-膜细胞严格的相互作用调控卵泡的生长发育,细胞与细胞之间通过各种分子信号作为桥梁,在卵泡液中构建成了庞大的信息交流枢纽,其中,microRNA的作用不可忽视。研究表明,前列腺癌细胞与骨细胞通过EVs中RNA进行潜在交流[89],这些交流作用对多种信号通路具有显著影响,在细胞内各种不同的生理过程中起调节作用,能够作用于颗粒细胞和卵泡内膜细胞[90],继而通过细胞机制来调控卵泡闭锁的进程。总之,卵泡液EVs中microRNA在卵泡液微环境中起细胞间通讯作用[7, 91]。
| Differentially expressed microRNAs | Gene ID |
| sEVs_F6 | bta-miR-26c; bta-let-7c; bta-let-7d; bta-let-7b; bta-miR-151-5p; bta-miR-7; bta-miR-34a; bta-miR-16b; bta-miR-15b; bta-miR-497; bta-miR-16a; bta-miR-125b; bta-miR-449a; bta-miR-204; bta-miR-574; bta-miR-127; bta-miR-3601; bta-miR-31; bta-miR-195; bta-let-7e; bta-miR-139; bta-miR-342; bta-miR-125a; bta-miR-30d; bta-miR-224; bta-miR-3432a; bta-miR-379; bta-miR-28; bta-miR-21-5p; bta-miR-455-3p; bta-miR-199b; bta-miR-145; bta-miR-199a-5p; bta-miR-193a-3p; bta-miR-1271; bta-miR-103; bta-miR-30e-5p; bta-miR-200a; bta-miR-33a; bta-miR-507b; bta-miR-362-5p; bta-miR-181b; bta-miR-339a; bta-miR-135a; bta-miR-340; bta-miR-153; bta-miR-491; bta-miR-196b; bta-miR-339b; bta-miR-2387; bta-miR-12023; bta-miR-182; bta-miR-345-5p; bta-miR-148c; bta-miR-708; bta-miR-181d; bta-miR-23b-3p; bta-miR-29b; bta-miR-96; bta-miR-500; bta-miR-206; bta-miR-29c; bta-miR-769 bta-miR-2898; bta-miR-2320-5p; bta-miR-495; bta-miR-223; bta-miR-331-3p; bta-miR-141; bta-miR-2284t-3p; bta-miR-1260b; bta-miR-2904; bta-miR-27a-5p; bta-miR-23b-5p; bta-miR-1249; bta-let-7i; bta-miR-26a; bta-miR-26b; bta-miR-98; bta-miR-374b; bta-miR-3604; bta-miR-411a; bta-miR-126-3p; bta-miR-10174-3p; bta-miR-99b; bta-miR-30b-5p; bta-miR-155; bta-miR-507-3p; bta-miR-30c; bta-miR-450a; bta-miR-451; bta-miR-17-5p; bta-miR-3613a; bta-miR-181a; bta-miR-151-3p; bta-miR-30a-5p; bta-miR-1; bta-miR-374a; bta-miR-383; bta-miR-194; bta-miR-126-5p; bta-miR-6120-3p; bta-miR-30f; bta-miR-2336; bta-miR-376b; bta-miR-6119-5p; bta-miR-450b; bta-miR-411b; bta-miR-505; bta-miR-411c-3p; bta-miR-2285bf; bta-miR-142-5p |
| sEVs_F8 | bta-miR-92a; bta-miR-424-5p; bta-miR-6123; bta-miR-93; bta-miR-660; bta-miR-128; bta-miR-19b; bta-miR-10b; bta-miR-2284y; bta-miR-335; bta-miR-380-3p; bta-miR-2284x; bta-let-7a-3p; bta-miR-2284ab; bta-miR-423-5p; bta-miR-1307; bta-miR-20a; bta-miR-423-3p; bta-miR-484; bta-miR-2299-3p; bta-miR-425-3p; bta-miR-152; bta-miR-2285av; bta-miR-130a bta-miR-410; bta-miR-671; bta-miR-369-3p; bta-miR-382; bta-miR-19a; bta-miR-376a bta-miR-2284aa; bta-miR-2483-3p; bta-miR-2284w; bta-miR-2285y; bta-miR-138; bta-miR-11986b; bta-miR-6522; bta-miR-6517; bta-miR-2285bc; bta-miR-760-3p; bta-miR-12031; bta-miR-2285ci; bta-miR-2313-3p; bta-miR-130b; bta-miR-2284h-5p; bta-miR-2285u; bta-miR-132; bta-miR-767; bta-miR-188; bta-miR-2285aj-5p; bta-miR-2285ak-5p; bta-miR-2382-5p; bta-miR-376d; bta-miR-2285bz; bta-miR-1197; bta-miR-2285m; bta-miR-2285p; bta-miR-2376; bta-miR-409b; bta-miR-365-5p; bta-miR-326; bta-miR-2382-3p; bta-miR-323b-3p; bta-miR-6536; bta-miR-3154; bta-miR-9851; bta-miR-2367-3p; bta-miR-656; bta-miR-20b bta-miR-2285be; bta-miR-124a; bta-miR-346; bta-miR-124b; bta-miR-2284m; bta-miR-12015; bta-miR-2450a |
