酿酒酵母细胞器区室化合成化学品的研究进展
栾韬
,
尹梦琦
,
王明
,
康秀龙
,
赵建志
,
鲍晓明
生物工程学报  2023, Vol. 39 2023, Vol. 39 Issue (6): 2334-2358 Issue (6): 2334-2358 |

随着合成生物学技术的快速发展,通过代谢工程改造微生物细胞工厂生产有价值的化合物来替代传统生产方法是更加环保可持续的解决方案[1]。根据代谢工程理念,在微生物中建立代谢途径并将代谢通量导向目标产物,可用于生物生产各种先进生物燃料、大宗化学品、药物和重组蛋白。此外,通过采取一系列有效措施对代谢途径进行优化,例如,控制酶的表达强度或改变关键酶的产物倾向、提高底物的摄入及转化能力、削弱内源竞争途径、重建辅因子平衡体系、缓解或绕过代谢瓶颈,以及减少中间体代谢物和产物的毒性等,能够达到提高目标化合物产量的目的[2-3]。
酿酒酵母(Saccharomyces cerevisiae)作为一种真核模式微生物,其对粗放工业生产环境中的低pH、高渗透压、乙醇及其他抑制物等具有较高的鲁棒耐受性,被认为是合成生物学底盘细胞的理想选择之一。此外,酿酒酵母具有完善的全基因组序列、详细的基因注释资源和简便的基因操作方法,这也促进了酿酒酵母代谢工程改造的发展。目前,许多生物合成途径已经在酿酒酵母细胞质中建立并取得不俗的进步,例如乙醇,青蒿酸、人胰岛素、肝炎疫苗、法呢烯和异丁醇等[4-16]。
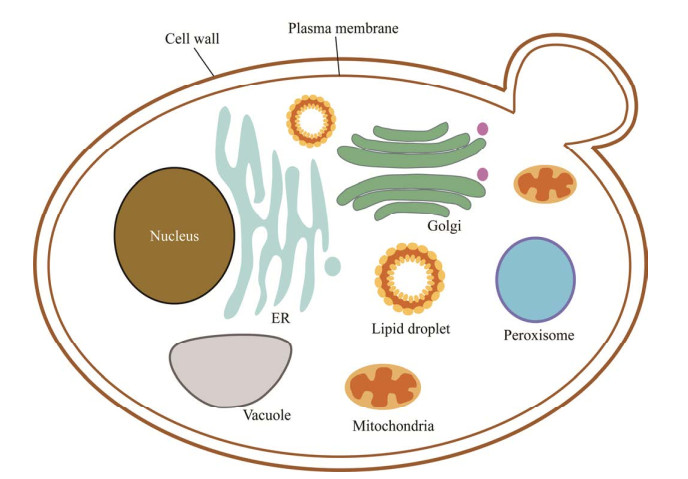
酿酒酵母细胞内具有复杂的膜系统,当合成目标产物所需的前体底物、酶和辅因子等内源性资源不平衡地分布在不同的细胞器区室中时,细胞器结构会造成一些不利的因素,导致特定化合物的合成效率偏低。例如,异源代谢途径的代谢物跨膜转运效率较低和部分代谢物不能跨膜运输、细胞质中前体供给不足以及相关酶过于分散等因素严重影响了目标途径的代谢效率。虽然提高相关酶的表达量、设计融合蛋白以及人造蛋白支架等策略在一定程度上能够提升产物合成效率,然而,前体供给不足等问题依然没有得到有效解决[17]。并且,随着代谢工程变得越来越复杂,更长和更复杂的异源代谢途径导入酿酒酵母细胞质中,与天然细胞功能发生串扰的可能性增加,也使细胞的代谢负担加大[18-20]。同时,异源代谢途径也难免会产生对细胞生长不利的中间体代谢物或(和)目标产物[21-22]。利用各种细胞器的功能特征解决这些阻碍代谢通量的问题,是酿酒酵母代谢工程改造的新的关注点[23-24]。细胞中存在各种功能不同的细胞器和细胞结构,包括线粒体(mitochondria)、过氧化物酶体(peroxisome)、高尔基体(golgi apparatus)、内质网(endoplasmic reticulum, ER)、脂滴(lipid droplets, LDs)、液泡(vacuole)和细胞壁(cell wall),它们结构复杂,每种细胞器都具有独特的内部环境以及特定的代谢途径与功能(图 1)。将代谢途径靶向细胞器中能够充分利用某些特定细胞器中不能转运出的关键前体物,增加酶和中间体代谢物的局部浓度,可以改善细胞质中合成目标化学品的前体物供应不足或异源酶及中间体代谢物浓度不足的劣势,使代谢通量和通路效率得到提高[25-26]。代谢途径的区室化,还能够减少通过其他途径消耗中间体代谢物,改善异源途径有毒中间体代谢物或产物对细胞造成的负面影响[2, 21, 27-29]。另外,细胞器之间的协作和物质交流对于区室化改造也是非常重要的,各个细胞器的动态协作也能提高产物的生物合成。通过代谢途径区室化的策略有望进一步提高目标化学品的产量,表 1总结了近年来通过酿酒酵母细胞器区室合成化学品的研究实例,并于文中综述了相关研究进展。
|
| 图 1 酿酒酵母中细胞器的结构 Fig. 1 Structure of organelles in Saccharomyces cerevisiae. |
| |
| Chemical | Classification of chemicals | Carbon source | Engineered organelle | Metabolic engineering methods in cell organelles | Purpose of transformation | Highest titer reported | References |
| Icaritin | Prenylflavonoid | Glucose | Mitochondria | Expressed gmomt2 | Use pH conditions to enhance enzyme activity | 7.2 mg/L | [30] |
| Sabinene | Terpenoids | Glucose | Mitochondria | Expressed t34sabs1, overexpression of aim25 | Improve precursor utilization and enhance compartmentalized pathways | 154.9 mg/L | [25] |
| Linalool | Terpenoids | Sucrose | Mitochondria | Expressed colis/erg20f96w/n127w, erg10, hmgs, thmg1, erg12, pmk, mvd1 and idi1, overexpression of colis, erg20f96w/Nn127w and colis/erg20f96w/n127w | Improve precursor utilization | 23.45 mg/L | [31] |
| Isoprene | Terpenoids | Glucose | Mitochondria | Overexpression of erg10, hmgs, thmg1, erg12, pmk, mvd1 and idi1 | Improve precursor utilization | 2 527 mg/L | [24] |
| Squalene | Terpenoids | Glucose | Mitochondria | Expressed erg10, erg13, thmg1, erg12, erg8, idi1, erg20, erg19 and erg9 | Improve precursor utilization | 21.1 g/L | [27] |
| 3-hydroxypropionic acid | Organic acid | Glucose | Mitochondria | Mutation mth1 and med2, overexpression of camcr | Improve energy and precursor supply | 2.04 g/L | [32] |
| Isobutanol, Isopentanol, 2-methyl-1-butanol | Alcohol | Glucose | Mitochondria | Overexpression of ilv2, ilv3, ilv5, α-kdc and adh | Increases local enzyme concentration and reduces loss of intermediates | (486±36) mg/L, (130±21) mg/L, (113±5) mg/L | [33] |
| Isopentanol | Alcohol | Glucose | Mitochondria | Overexpression of leu4, leu1 and leu2 | Prevent early termination in recursive elongation pathways and enhance product specificity | (1.24±0.06) g/L | [34] |
| Isobutanol | Alcohol | Xylose | Mitochondria | Expressed ilv2, ilv5, ilv3, kivd and adhare1 | Improve precursor utilization | 2.60 g/L | [35] |
| Isobutanol, 2-methyl-1-butanol | Alcohol | Xylose | Mitochondria | Δbat1, Δald6, Δpho13, Expressed ilv2, ilv5 and ilv3, overexpression of aro10, lladhare1 and llkivd | Improve the utilization of precursors and isolate metabolic pathways | (3.10±0.18) g/L, (0.91±0.02) g/L | [36] |
| Chemical | Classification of chemicals | Carbon source | Engineered organelle | Metabolic engineering methods in cell organelles | Purpose of transformation | Highest titer reported | References |
| S-adenosylmethionine enzyme | Protein | Glucose | Mitochondria | Expressed nifu, nifs and fdxn | Use of Fe-S clusters | [37] | |
| (R)-(+)-limonene, trans-isopiperitenol, geraniol, 8-hydroxygeraniol, cannabigerolic acid | Terpenoids | Glucose | Peroxisome | Expressed cllims, erg20n127w, idi1, erg19, erg8, erg12, efmvae, efmvas, tccpr, mslimh, mslims, obgers, crg8oh, crcpr and cspt4, overexpression of efmvae, efmvas, erg20n127w, cllims and erg12 | Improve precursor utilization | 2.58 g/L, 19.24 mg/L, 5.52 g/L, 25.11 mg/L, 0.82 mg/L | [1] |
| Geraniol | Terpenoids | Glucose | Peroxisome | Δpex30, Δpex31, Δpex32, Δatg36, overexpression of bul1 | Increase the number of peroxisomes and improve strain tolerance | 2.75 mg/L | [38] |
| α-humulene | Terpenoids | Glucose | Peroxisome | Overexpression of erg10, erg13, thmg1, erg12, erg8, mvd1, idi1, erg20 and zss1 | Improve precursor utilization | 1 726.78 mg/L | [39] |
| Squalene | Terpenoids | Glucose | Peroxisome | Overexpression of erg10, erg13, thmg1, nadh-hmgr, erg12, erg8, mvd1, idi1, erg20, erg9, ant1, idp2, idp3, acl1, acs1 and Δcit2 | Capable of storing lipophilic compounds and improve precursor utilization | 11.00 g/L | [40] |
| (2S)-naringenin | Prenylflavonoid | Glucose | Peroxisome | Overexpression of pex11, fox1, fox2 and fox3 | Increase precursor supply | 1 129.44 mg/L | [41] |
| Medium chain fatty alcohols | Fatty alcohol | Glucose | Peroxisome | Overexpression of pex7 and acc1, expressed tafar | Improve positioning efficiency and increase precursor supply | 1.30 g/L | [42] |
| (S)-norcoclaurine | Alkaloid | Glucose | Peroxisome | Overexpression of adr1, oaf1 and pip2, expressed ncs | Separate toxic products | 47% increase | [43] |
| Ethanol | Alcohol | Xylose | Peroxisome | Overexpression of pex34 | Increase the number of peroxisomes | 8 g/L | [44] |
| Opioid | Opiates | Glucose | ER | Overexpression of cor | Enhance product specificity | 131 mg/L | [45] |
| Medicagenic acid, artemisinic acid, saponins | Terpenoids | Glucose | ER | Δpah1 | Increase the volume of the endoplasmic reticulum | 27.10 mg/L, 2-fold more the wild-type strain, 16-fold increase | [46] |
| Chemical | Classification of chemicals | Carbon source | Engineered organelle | Metabolic engineering methods in cell organelles | Purpose of transformation | Highest titer reported | References |