从细胞释放开始,成熟的microRNA就参与到细胞间的信息传递中。事实上,对卵泡液中EVs的鉴定及其携带的microRNA的研究可以探索生殖疾病的诊断,为辅助生殖治疗提供卵母细胞质量检测的生物标志物,为哺乳类动物生殖生物学研究以及相关繁殖障碍的预警、预防提供依据[92]。
结合国内外研究现状和本实验室已经开展的卵泡液细胞外囊泡的系列研究,对卵泡液EVs中microRNA参与生殖调控及生殖疾病诊断需要开展以下工作:(1) 卵泡液EVs中microRNA与卵泡发育以及超数排卵效果的关联研究。(2) 卵泡闭锁或者卵泡发育不良与卵泡液EVs中microRNA的关联研究。(3) 卵泡液EVs在卵泡发育过程中颗粒细胞、卵母细胞以及膜间质细胞之间互作与细胞信息传递过程中microRNA作用,卵泡液EVs中microRNA是如何调控细胞通信并影响受体细胞的基因表达和功能改变?(4) 卵泡液EVs中除microRNA以外,携带的其他生物成分发挥了什么功能?这些成分对卵泡闭锁、生殖调控及其生殖疾病诊断有什么意义?(5) 卵泡液EVs可以穿越生物屏障,它是否可以作为药物载体参与卵泡闭锁、生殖调控及生殖疾病治疗?卵泡液EVs中microRNA是否可以作为生殖疾病发病机制、诊断、预后和治疗的生物标志物?(6) 在卵泡发育不同阶段,不同物种间卵泡液EVs中microRNA表达谱的差异分析以及可能参与的生殖调控通路研究等。
理解卵泡液EVs发挥作用的分子和细胞机制,了解卵泡液EVs中microRNA如何通过细胞机制调节卵泡闭锁的发生,对于选择最佳质量的生殖细胞具有重要的临床意义,对于畜牧繁殖技术和人类辅助生殖技术发展具有理论指导,可为卵泡发育调控提供新的研究方向和思路。
| [1] |
Matsuda F, Inoue N, Manabe N, et al. Follicular growth and atresia in mammalian ovaries: regulation by survival and death of granulosa cells. J Reprod Dev, 2012, 58(1): 44-50. DOI:10.1262/jrd.2011-012
|
| [2] |
Yu YS, Sui HS, Han ZB, et al. Apoptosis in granulosa cells during follicular atresia: relationship with steroids and insulin-like growth factors. Cell Res, 2004, 14(4): 341-346. DOI:10.1038/sj.cr.7290234
|
| [3] |
Zhang JB, Xu YX, Liu HL, et al. microRNAs in ovarian follicular atresia and granulosa cell apoptosis. Reprod Biol Endocrinol, 2019, 17(1): 9. DOI:10.1186/s12958-018-0450-y
|
| [4] |
Vader P, Breakefield XO, Wood MJA. Extracellular vesicles: emerging targets for cancer therapy. Trends Mol Med, 2014, 20(7): 385-393. DOI:10.1016/j.molmed.2014.03.002
|
| [5] |
Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol, 2013, 200(4): 373-383. DOI:10.1083/jcb.201211138
|
| [6] |
Colombo M, Raposo G, Théry C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol, 2014, 30: 255-289. DOI:10.1146/annurev-cellbio-101512-122326
|
| [7] |
Aiello A, Giannessi F, Percario ZA, et al. An emerging interplay between extracellular vesicles and cytokines. Cytokine Growth Factor Rev, 2020, 51: 49-60. DOI:10.1016/j.cytogfr.2019.12.003
|
| [8] |
Chung IM, Rajakumar G, Venkidasamy B, et al. Exosomes: current use and future applications. Clin Chim Acta, 2020, 500: 226-232. DOI:10.1016/j.cca.2019.10.022
|
| [9] |