| Squalene, protopanaxadiol | Terpenoids | Glucose | ER | Overexpression of ino2 | Increase the volume of the endoplasmic reticulum | (634±11) mg/L, (12.10±1.00) mg/L | [47] |
| Carnosic acid, ferruginol | Terpenoids | Glucose | ER | Overexpression of ino2 and hac1 | Increase the volume of the endoplasmic reticulum and promote protein folding to reduce stress | 75.18 mg/L, 36.29 mg/L | [48] |
| α-amylase | Protein | Glucose | Golgi | Overexpression of sec16 and glo3 | Enhance protein secretion | 25.10% increase | [49] |
| α-amylase, insulin precursor | Protein | Glucose | Golgi | Overexpression of sec1 and sly1 | Enhance protein secretion | 238 mg/L, 84 mg/L | [14] |
| α-amylase | Protein | Glucose | Golgi | Δvps5, Δcps17 | Reduce protein retention in cells | 2.50 g/L | [50] |
| α-amyrin | Terpenoids | Glucose | LDs | Overexpression of dga1 | Expand intracellular storage capacity | (1 107.9±76.8) mg/L | [51] |
| Squalene | Terpenoids | Glucose | LDs | Overexpression of dga1 | Expand intracellular storage capacity | 255.11 mg/L | [52] |
| Lycopene | Carotenoids | Glucose | LDs | Overexpression of ole1, pah1, dga1, acc1 and Δfld1 | Make lipid droplets larger and increase capacity | 2.37 g/L and 73.30 mg/g DCW | [53] |
| Ergosterol | Terpenoids | Glucose | LDs | Overexpression of are2, upc2-1 and acc1 | Make lipid droplets larger and increase capacity | 2 986.70 mg/L and 29.50 mg/g DCW | [54] |
| β-carotene | Carotenoids | Glucose | LDs | Overexpression of are1, are2, Δdpp1, Δlpp1, Δpah1 | Regulation of lipid metabolic pathways | 8.98 mg/g DCW | [55] |
| β-carotene | Carotenoids | Glucose, 2 mmol/L exogenous oleic acid | LDs | Δdpp1, Δlpp1, Δerg9, expressed izh1 | Expand intracellular storage capacity | 11.40 mg/g DCW and 142 mg/L | [56] |
| PPD-type saponin ginsenoside compound K | Terpenoids | Glucose | LDs | Expressed ppds | Improve precursor utilization | 5 g/L | [29] |
| 7-dehydrocholesterol | Sterol | Glucose | LDs | Expressed erg2, erg3, dhcr24, erg25, erg26 and erg27 | Improve the conversion efficiency of intermediates | 360.60 mg/L | [57] |
| Chemical | Classification of chemicals | Carbon source | Engineered organelle | Metabolic engineering methods in cell organelles | Purpose of transformation | Highest titer reported | References |
| p-coumaric acid | Carboxylic acid compounds | Glucose | Vacuolar | Expressed fjtal | Promote effective conversion of tyrosine | 593.04 mg/L | [58] |
| Hyoscyamine, scopolamine | Alkaloid | Glucose | Vacuolar | Expressed abls and leu2, overexpression of ntjat1 | Improve vacuolar transport efficiency | 30‒80 μg/L | [59] |
| Hyoscyamine, scopolamine | Alkaloid | Glucose | Vacuolar | Expressed ntjat1, ntmate2, abpup1 and ablp1 | Improve vacuolar transport efficiency | 480 μg/L, 172 μg/L | [60] |
| Ethanol | Alcohol | Glucose | Cell wall | mot3 in certain locations sequence changed | Improve the robustness of strains | 14.50 g/L | [61] |
| Squalene | Terpenoids | Glucose | Cell wall | Δecm33 | Improve the robustness of strains | 12% increase | [62] |
| Cellobiohydrolase | Protein | Glucose | Cell wall | Δcwp2 | Enhance enzyme activity | Enzyme activity by 85.90% | [63] |
| Ethanol | Alcohol | Xylose | Cell wall | Overexpression of ccw12 | Improve the robustness of strains | 0.50 g/g total sugars, 0.49 g/g total sugars | [64] |
| Ethanol | Alcohol | Cellulose | Cell wall | Sed1-anchoring domain repositioning egi1, Sag1-anchoring domain repositioning bgl1 | Improve cellulolytic capacity | 30% increase | [65] |
| β-glucosidase | Protein | Glucose | Cell wall | Δccw12, Δccw14, expressed bgl1 | Alteration of cell wall morphology | 1.4-fold higher | [66] |
| d-glucaric acid | Protein | Glucose | Synthetic organelles based on Murine polyomavirus virus-like particles | Δvp1, expressed miox | Capture and localization of proteins | 20% increase | [67] |
线粒体基质由两层膜包裹,与细胞质内环境相比,具有更高的pH值、更低的氧浓度和更高的氧化还原电位,可通过三羧酸(tricarboxylic acid, TCA)循环和氧化磷酸化的过程,产生大量的能量物质,是细胞的动力车间[68]。此外,线粒体还参与多种化合物的生物合成过程,如乙酰辅酶A、支链氨基酸、血红素、脂质、丙酮酸、铁硫(Fe-S)簇、NADH、NAD+、黄素腺嘌呤二核苷酸和α-酮异戊酸,这能为许多化合物的合成提供关键前体代谢物和氧化还原辅因子[7, 69],对产物的高效合成至关重要。
1.2 线粒体中合成以乙酰辅酶A为前体的化学品乙酰辅酶A是中心碳代谢的重要中间代谢物,也是糖代谢流向脂类分子合成的节点,这也自然成为脂肪酸衍生物和萜类化合物等多种高值化学品的前体代谢物[70]。在细胞器中乙酰辅酶A的产生途径有3条,一是在线粒体中丙酮酸脱氢酶复合物催化丙酮酸形成乙酰辅酶A[68];二是在线粒体中乙酰辅酶A水解酶催化琥珀酰辅酶A和乙酸底物,将琥珀酰辅酶A的辅酶A基团转移到乙酸,形成乙酰辅酶A[71];三是在过氧化物酶体中脂肪酸β-氧化产生乙酰辅酶A。在酿酒酵母中,乙酰辅酶A的分布是高度区域化的,主要分布在细胞质、线粒体、过氧化物酶体和细胞核中,且不同区室的乙酰辅酶A的浓度差别较大,据报道其在线粒体中的含量是细胞质中的20–30倍[72]。然而乙酰辅酶A不能跨膜转运的特点,导致不同区域内不能互相穿梭利用[73]。利用线粒体中乙酰辅酶A的有效措施,是直接在线粒体中构建下游代谢途径,转化产生可以自由跨膜扩散或通过转运蛋白实现跨膜运输到细胞质的前体或目标产物[21]。例如,利用细胞色素氧化酶等定位线粒体的蛋白N端信号肽,将乙酰辅酶A下游的经甲羟戊酸(mevalonic acid, MVA)途径或甲基赤藓糖磷酸(methylerythritol phosphate, MEP)途径中的全部酶定位表达到线粒体中,实现了在线粒体中表达一些亲脂性萜类化合物(如桧烯、芳樟醇、异戊二烯、角鲨烯),并利用其亲脂性能自由跨膜扩散的特点,合并在细胞质建立的同样代谢途径,明显提升目标产物的产量[24-25, 27, 31, 74]。利用这种策略,Thomik等[25]通过在细胞质和线粒体共表达N-截短的桧烯合酶(t34SabS1),并超表达线粒体功能蛋白Aim25p,使桧烯的滴度达到了154.9 mg/L,是初始菌株的60倍;Zhang等[31]在线粒体中超表达法尼基焦磷酸合成酶(farnesyl pyrophosphate synthetase, ERG20)突变体ERG20F96W/N127W和来自肉桂的芳樟醇合成酶(linalool synthase, CoLIS)的融合蛋白CoLIS/ERG20F96W/N127W,并建立细胞质芳樟醇的合成途径,使补料分批发酵的芳樟醇滴度达到23.45 mg/L;Lv等[24]杂交构建二倍体细胞质和线粒体双重代谢菌株,该策略相对于仅使用线粒体或细胞质工程的重组菌株,分别增加了2.1倍和1.6倍的异戊二烯产量,补料分批发酵生产了2 527 mg/L的异戊二烯;Zhu等[27]利用线粒体改造策略生产角鲨烯,并在细胞质中超表达截短的3-羟基-3-甲基戊二酰辅酶A还原酶(tHMG1),减轻MVA通路有毒化合物对细胞生长和角鲨烯生产能力的负面影响,使角鲨烯效价达到了21.1 g/L。将某些亲脂性萜类化合物的合成途径构建到线粒体区室中,可充分利用区室中的乙酰辅酶A前体底物,使目标化合物的合成相较于细胞质工程得到一定程度的提高。但是,萜类化合物的一些中间体代谢物对细胞是有毒性的,尤其是在线粒体中,能够影响线粒体的正常功能,以至于影响产物的积累。因此,未来研究需通过更加精细的代谢调控,进一步提高代谢通量,减少中间产物的积累,以及超表达或(和)敲除某些关键基因,能够减轻有毒化合物对细胞生长和产物积累的负面影响,为更多的萜类化合物的生物合成提供了策略。
1.3 线粒体中合成以丙酮酸为前体的化学品丙酮酸是线粒体中TCA循环正常运转的关键前体物。线粒体中丰富的丙酮酸为生物燃料异丁醇和异戊醇的生物合成奠定了基础[34, 75]。通过将合成途径的酶基因(包括乙酰乳酸合酶ILV2、酮醇酸还原异构酶ILV5、二羟酸脱水酶ILV3、乙醇脱氢酶ADH及α-酮异戊酸脱羧酶KDC)定位到线粒体中表达,Avalos等[33]利用此策略使菌株合成了635 mg/L的异丁醇并产生了130 mg/L的异戊醇。Hammer等[34]进一步把负责2-酮酸延伸的3种递归酶(LEU4, LEU1, LEU2)定位到线粒体中表达,增加了异戊醇合成途径的通量并有效地减少了中间体代谢物2-异丙基苹果酸合酶2-IPMS的损耗,使异戊醇产量达到了1 241 mg/L。因此,将完整的代谢途径靶向到线粒体中,能够增加酶和中间体代谢物的局部浓度,绕过竞争通路,减少中间体代谢物的损失,使产物得到更多的积累。