Delauzun V, Amigues B, Gaubert A, et al. Extracellular vesicles as a platform to study cell-surface membrane proteins. Methods, 2020, 180: 35-44. DOI:10.1016/j.ymeth.2020.03.004
|
| [10] |
Becker A, Thakur BK, Weiss JM, et al. Extracellular vesicles in cancer: cell-to-cell mediators of metastasis. Cancer Cell, 2016, 30(6): 836-848. DOI:10.1016/j.ccell.2016.10.009
|
| [11] |
Yáñez-Mó M, Siljander PRM, Andreu Z, et al. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles, 2015, 4: 27066. DOI:10.3402/jev.v4.27066
|
| [12] |
Skotland T, Sagini K, Sandvig K, et al. An emerging focus on lipids in extracellular vesicles. Adv Drug Deliv Rev, 2020, 159: 308-321. DOI:10.1016/j.addr.2020.03.002
|
| [13] |
王亨琴, 王晓梅, 孟凯, 等. 卵泡液中细胞外囊泡及其携带的microRNA对卵泡发育的作用. 生物工程学报, 2020, 36(4): 632-642. Wang HQ, Wang XM, Meng K, et al. Effect of extracellular vesicles and microRNAs in follicular fluid on follicular development. Chin J Biotech, 2020, 36(4): 632-642 (in Chinese). |
| [14] |
Hung WT, Hong X, Christenson LK, et al. Extracellular vesicles from bovine follicular fluid support cumulus expansion. Biol Reprod, 2015, 93(5): 117.
|
| [15] |
Hailay T, Hoelker M, Poirier M, et al. Extracellular vesicle-coupled miRNA profiles in follicular fluid of cows with divergent post-calving metabolic status. Sci Rep, 2019, 9(1): 12851. DOI:10.1038/s41598-019-49029-9
|
| [16] |
Hung WT, Navakanitworakul R, Khan T, et al. Stage-specific follicular extracellular vesicle uptake and regulation of bovine granulosa cell proliferation. Biol Reprod, 2017, 97(4): 644-655. DOI:10.1093/biolre/iox106
|
| [17] |
丁强, 李亚新, 陈玉林. 山羊卵巢卵泡液中外泌体分离鉴定及其对颗粒细胞的影响. 中国畜牧兽医学会养羊学分会会议论文集. 石家庄: 中国畜牧兽医, 2017: 129.
|
| [18] |
Grzesiak M, Popiolek K, Knapczyk-Stwora K. Extracellular vesicles in follicular fluid of sexually mature gilts' ovarian antral follicles-identification and proteomic analysis. J Physiol Pharmacol, 2020, 71(1): 2020Feb; 71(1).
|
| [19] |
Matsuno Y, Kanke T, Maruyama N, et al. Characterization of mRNA profiles of the exosome-like vesicles in porcine follicular fluid. PLoS One, 2019, 14(6): e0217760. DOI:10.1371/journal.pone.0217760
|
| [20] |
Matsuno Y, Onuma A, Fujioka YA, et al. Effects of exosome-like vesicles on cumulus expansion in pigs in vitro. J Reprod Dev, 2017, 63(1): 51-58. DOI:10.1262/jrd.2016-124
|
| [21] |
Simon C, Greening DW, Bolumar D, et al. Extracellular vesicles in human reproduction in health and disease. Endocr Rev, 2018, 39(3): 292-332. DOI:10.1210/er.2017-00229
|
| [22] |
Machtinger R, Laurent LC, Baccarelli AA. Extracellular vesicles: roles in gamete maturation, fertilization and embryo implantation. Hum Reprod Update, 2016, 22(2): 182-193.