1.4 不同碳源对线粒体代谢及产物合成的影响酿酒酵母在进行葡萄糖代谢时,细胞会处于强酵解状态,糖异生途径、TCA循环和乙醛酸循环中的基因表达会受到阻遏,线粒体活性以及细胞生长也会受到抑制,代谢通量大部分流向发酵产物乙醇,该现象被称为Crabtree效应[76-78]。通过改造参与葡萄糖信号传导的转录因子mth1和RNA聚合酶II介质复合物的亚基med2这2个等位基因可以减轻Crabtree效应,Zhang等[32]研究发现mth1和med2等位基因的突变体mth1a81d和med2*432y在菌株中会产生协同效应,以缓解Crabtree效应,同时改善细胞生长,使更多的碳通量引入线粒体并增强能量和线粒体乙酰辅酶A的合成,通过将丙二酰辅酶A还原酶靶向线粒体中表达,工程菌株利用葡萄糖产生了2.04 g/L滴度的3-羟基丙酸,是野生型菌株的5.4倍。当酿酒酵母利用木糖代谢时,与葡萄糖相比,木糖代谢过程中细胞整体呈现出Crabtree-negative代谢特征,且木糖代谢中线粒体更加活跃,通过呼吸测定实验表明:木糖代谢的酵母线粒体活性能够提高多达7.3倍,丙酮酸由胞质到线粒体的运输量和TCA循环代谢通量也明显增强,为以丙酮酸为前体物的有机酸(醇)类化合物生物合成提供了有利条件[36, 71, 79-80]。通过将异丁醇合成途径定位到木糖利用菌株的线粒体中表达,Lane等[35]使异丁醇的产量达到了2.6 g/L。Zhang等[36]在此基础上进一步敲除bat1 (编码线粒体支链氨基酸转氨酶)、ald6 (编码胞质醛脱氢酶)和pho13 (编码碱性磷酸酶)来优化代谢途径,使异丁醇的产量达到了3.1 g/L。基于木糖代谢能够增强丙酮酸进入线粒体的运输量,进而也会提高线粒体中乙酰辅酶A的积累,这有利于提高萜类化合物在线粒体区室中的合成。本课题组通过前期在葡萄糖和木糖共利用酿酒酵母细胞质中合成单萜香叶醇的研究基础上,通过信号肽将以乙酰辅酶A为前体物到香叶醇全合成途径的酶基因定位表达到线粒体区室内,构建木糖利用酿酒酵母线粒体合成香叶醇菌株。研究发现,线粒体改造后的菌株香叶醇产量很低且细胞生长缓慢,细胞的代谢负担加重,不利于产物的合成。通过调整策略将香叶醇合成酶和偏好香叶基焦磷酸(geranyl diphosphate, GPP)合成酶活性的ERG20WW的融合蛋白(tVoGES-GGGS-ERG20WW)以及异戊烯基焦磷酸异构酶(IDI1)定位到线粒体中表达,结合细胞质中表达香叶醇合成酶,使菌株生长得到改善且香叶醇产量得到大幅提升,但仍无法达到细胞质改造菌株的产量,说明菌株在木糖代谢时线粒体中乙酰辅酶A的通量大部分流向TCA循环,而流向目标化合物的合成的通量较少。综上所述,通过对酿酒酵母在不同碳源条件下的代谢特征的研究,发现木糖条件下线粒体活性会得到提高,部分前体代谢物的代谢通量也会增强,将目标化合物的代谢途径靶向线粒体中表达,在一定程度上能够使产量得到提高,但有些化合物的合成与线粒体自身代谢途径竞争前体代谢物和能量,势必会影响目标化合物的积累。另外,线粒体内部空间非常拥挤,当引入较长、较复杂的外源代谢途径时,可能会导致线粒体代谢压力较大[30],从而影响正常的生理功能。因此,在未来的研究中,需进一步对线粒体中代谢途径进行优化,提高关键酶的催化效率,并且针对不同碳源设计与之匹配的目标产物合成途径,使代谢通量尽可能流向目标产物,同时保障线粒体维持其基本生理所需的代谢物和辅因子等,最大限度提高线粒体合成化学品的效率。
1.5 线粒体其他特殊环境特点对化学品合成的影响线粒体区室内具有较高的pH值,这为高pH需求化合物的合成提供了有利场所,例如,淫羊藿苷的生物合成。Wang等[81]在高效生产8-异戊二烯山奈酚的酵母菌株的基础上,将对低pH敏感且在酵母细胞质中表达时会失去活性的异源甲基转移酶(GmOMT2)定位表达在酿酒酵母线粒体中,催化8-异戊二烯山奈酚生成淫羊藿苷,使重组菌株利用葡萄糖产生了7.2 mg/L淫羊藿苷。
另外,线粒体Fe-S簇蛋白是TCA循环和电子传递链的重要组成部分,它们还参与线粒体内某些氨基酸、血红素、钼辅因子、硫辛酸和生物素的合成[82]。有研究发现,线粒体中Fe-S簇提供了一个合适的环境来合成固氮酶生物合成的前体甲硫氨酸酶(S-adenosylmethionine enzyme, NifB)[37]。NifB在固氮酶生物合成中占有重要的地位,NifB催化8Fe-9S-C簇形成NifB-co,它构成了3种固氮酶类型的活性位点辅因子的核心。Burén等[37]将固氮酶基因转移到酿酒酵母线粒体中表达,使细胞在有氧条件下,产生NifB,该研究为实现在真核生物中固定N2提供了可行方案。
2 过氧化物酶体区室合成化学品 2.1 过氧化物酶体的结构与功能氧化物酶体是由单层膜包裹并含有产生H2O2的氧化酶和降解H2O2的过氧化氢酶的细胞器,它的大小和数量根据生长条件而动态变化。氧化物酶体的膜结构对许多分子量低于700 Da的小分子物质具有渗透性,并对分子量较大的溶质产生屏障。氧化物酶体可以容纳一定量的疏水性化合物参与许多代谢反应,包括脂肪酸β-氧化、乙醛酸循环和甲醇代谢,以及相关联的生物合成过程[40, 43, 83]。过氧化物酶体的脂肪酸β-氧化能够产生乙酰辅酶A,是许多化合物合成的前体物。过氧化物酶体NADP依赖性异柠檬酸脱氢酶(isocitrate dehydrogenase, IDP2和IDP3),能够为过氧化物酶体提供氧化还原状态,满足生物合成过程对NADPH的需求[84]。由于过氧化物酶体膜对NADPH和乙酰辅酶A不可渗透,依靠能够定位过氧化物酶体基质的信号肽Pts1p和Pts2p,以及定位到过氧化物酶体膜的信号肽mPTS,可将代谢途径有效引入和定位到过氧化物酶体[43, 85]。
2.2 过氧化物酶体中合成萜类化合物过氧化物酶体的脂肪酸β-氧化产生的乙酰辅酶A,是萜类化合物合成的有益要素,激发了在过氧化物酶体区室合成萜类化合物的研究热情。Dusséaux等[1]将MVA途径和一些单萜化合物合成酶定位表达到过氧化物酶体中,成功在过氧化物酶体中合成了香叶基二磷酸衍生化合物,包括香叶醇、(R)-(+)-柠檬烯、反式异胡椒烯醇、单萜吲哚生物碱的前体8-羟基香叶醇和大麻素前体大麻二酚酸。并在半连续补料分批发酵条件下产生了5.5 g/L香叶醇和2.6 g/L (R)-(+)-柠檬烯。通过对过氧化物酶体生物发生因子基因pex30、pex31、pex32和过氧化物酶体吞噬受体基因atg36的单一或多重缺失的结果显示能够增加过氧化物酶体的数量,结合超表达截短的α-阻遏蛋白接头bul1,可将E3泛素连接酶(RSP5)与其底物连接起来进行泛素化,防止转运蛋白降解,提高酿酒酵母细胞对香叶醇的耐受性,使香叶醇滴度得到进一步提高[38]。Zhang等[39]将MVA途径和α-葎草烯合酶通过增强型过氧化物酶体信号肽ePTS1靶向定位表达于过氧化物酶体中,结合细胞质中α-葎草烯合成途径,使重组菌株产生了160 mg/L的α-葎草烯,与细胞质工程菌株相比,α-葎草烯的产量增加了2.5倍,并在5 L生物反应器中补料分批发酵获得了1 726.78 mg/L滴度的α-葎草烯。Liu等[40]在过氧化物酶体中合成角鲨烯,通过超表达idp2和idp3增加NADPH向过氧化物酶体的转运、超表达ant1 (编码过氧化物酶体腺嘌呤核苷酸转运蛋白)增加ATP的供应,改善过氧化物酶体中角鲨烯合成,并杂交构建二倍体细胞质和过氧化物酶体双重代谢菌株,使角鲨烯滴度达到了11.00 g/L。过氧化物酶体的脂肪酸β-氧化产生的乙酰辅酶A、辅因子和能量等,以及其大小和数量可以根据生长状态进行动态调整,增加产物的存储空间,为萜类化合物的合成提供了有利条件。
2.3 过氧化物酶体区隔有毒化合物在化学品合成过程中,异源蛋白质的负荷和对细胞有毒性的某些代谢物也是提高目标化合物产量所需要面对的难题。过氧化物酶体与其他细胞器不同,即使内部代谢途径完全被破坏,也不会对细胞生长产生不利影响,使之更有利于分隔有毒的化合物[19]。Sibirny等[43]利用信号肽ePTS1将有毒的去甲克劳林合成酶(nocloline synthetase, NCS)定位表达到过氧化物酶体中,达到改善菌株生长的目的,同时利用过氧化物酶体膜的特性,允许小分子底物和产物能够从过氧化物酶体中扩散流出,使苄基异喹啉生物碱滴度提高了47%。过氧化物酶体良好的分隔有毒物质的能力,也为其他有毒化合物的合成提供了选择。
2.4 过氧化物酶体合成其他类化合物除了合成萜类化合物以外,利用脂肪酸β-氧化产生的乙酰辅酶A还可以促进黄酮类化合物(2S)-柚皮素的合成。Zhang等[41]超表达脂肪酸β-氧化途径关键的过氧化物酶体增殖和游离脂肪酸转运途径蛋白(Pex11p)、酰基辅酶A氧化酶(fatty-acyl coenzyme A oxidase, FOX1)、羟酰基辅酶A脱氢酶/烯酰基辅酶A水合酶(3-hydroxyacyl-CoA dehydrogenase and enoyl-CoA hydratase, FOX2)和乙酰辅酶A酰基转移酶(peroxisomal oxoacyl thiolase, FOX3),增加了过氧化物酶体脂肪酸β-氧化前体物的供给,进一步增加乙酰辅酶A的供应,促进(2S)-柚皮素的产生,在葡萄糖补料分批发酵条件下,菌株(2S)-柚皮素滴度从改造之前的703.53 mg/L提高到了1 129.44 mg/L。过氧化物酶体也是脂肪酸降解的区室,而降解产物正是转化脂肪酸衍生物的底物。Sheng等[42]将脂肪酰基辅酶A还原酶(TaFAR)靶向表达于酿酒酵母的过氧化物酶体中,通过超表达过氧化物酶体中的结构蛋白Pex7p和乙酰辅酶A羧化酶(acetyl-CoA carboxylase, ACC1),提高靶向过氧化物酶体酶的效率并优化培养基条件,使在补料分批发酵时,中长链脂肪醇产量超过1.3 g/L。
过氧化物酶体在利用木糖的工程酿酒酵母菌株生产乙醇中也发挥着重要作用。Dzanaeva等[44]研究发现,过氧化物酶体的缺失会降低木糖的乙醇产量,过氧化物酶体膜蛋白基因pex3或编码过氧化氢酶基因cta1的缺失也会阻碍乙醇生产。而超表达过氧化物酶体膜蛋白Pex34p,使过氧化物酶体的大小增大,提高了转化木糖生产乙醇的效率,使乙醇产量达到了8 g/L,是亲本菌株的1.4倍。未来,通过改善前体物和辅因子的供应、优化蛋白质输入机制、控制特异性自噬降解过程将进一步改善目标产物的滴度,过氧化物酶体区室化改造是酿酒酵母生物合成化学品的一种非常有前途的方法。
3 内质网改造促进化学品合成 3.1 内质网的结构与功能内质网是真核细胞中最大的细胞器,是多重结合且广泛动态分布的膜系统。内质网膜系统网络遍布整个细胞内部,与其他细胞器关联结合密切,在信息传递、物质和能量交换过程中扮演重要角色,并执行多种重要的生物学功能,包括蛋白质折叠和转运、脂质合成、囊泡运输及钙离子(Ca2+)储存和释放,以及参与多种信号转导途径的调控过程[86-87]。内质网内蛋白多要被分泌到细胞外,蛋白积累也容易造成内质网胁迫,故不适合在其内部构建代谢途径,而内质网内在的一些稳态变化往往对提高化学品合成有积极的意义。
3.2 细胞色素P450酶的表达酿酒酵母中,主要位于内质网的能催化羟化、环氧化、脱烷基化、碳-碳偶联、氧化裂解等多种不同反应的“红色万能酶”细胞色素P450酶,在次级代谢物(包括萜类、黄酮类、生物碱、阿片类和酚类化合物)的生物合成中发挥着重要作用[88-89]。例如,Thodey等[45]通过表达一种细胞色素P450酶,以催化阿片类生物合成途径中关键中间体代谢物(R)-网碱转化为清风藤碱,并将可待因还原酶(codeine reductase, COR)靶向内质网膜进行表达,使吗啡的特异性合成得到提高,并在此基础上使阿片类药物的总滴度达到了131 mg/L;通过细胞色素P450酶的催化可将来自MVA或MEP途径的萜类前体物修饰、剪裁和调整以形成萜类化合物,如三萜皂苷、倍半萜青蒿酸、原人参二醇、酚类三环二萜鼠尾草酸等[90-91]。然而,在酿酒酵母中表达异源细胞色素P450酶存在一些酶促反应效率较低以及对NADPH细胞色素P450还原酶的依赖性等局限性,并且细胞色素P450酶的超表达通常会导致内质网的异常形态和增殖[92-93]。因此,在未来的研究中,需要进一步深入研究内质网的生理机制和调控网络,努力改善细胞色素P450酶的表达,减轻对内质网的负面影响。
3.3 扩张内质网体积提高化学品产量由于内质网的体积空间是蛋白质折叠能力的决定因素,利用内质网体积的扩张,增强内质网内源性和异源性蛋白的合成和折叠能力,能够缓解酶表达量不足带来的代谢限制并改善细胞色素P450酶的表达和折叠,可以进一步提高目标化合物的生产[94-98]。Arendt等[46]通过敲除磷脂酸磷酸酶(phosphatidic acid phosphohydrolase, PAH1)导致内质网急剧扩张,促进了三萜类化合物、三萜皂苷以及倍半萜青蒿酸的积累。Kim等[47]超表达激活脂质生物合成的转录因子Ino2p,促进磷脂合成,从而增加内质网膜的面积,使角鲨烯和原人参二醇产量分别提高到了634 mg/L和12.1 mg/L,与对照菌株相比,产量分别提高了71倍和8倍。Wei等[48]利用相同策略,并超表达促进蛋白质折叠的转录因子Hac1p,激活蛋白质折叠相关基因的转录,激活内质网中的未折叠蛋白应答,减少内质网扩张带来的压力,使铁锈醇滴度增加到了36.29 mg/L,并在5 L分批补料发酵中生产了75.18 mg/L鼠尾草酸。利用酿酒酵母内质网进行代谢工程,为提高化合物的产量提供了新选择。通过在内质网表达异源细胞色素P450酶,多种高值化合物可在酿酒酵母中进行合成。通过扩大内质网的体积,部分化学品的合成得到提高,但过度扩大也会对细胞生长产生负面影响。因此,需要保持内质网生理状态的稳定性,来提高酵母细胞工厂合成目标产物的能力。
4 高尔基体改造提高蛋白质分泌表达 4.1 高尔基体的结构与功能高尔基体是一个短暂的、动态的细胞器。在酿酒酵母细胞中,高尔基体呈分散的形式分布,由多个独立膜结合的池组成,显示出横向互连、开窗和管状的结构[99-100]。高尔基体是囊泡运输网络的中心,和内质网协同工作以处理、分选和运输新合成的蛋白质和脂质[101]。在蛋白质分泌过程中,内质网新合成的分泌蛋白被输送到高尔基体,在那里它们被加工和修饰[102]。加工完成后,货物蛋白在反式高尔基网络(trans-Golgi network, TGN)中被分拣到运输载体囊泡中,完成分泌表达[99]。但是,比较而言酿酒酵母的蛋白分泌能力较差,导致异源蛋白胞外表达的水平相对较低,并且异源蛋白的积累也会对细胞造成压力[103]。
4.2 优化基于高尔基的蛋白质分泌途径提高产物的合成通过改善包被着外壳蛋白复合物Ⅱ (coat protein complex II, COPII)和外壳蛋白复合物I (coat protein complex I, COPI)的囊泡之间的顺序耦合,协调内质网和高尔基体之间的双向囊泡运输,以及加强内质网到高尔基体和高尔基体到质膜过程中的蛋白质转运方式,是改善酿酒酵母异源蛋白质生产的一种策略。通过超表达内质网外周蛋白Sec16p,加强内质网到高尔基体之间的顺行运输,可以促进COPII囊泡的形成,增加重组蛋白的分泌[104]。Bao等[49]在超表达sec16基因的菌株中超表达ADP-核糖基化因子GTP激活蛋白Glo3p来改善COPI囊泡介导的逆行运输,增加了酵母中异源蛋白α-淀粉酶的分泌。Nielsen[14]超表达从高尔基体到细胞膜的囊泡运输以及内源性蛋白转化酶分泌的Sec1/Munc18 (SM)蛋白(SEC1)刺激从高尔基体到胞吐作用的囊泡运输增强了胰岛素前体、α-淀粉酶和内源性蛋白质转化酶的分泌,并超表达从内质网到高尔基体的囊泡融合的SM蛋白(SLY1)改善了内质网到高尔基体的运输,进一步提高了α-淀粉酶的产量,使菌株在葡萄糖限制补料分批发酵中产生了238 mg/L α-淀粉酶,比对照菌株提高了16%,且重组胰岛素前体菌株产生了84 mg/L的胰岛素前体,比对照菌株提高了34%。通过对核内体到高尔基体运输的修饰,可以在增加蛋白质分泌的同时有效地减少了蛋白质的滞留,进一步提高异源蛋白的分泌。Huang等[50]敲除参与高尔基体和核内体之间的转运基因vps5和vps17,结果显示减少了α-淀粉酶在细胞内滞留及增强了胞外分泌量,改造后的菌株α-淀粉酶的产量较对照菌株提高了2倍多,在补料分批发酵中α-淀粉酶的产量达到了2.5 g/L,并且在发酵过程中α-淀粉酶保留在细胞内的比例不到10%。