|
| [23] |
De Almeida Monteiro Melo Ferraz M, Fujihara M, Nagashima JB, et al. Follicular extracellular vesicles enhance meiotic resumption of domestic cat vitrified oocytes. Sci Rep, 2020, 10(1): 8619. DOI:10.1038/s41598-020-65497-w
|
| [24] |
Da Silveira JC, Veeramachaneni DNR, Winger QA, et al. Cell-secreted vesicles in equine ovarian follicular fluid contain miRNAs and proteins: a possible new form of cell communication within the ovarian follicle. Biol Reprod, 2012, 86(3): 71.
|
| [25] |
付衍辉. 猪卵泡闭锁过程中部分相关基因表达特征研究[D]. 南京: 南京农业大学, 2011. Fu YH. The expression characteristics of partial gene during the porcine follicualr atresia[D]. Nanjing: Nanjing Agricultural University, 2011 (in Chinese). |
| [26] |
Brzyski RG, Muasher SJ, Droesch K, et al. Follicular atresia associated with concurrent initiation of gonadotropin-releasing hormone agonist and follicle-stimulating hormone for oocyte recruitment. Fertil Steril, 1988, 50(6): 917-921. DOI:10.1016/S0015-0282(16)60372-2
|
| [27] |
Taya K, Sasamoto S. Selective release of FSH in lactating rats during the period of follicular atresia induced by the administration of antiserum to LH-releasing hormone. J Endocrinol, 1988, 118(3): 455-464. DOI:10.1677/joe.0.1180455
|
| [28] |
Santonocito M, Vento M, Guglielmino MR, et al. Molecular characterization of exosomes and their microRNA cargo in human follicular fluid: bioinformatic analysis reveals that exosomal microRNAs control pathways involved in follicular maturation. Fertil Steril, 2014, 102(6): 1751-1761.e1. DOI:10.1016/j.fertnstert.2014.08.005
|
| [29] |
Ana Clara Faquineli Cavalcante Mendes De Ávila, Bridi A, Andrade GM, et al. Estrous cycle impacts microRNA content in extracellular vesicles that modulate bovine cumulus cell transcripts during in vitro maturation. Biol Reprod, 2019, 102(2): 362-375.
|
| [30] |
Navakanitworakul R, Hung WT, Gunewardena S, et al. Characterization and small RNA content of extracellular vesicles in follicular fluid of developing bovine antral follicles. Sci Rep, 2016, 6: 25486. DOI:10.1038/srep25486
|
| [31] |
Donadeu FX, Mohammed BT, Ioannidis J. A miRNA target network putatively involved in follicular atresia. Domest Anim Endocrinol, 2017, 58: 76-83. DOI:10.1016/j.domaniend.2016.08.002
|
| [32] |
Sohel MMH, Hoelker M, Noferesti SS, et al. Exosomal and non-exosomal transport of extra-cellular microRNAs in follicular fluid: implications for bovine oocyte developmental competence. PLoS One, 2013, 8(11): e78505. DOI:10.1371/journal.pone.0078505
|
| [33] |
Da Silveira J, Andrade GM, Perecin F, et al. Isolation and analysis of exosomal microRNAs from ovarian follicular fluid. Methods Mol Biol, 2018, 1733: 53-63.
|
| [34] |
Kamalidehghan B, Habibi M, Afjeh SS, et al. The importance of small non-coding RNAs in human reproduction: a review article. Appl Clin Genet, 2020, 13: 1-11. DOI:10.2147/TACG.S207491
|
| [35] |
梁学超, 蒋明, 罗玉茹, 等. 猪卵巢发育的组织学变化及卵泡闭锁规律研究. 畜牧兽医学报, 2017, 48(10): 1863-1870. Liang XC, Jiang M, Luo YR, et al. Study on histology and patterns of follicular atresia during ovarian development in pig. Chin J Animal Vet Sci, 2017, 48(10): 1863-1870 (in Chinese). DOI:10.11843/j.issn.0366-6964.2017.10.009 |
| [36] |
Andronico F, Battaglia R, Ragusa M, et al. Extracellular vesicles in human oogenesis and implantation. Int J Mol Sci, 2019, 20(9): 2162. DOI:10.3390/ijms20092162
|
| [37] |
Xu L, Sun HX, Zhang M, et al. microRNA-145 protects follicular granulosa cells against oxidative stress-induced apoptosis by targeting Krüppel-like factor 4. Mol Cell Endocrinol, 2017, 452: 138-147. DOI:10.1016/j.mce.2017.05.030
|
| [38] |
Liu JY, Tu F, Yao W, et al. Conserved miR-26b enhances ovarian granulosa cell apoptosis through HAS2-HA-CD44-caspase-3 pathway by targeting HAS2. Sci Rep, 2016, 6: 21197. DOI:10.1038/srep21197
|
| [39] |
Zhang L, Gao J, Cui S. miR-21 is involved in norepinephrine-mediated rat granulosa cell apoptosis by targeting SMAD7. J Mol Endocrinol, 2017, 58(4): 199-210. DOI:10.1530/JME-16-0248
|
| [40] |
Da Silveira JC, Carnevale EM, Winger QA, et al. Regulation of ACVR1 and ID2 by cell-secreted exosomes during follicle maturation in the mare. Reprod Biol Endocrinol, 2014, 12: 44. DOI:10.1186/1477-7827-12-44
|
| [41] |
Yao W, Pan ZX, Du X, et al. miR-181b-induced SMAD7 downregulation controls granulosa cell apoptosis through TGF-β signaling by interacting with the TGFBR1 promoter. J Cell Physiol, 2018, 233(9): 6807-6821. DOI:10.1002/jcp.26431
|
| [42] |
Li Q, Du X, Liu L, et al. Upregulation of miR-146b promotes porcine ovarian granulosa cell apoptosis by attenuating CYP19A1. Domest Anim Endocrinol, 2021, 74: 106509. DOI:10.1016/j.domaniend.2020.106509
|
| [43] |
Wang PJ, Liu SJ, Zhu C, et al. miR-29 regulates the function of goat granulosa cell by targeting PTX3 via the PI3K/AKT/mTOR and Erk1/2 signaling pathways. J Steroid Biochem Mol Biol, 2020, 202: 105722. DOI:10.1016/j.jsbmb.2020.105722
|
| [44] |
Zhu WH, Yang M, Shang JN, et al. miR-222 inhibits apoptosis in porcine follicular granulosa cells by targeting the THBS1 gene. Anim Sci J, 2019, 90(6): 719-727. DOI:10.1111/asj.13208
|
| [45] |
Chen HY, Liu C, Jiang H, et al. Regulatory role of miRNA-375 in expression of BMP15/GDF9 receptors and its effect on proliferation and apoptosis of bovine cumulus cells. Cell Physiol Biochem, 2017, 41(2): 439-450. DOI:10.1159/000456597
|
| [46] |
Guo TY, Zhang JB, Yao W, et al. CircINHA resists granulosa cell apoptosis by upregulating CTGF as a CeRNA of miR-10a-5p in pig ovarian follicles. Biochim Biophys Acta Gene Regul Mech, 2019, 1862(10): 194420. DOI:10.1016/j.bbagrm.2019.194420
|
| [47] |
Battaglia R, Musumeci P, Ragusa M, et al. Ovarian aging increases small extracellular vesicle CD81+ release in human follicular fluid and influences miRNA profiles. Aging, 2020, 12(12): 12324-12341. DOI:10.18632/aging.103441
|
| [48] |
Martinez RM, Hauser R, Liang LM, et al. Urinary concentrations of phenols and phthalate metabolites reflect extracellular vesicle microRNA expression in follicular fluid. Environ Int, 2019, 123: 20-28. DOI:10.1016/j.envint.2018.11.043
|
| [49] |
Xiao GY, Cheng CC, Chiang YS, et al. Exosomal miR-10a derived from amniotic fluid stem cells preserves ovarian follicles after chemotherapy. Sci Rep, 2016, 6: 23120. DOI:10.1038/srep23120
|
| [50] |