此外,高尔基体的形态、数量的大小可以通过基因改造进行改变,但是这些表型的改变对蛋白质分泌的影响机制尚不清楚。其他机制包括细胞内降解或蛋白质合成增加也可能影响蛋白质的滞留和分泌[50]。在未来的研究中,基于异源蛋白分泌途径的改善以及其他提高蛋白质分泌的策略,酿酒酵母将在异源蛋白质的生产中发挥巨大潜力。
5 脂滴改造促进亲脂性化合物的合成 5.1 脂滴的结构与功能脂滴是一种具有独特结构的动态细胞器,起源于内质网,由疏水性中性脂质、三酰基甘油和甾醇酯组成,被内质网衍生的磷脂单层膜包围,参与疏水性中性脂质的生物合成和分解以及磷脂和甾醇的合成,在脂质和细胞能量代谢中发挥核心作用[29, 105-111]。酿酒酵母作为一种非产油酵母,虽然对亲脂性化合物的产生能力有限,但脂滴作为细胞内脂质的储存室,能够将亲脂性化合物储存在其中,这对于提高亲脂性化合物的合成有很大的启发[112]。
5.2 增加脂滴容量提高化合物产量在脂滴中脂质储存水平会受到脂滴储存空间的限制,通过细胞类型、营养可用性和代谢状态调节脂滴的脂质组成、大小和数量,可使脂滴容量增加,来容纳更多的亲脂性化合物[55, 113]。例如超表达二酰基甘油酰基转移酶(diacylglycerol acyltransferase, DGA1),能够增加细胞内脂滴的数量,提高α-香树脂醇和角鲨烯的产量[51-52],最终在补料分批发酵中产生了1 107.9 mg/L α-香树脂醇;使角鲨烯产量达到了255.11 mg/L。在超表达dga1基础上,Ma等[53]进一步超表达acc1、脂肪酸去饱和酶基因ole1和敲除内质网中一种膜蛋白基因fld1 (Seipin),改善脂滴的主要成分三酰基甘油的积累,促使脂滴变大,容量增加,进而使工程菌的番茄红素产量达到了70.5 mg/g DCW,较原高产菌株提高了25%,补料分批发酵番茄红素产量达到了2.37 g/L和73.3 mg/g DCW。通过增加脂滴的大小和容量,使其能够容纳更多的亲脂性化合物,增加细胞的承载能力,运用此策略可以结合其他细胞器改造,进一步增加一些亲脂性化合物的积累,例如线粒体或过氧化物酶体工程改造相结合,来提高亲脂性萜类化合物的合成。
5.3 调节脂质代谢提高化合物产量脂滴在脂质代谢中具有重要作用,脂质代谢网络的调节可以有效地影响亲脂性化合物的生物合成。例如Sun等[54]超表达全局调节因子等位基因upc2-1、甾醇酰基转移酶基因are2和acc1,增强脂肪酸的生物合成途径并扩大脂滴储存容量,使菌株麦角甾醇产量增加到了40.6 mg/g DCW,比初始菌株高4.2倍,采用两阶段补料策略进行高密度细胞发酵,使菌株麦角甾醇产量达到了2 986.7 mg/L和29.5 mg/g DCW。Zhao等[55]超表达甾醇酰基转移酶基因are1和are2,使细胞内甾醇酯水平提高。并且磷脂酸磷酸酶基因pah1、dpp1和lpp1的缺失,提高了磷脂水平。结合这两种策略,增加了细胞内类胡萝卜素的积累,使β-胡萝卜素产量达到了8.98 mg/g DCW,相比于初始菌株提高了2.4倍。此外,Bu等[56]利用外源添加2 mmol/L油酸的策略,减少了β-胡萝卜素与三酰基甘油生物合成对前体物的竞争,显著改善了三酰基甘油的代谢,并结合代谢工程方法,使菌株产生了11.4 mg/g DCW和142 mg/L的β-胡萝卜素。在增加脂滴的大小和容量的基础上,进一步提高脂质代谢通量,能够使亲脂性化合物的积累得到进一步提高。
5.4 脂滴中调节途径代谢通量对于复杂的代谢途径,除了调节、优化脂质的合成途径以及使脂滴容量增加外,将代谢途径区室化改造来调节代谢通量,能够缓解亲脂性化合物合成中的表达瓶颈。Shi等[29]使用信号肽Pln1p将细胞色素P450酶原人参二醇(protopanaxadiol, PPD)合酶(PPDS)靶向脂滴进行表达,使底物达马烯二醇-II转化为PPD的效率提高了394%,在以PPD为底物生产PPD型皂苷人参皂甙化合物K (PPD-type saponin ginsenoside compound K, CK)的菌株中,CK滴度达到了5 g/L。Guo等[57]将erg2 (编码C-8甾醇异构酶)、erg3 (C-5甾醇去饱和酶)、dhcr24 (Δ24-脱氢胆固醇还原酶)、erg25 (编码C-4甲基甾醇氧化酶)、erg26 (编码C-3甾醇脱氢酶)和erg27 (编码3-酮甾醇还原酶)基因靶向脂滴进行表达,提高了中间代谢物的转化效率,使甾醇7-脱氢胆固醇产量从最初的187.7 mg/L提高至360.6 mg/L。通过将部分代谢途径靶向到脂滴中,能够增加酶和中间体代谢物的局部浓度,提高了中间代谢物的转化效率,使产物得到更多的积累,为异源亲脂性化合物(如天然橡胶[114]等)的合成提供了思路。
6 液泡改造提高化学品合成 6.1 液泡的结构与功能酿酒酵母液泡是细胞中酸性最强的细胞器,pH值在5–6.5之间,是碱性氨基酸、多聚磷酸盐、Ca2+和许多金属离子的主要储存场所,区室内含有多种水解酶,可降解蛋白质、核酸和脂质等大分子物质,并能分隔多种有毒物质[115-119]。液泡参与细胞质离子浓度和pH值的稳态调节,维持细胞内渗透压平衡,并在细胞应激反应中发挥着多重作用。当细胞在营养丰富的条件下,大部分氨基酸在液泡中积累,而在营养缺乏时,液泡中氨基酸会转运到细胞质中保证细胞生长[120]。
6.2 优化液泡转运机制提高产物积累由于液泡是部分氨基酸的主要存储场所,因此液泡的定位表达策略往往能够补偿关键酶催化能力不足的问题,促进液泡中氨基酸有效转化,从而提高前体的利用率及目标产物的积累。张思琪等[58]利用羧肽酶Y的锚定肽将酪氨酸解氨酶(FjTAL)定位表达到液泡中,提高了液泡中酪氨酸利用率,使以酪氨酸为前体物合成对香豆酸的产量提高至593.04 mg/L。同时,液泡膜上特定的氨基酸转运蛋白对细胞器之间物质的转运发挥着重要作用,优化液泡转运蛋白运输机制,可以增强代谢产物的运输和生产。Srinivasan等[59]引入植物液泡转运蛋白来改善酿酒酵母液泡转运蛋白运输机制的限制,提高了托烷生物碱的生物合成,使生物碱莨菪碱滴度达到了30–80 μg/L。进一步发现2种转运蛋白AbPUP1和AbLP1能促进液泡输出和细胞对石蕊碱和莨菪碱的再次摄取来增加工程酵母中的托烷生物碱产量[60]。将来自4种不同植物中转运蛋白(NtJAT1、NtMATE2、AbPUP1和AbLP1)的表达结合起来,能够促进细胞器之间通路中间体代谢物的交换,使莨菪碱和东莨菪碱产量分别达到了480 μg/L和172 μg/L。
目前,大多数研究是将产物合成的关键酶在液泡中定位表达,并通过简单优化液泡的转运机制改善代谢产物的运输和生产。有趣的是,液泡的大小和数量会随着细胞外部环境的变化而变化,这有利于液泡通路的理性设计和改造,以期增强液泡合成产物的效率,但对于生物合成的分析和调控机制还需深入研究。此外,液泡酸性内环境的特点可为pH敏感的化学品提供理想的合成场所。为了进一步提高液泡合成相关化学品的能力,优化液泡对产物转运过程及其与其他细胞器之间的协作是今后研究的重要方面之一,并结合液泡的特点针对性地选择合成特定化学品。