Sun B, Ma YJ, Wang F, et al. miR-644-5p carried by bone mesenchymal stem cell-derived exosomes targets regulation of p53 to inhibit ovarian granulosa cell apoptosis. Stem Cell Res Ther, 2019, 10(1): 360. DOI:10.1186/s13287-019-1442-3
|
| [51] |
Pasquariello R, Manzoni EFM, Fiandanese N, et al. Implications of miRNA expression pattern in bovine oocytes and follicular fluids for developmental competence. Theriogenology, 2020, 145: 77-85. DOI:10.1016/j.theriogenology.2020.01.027
|
| [52] |
Guo TY, Huang L, Yao W, et al. The potential biological functions of circular RNAs during the initiation of atresia in pig follicles. Domest Anim Endocrinol, 2020, 72: 106401. DOI:10.1016/j.domaniend.2019.106401
|
| [53] |
Tesfaye D, Hailay T, Salilew-Wondim D, et al. Extracellular vesicle mediated molecular signaling in ovarian follicle: implication for oocyte developmental competence. Theriogenology, 2020, 150: 70-74. DOI:10.1016/j.theriogenology.2020.01.075
|
| [54] |
De Ávila A, Bridi A, Andrade GM, et al. Estrous cycle impacts microRNA content in extracellular vesicles that modulate bovine cumulus cell transcripts during in vitro maturation. Biol Reprod, 2020, 102(2): 362-375. DOI:10.1093/biolre/ioz177
|
| [55] |
Hatzirodos N, Hummitzsch K, Irving-Rodgers HF, et al. Transcriptome profiling of the theca interna in transition from small to large antral ovarian follicles. PLoS One, 2014, 9(5): e97489. DOI:10.1371/journal.pone.0097489
|
| [56] |
Donadeu FX, Fahiminiya S, Esteves CL, et al. Transcriptome profiling of granulosa and theca cells during dominant follicle development in the horse. Biol Reprod, 2014, 91(5): 111.
|
| [57] |
张家庆, 王献伟, 李文嘉, 等. 猪let-7a靶基因预测及生物信息学分析. 家畜生态学报, 2020, 41(4): 14-21. Zhang JQ, Wang XW, Li WJ, et al. Target gene prediction and bioinformatics analysis of ssc-let-7a. J Domest Animal Ecol, 2020, 41(4): 14-21 (in Chinese). DOI:10.3969/j.issn.1673-1182.2020.04.003 |
| [58] |
Zhou JL, Liu JY, Pan ZX, et al. The let-7g microRNA promotes follicular granulosa cell apoptosis by targeting transforming growth factor-β type 1 receptor. Mol Cell Endocrinol, 2015, 409: 103-112. DOI:10.1016/j.mce.2015.03.012
|
| [59] |
Chun SY, Eisenhauer KM, Minami S, et al. Hormonal regulation of apoptosis in early antral follicles: follicle-stimulating hormone as a major survival factor. Endocrinology, 1996, 137(4): 1447-1456. DOI:10.1210/endo.137.4.8625923
|
| [60] |
Cao R, Wu WJ, Zhou XL, et al. Expression and preliminary functional profiling of the let-7 family during porcine ovary follicle atresia. Mol Cells, 2015, 38(4): 304-311. DOI:10.14348/molcells.2015.2122
|
| [61] |
Yao W, Wang S, Du X, et al. SMAD4 inhibits granulosa cell apoptosis via the miR-183-96-182 cluster and FoxO1 axis. Reprod Sci, 2021, 2021Jul21.
|
| [62] |
Yao W, Pan ZX, Du X, et al. NORHA, a novel follicular atresia-related lncRNA, promotes porcine granulosa cell apoptosis via the miR-183-96-182 cluster and FoxO1 axis. J Anim Sci Biotechnol, 2021, 12(1): 103. DOI:10.1186/s40104-021-00626-7
|
| [63] |
Ma MN, Zhang JB, Gao XM, et al. miR-361-5p mediates SMAD4 to promote porcine granulosa cell apoptosis through VEGFA. Biomolecules, 2020, 10(9): 1281. DOI:10.3390/biom10091281
|
| [64] |
Yao W, Pan ZX, Du X, et al. miR-181b-induced SMAD7 downregulation controls granulosa cell apoptosis through TGF-β signaling by interacting with the TGFBR1 promoter. J Cell Physiol, 2018, 233(9): 6807-6821. DOI:10.1002/jcp.26431
|
| [65] |
Du X, Liu L, Wu WJ, et al. SMARCA2 is regulated by NORFA/miR-29c, a novel pathway related to female fertility, controls granulosa cell apoptosis. J Cell Sci, 2020, 133(23): 249961.
|
| [66] |
Du X, Zhang LF, Li XY, et al. TGF-β signaling controls FSHR signaling-reduced ovarian granulosa cell apoptosis through the SMAD4/miR-143 axis. Cell Death Dis, 2016, 7(11): e2476. DOI:10.1038/cddis.2016.379
|
| [67] |
Liu JY, Du X, Zhou JL, et al. microRNA-26b functions as a proapoptotic factor in porcine follicular granulosa cells by targeting Sma-and Mad-related protein 4. Biol Reprod, 2014, 91(6): 146.
|
| [68] |
Zhong YY, Li LY, Chen ZT, et al. MIR143 inhibits steroidogenesis and induces apoptosis repressed by H3K27me3 in granulosa cells. Front Cell Dev Biol, 2020, 8: 565261. DOI:10.3389/fcell.2020.565261
|
| [69] |
Sen A, Prizant H, Light A, et al. Androgens regulate ovarian follicular development by increasing follicle stimulating hormone receptor and microRNA-125b expression. PNAS, 2014, 111(8): 3008-3013. DOI:10.1073/pnas.1318978111
|
| [70] |
Nie MY, Yu S, Peng S, et al. miR-23a and miR-27a promote human granulosa cell apoptosis by targeting SMAD5. Biol Reprod, 2015, 93(4): 98.
|
| [71] |
Huo SW, Qi HR, Si YX, et al. microRNA 26a targets Ezh2 to regulate apoptosis in mouse ovarian granulosa cells. Syst Biol Reprod Med, 2021, 67(3): 221-229. DOI:10.1080/19396368.2021.1895362
|
| [72] |
Glamoclija V, Vilović K, Saraga-Babić M, et al. Apoptosis and active caspase-3 expression in human granulosa cells. Fertil Steril, 2005, 83(2): 426-431. DOI:10.1016/j.fertnstert.2004.06.075
|
| [73] |
Reynaud K, Driancourt MA. Oocyte attrition. Mol Cell Endocrinol, 2000, 163(1/2): 101-108.
|
| [74] |
Liu JY, Li XY, Yao Y, et al. miR-1275 controls granulosa cell apoptosis and estradiol synthesis by impairing LRH-1/CYP19A1 axis. Biochim Biophys Acta Gene Regul Mech, 2018, 1861(3): 246-257. DOI:10.1016/j.bbagrm.2018.01.009
|
| [75] |
Yang ML, Lin L, Sha CL, et al. Bone marrow mesenchymal stem cell-derived exosomal miR-144-5p improves rat ovarian function after chemotherapy-induced ovarian failure by targeting PTEN. Lab Invest, 2020, 100(3): 342-352. DOI:10.1038/s41374-019-0321-y
|
| [76] |
Zhang XD, Chen YG, Yang M, et al. miR-21-5p actions at the Smad7 gene during pig ovarian granulosa cell apoptosis. Anim Reprod Sci, 2020, 223: 106645. DOI:10.1016/j.anireprosci.2020.106645
|
| [77] |
Li QQ, Du X, Liu L, et al. miR-126* is a novel functional target of transcription factor SMAD4 in ovarian granulosa cells. Gene, 2019, 711: 143953. DOI:10.1016/j.gene.2019.143953
|
| [78] |
Zhang P, Wang J, Lang H, et al. microRNA-205 affects mouse granulosa cell apoptosis and estradiol synthesis by targeting CREB1. J Cell Biochem, 2018, 2018Dec16.
|
| [79] |
Guo LW, Huang QX, Zhao J, et al. microRNA-10b promotes the apoptosis of bovine ovarian granulosa cells by targeting plasminogen activator inhibitor-1. Theriogenology, 2021, 176: 206-216. DOI:10.1016/j.theriogenology.2021.09.035
|
| [80] |
Li QQ, Du X, Pan ZX, et al. The transcription factor SMAD4 and miR-10b contribute to E2 release and cell apoptosis in ovarian granulosa cells by targeting CYP19A1. Mol Cell Endocrinol, 2018, 476: 84-95. DOI:10.1016/j.mce.2018.04.012
|
| [81] |
陈慧芳, 黄绮亮, 胡智超, 等. 外泌体microRNA在猪成熟和闭锁卵泡中的表达差异及功能分析. 中国农业科学, 2021, 54(21): 4664-4676. Chen HF, Huang QL, Hu ZC, et al. Expression differences and functional analysis of exosomes microRNA in porcine mature and atretic follicles. Sci Agric Sin, 2021, 54(21): 4664-4676 (in Chinese). DOI:10.3864/j.issn.0578-1752.2021.21.015 |
| [82] |
Cui ZF, Liu LB, Kwame Amevor F, et al. High expression of miR-204 in chicken atrophic ovaries promotes granulosa cell apoptosis and inhibits autophagy. Front Cell Dev Biol, 2020, 8: 580072. DOI:10.3389/fcell.2020.580072
|
| [83] |
Ma LZ, Tang XR, Guo S, et al. miRNA-21-3p targeting of FGF2 suppresses autophagy of bovine ovarian granulosa cells through AKT/mTOR pathway. Theriogenology, 2020, 157: 226-237. DOI:10.1016/j.theriogenology.2020.06.021
|
| [84] |
Andreu-Vieyra CV, Habibi HR. Factors controlling ovarian apoptosis. Can J Physiol Pharmacol, 2000, 78(12): 1003-1012. DOI:10.1139/y00-101
|
| [85] |
Singh J, Paul A, Thakur N, et al. Localization of IGF proteins in various stages of ovarian follicular development and modulatory role of IGF-I on granulosa cell steroid production in water buffalo (Bubalus bubalis). Anim Reprod Sci, 2015, 158: 31-52. DOI:10.1016/j.anireprosci.2015.04.006
|
| [86] |
Duan CM, Xu QJ. Roles of insulin-like growth factor (IGF) binding proteins in regulating IGF actions. Gen Comp Endocrinol, 2005, 142(1/2): 44-52.
|
| [87] |
姚郅璆, 司文宇, 方富贵. IGF系统与雌性哺乳动物生殖. 生理科学进展, 2019, 50(3): 211-215. Yao ZQ, Si WY, Fang FG. IGF system and reproduction in female mammals. Prog Physiol Sci, 2019, 50(3): 211-215 (in Chinese). DOI:10.3969/j.issn.0559-7765.2019.03.011 |
| [88] |
Wang XM, Meng K, Wang HQ, et al. Identification of small extracellular vesicle subtypes in follicular fluid: insights into the function and miRNA profiles. J Cell Physiol, 2021, 236(8): 5633-5645. DOI:10.1002/jcp.30251
|
| [89] |
Probert C, Dottorini T, Speakman A, et al. Communication of prostate cancer cells with bone cells via extracellular vesicle RNA: a potential mechanism of metastasis. Oncogene, 2019, 38(10): 1751-1763. DOI:10.1038/s41388-018-0540-5
|
| [90] |
Zielak-Steciwko AE, Browne JA. How to explore the function and importance of microRNAs: microRNAs expression profile and their target/pathway prediction in bovine ovarian cells. Methods Mol Biol, 2018, 1733: 93-105.
|
| [91] |
Bayraktar R, Van Roosbroeck K, Calin GA. Cell-to-cell communication: microRNAs as hormones. Mol Oncol, 2017, 11(12): 1673-1686. DOI:10.1002/1878-0261.12144
|
| [92] |
Martinez RM, Baccarelli AA, Liang LM, et al. Body mass index in relation to extracellular vesicle-linked microRNAs in human follicular fluid. Fertil Steril, 2019, 112(2): 387-396.e3. DOI:10.1016/j.fertnstert.2019.04.001
|
 2022, Vol. 38
2022, Vol. 38