7 细胞壁工程强化化学品的生物合成细胞壁是一个复杂的细胞结构,在细胞生长和分裂过程中决定了细胞的形状和完整性。细胞壁占酿酒酵母细胞干重的15%–30%,由含有β-1, 3-葡聚糖、β-1, 6-葡聚糖和几丁质的内层和含有甘露糖聚蛋白的外层组成[121-122]。内层主要负责细胞壁的机械强度,而外层则起到屏障的作用,限制细胞内物质流出。细胞壁形成的物理屏障,是细胞抵御外部环境胁迫的第一道防线,细胞壁完整性(cell wall integrity, CWI)途径的细胞壁补偿机制可以响应细胞壁损伤,维持细胞稳态[123-125]。对细胞壁稳态扰动的细胞壁工程可以提高细胞内目标化学品的生产。(1) 增强细胞对抑制物应激反应,提高对某些抑制物的耐受性,减轻毒性化合物对细胞造成的损害。Kahar等[61]通过在有抑制物的培养基中筛选出一株高鲁棒性的菌株,对其研究发现,参与细胞壁产生的朊病毒转录抑制蛋白基因mot3的突变,能够增强酿酒酵母菌株细胞壁的疏水性,引起絮凝反应激发,使菌株鲁棒性得到加强,在含有抑制物的培养基中,24 h消耗30 g/L的葡萄糖,产生了14.5 g/L的乙醇。Son等[62]敲除CWI途径的关键调节因子Ecm33p激活CWI途径恢复膜刚性,使细胞对高渗透压和高浓度盐离子的耐受性显著增强,进一步提高角鲨烯生产效率,与亲本菌株相比产量增加了约12%。(2) 破坏细胞壁完整性,提高蛋白分泌表达,增加了酵母中异源蛋白的产量。Li等[63]破坏编码细胞壁甘露糖蛋白基因cwp2,使酿酒酵母中纤维二糖水解酶的产量得到增加。许多研究发现,使用糖基磷脂酰肌醇(glycosylphosphatidylinositol, GPI)锚定技术在细胞表面表达功能蛋白是构建具有特殊功能的酵母细胞的一种很有前景的方法,该技术主要依赖于通过与基因融合的锚定蛋白[通常是GPI-锚定细胞壁蛋白(GPI-anchored cell wall proteins, GPI-CWP)]连接在细胞壁上表达靶蛋白。Inokuma等[65]使用不同的锚定域Sed1p和Sag1p将外切和内切纤维素酶定位到细胞壁进行表达,提高了重组菌株的纤维素分解能力,在对预处理后的稻草进行水热和球磨处理,同时糖化和发酵过程中乙醇滴度提高了30%。GPI锚定的甘露糖蛋白(covalently linked cell wall protein, CCW12)是酿酒酵母细胞壁结构的重要成,超表达ccw12能够提高菌株对抑制物的耐受性和细胞壁稳定性。Kong等[64]在利用木糖菌株中超表达ccw12,改造后的菌株在玉米秸秆水解物中48 h内产生了0.503 g/g总糖或理论值的98.8%的最佳乙醇产量。在玉米、芒草、玉米芯和小麦秸秆的各种工业水解物中表现良好,在玉米芯水解物中12 h内的最佳乙醇产量为0.49 g/g总糖,并对未脱毒的水解物表现出很好的适应性。然而,与之不同的是Inokuma等[66]研究发现,在酿酒酵母菌株中共敲除ccw12和ccw14 (编码非酶GPI-CWP),能使菌株细胞壁厚度增加,并使异源β-葡萄糖苷酶表达的数量及其活性比亲本菌株提高约1.4倍,但细胞壁形态变化和异源蛋白分泌能力增加的分子机制仍不清楚。
通过操纵细胞壁相关蛋白的比例与含量来强化细胞的鲁棒性,对增加酵母菌株中异源蛋白和高值化合物的积累有很大的帮助,但目前相关作用机制尚不清晰。特别是蛋白类化合物的合成和分泌过程中细胞壁蛋白是如何发挥作用的还有待深入研究。
8 人工合成细胞器的研究与应用人工合成细胞器是指可以参与代谢途径的人工生物催化隔室,将生物代谢途径封装到人工合成细胞器中,是增强生物化学品生产的一种有前途的策略。使用人工合成细胞器可以减少与宿主细胞代谢的不良串扰,并能更好地控制反应环境。基于人工合成细胞器的可编程性意味着它们对代谢物的渗透性和表面结构可以被调整利于特定的反应。例如,通过设计细胞器孔径的大小和电荷以利于底物的流入和中间体代谢物的流出等[126]。Cheah等[67]利用鼠多瘤病毒样颗粒(murine polyomavirus virus-like particles, MPyV-VLPs)设计出人工代谢纳米区室。并将一种天然不稳定的肌醇加氧酶(inositol oxygenase, MIOX),组装进人工代谢纳米区室中用于生产d-葡糖二酸。该研究是在酿酒酵母中首次展示可以参与代谢途径的人工合成细胞器,并表明代谢物可以通过MPyV外壳扩散,尽管表达的蛋白质水平较低,但仍增加了目标产物滴度。人工合成细胞器为酿酒酵母这一重要的生物底盘细胞扩展了体内蛋白质支架和分隔工具。而对于一些细胞内特定的反应需要具体调整人工合成细胞器的结构等一些机制问题还需进一步研究阐明。
9 展望近年来,随着对酿酒酵母中不同细胞器的深入研究,各个细胞器的结构和功能得到了更好的认知。通过细胞器工程合成化学品的关键是识别并调节细胞内转运的细胞器膜蛋白,通过快速有效的蛋白质转运机制,将代谢途径的相关酶直接定位到细胞器中表达,然后利用各种细胞器独特的功能与丰富的底物,目前多种化学品的生物代谢途径已经在酿酒酵母细胞器中构建[18, 127]。随后,利用代谢工程技术进一步对区室中的代谢途径以及细胞器的结构进行优化,使酿酒酵母可以高效合成目标化合物,以及一些特定性能得到加强(例如,鲁棒性)。本文通过重点介绍了酿酒酵母的各种细胞器的结构、功能以及化学品合成的代谢工程改造策略等,并分别阐述了不同细胞器的环境特点及其适合的化学品种类。例如,线粒体中充足的乙酰辅酶A、丙酮酸等前体物、辅因子和能量等,可为萜类化合物、醇类化合物和其他化合物的生物合成提供有利条件;过氧化物酶体脂肪酸β-氧化产生的乙酰辅酶A、辅因子和能量等,可为萜类化合物、黄酮类化合物和脂肪酸衍生物的合成提供有利条件,并能有效地区隔有毒化合物;内质网中表达异源细胞色素P450酶,对许多化合物的合成至关重要,通过扩大内质网的体积,能够增强内质网内源性和异源性蛋白的合成和折叠能力,提高目标化合物的积累;改善高尔基体的蛋白质转运方式,可以提高细胞蛋白质分泌;增加脂滴的大小、体积和脂质代谢通量,能够提高亲脂性化合物的积累;优化液泡转运机制,改善代谢产物的运输和生产,提高目标产物的积累;操纵细胞壁相关蛋白来加强细胞的鲁棒性,对增加产物的积累有很大的帮助。虽然部分细胞器代谢途径通量无法超过细胞质途径,但可与细胞质形成协同增强的效果,从而整体上提高了目标产物的产量。由于细胞器之间代谢通路的复杂性,以及当代谢途径的内部资源分布在由高选择性、不渗透性膜隔开的不同细胞器时,酵母的细胞器结构会给代谢工程带来很多挑战。例如,不同细胞器对不同物质的存储方式不同;对蛋白质的定位和数量的了解不足,无法指导通路的构建和优化或导致通路通量不平衡;有毒代谢物引入关键细胞器会影响其正常功能;酿酒酵母有些细胞器是高度动态的,并且随着生长条件、碳源、代谢状态、外部环境和其他因素在数量、大小、体积、定位和活性方面发生改变[2];即使细胞器数量和体积扩大,特定细胞器中的最大蛋白质负载可能会变得有限,导致生长负担等。为了应对这些挑战,需要对代谢物运输、辅因子生物合成和运输、定位信号肽选择、细胞器膜的渗透性、各个细胞器的生理学特性和细胞代谢进行更系统的研究,以进行合理的途径改造[128]。
展望未来,虽然在酿酒酵母细胞器工程改造合成化学品的研究中取得了阶段性的成果,但要想显著提高目标产物的产量,笔者认为未来的研究方向可着重从如下几个方面考虑:(1) 将新的生物合成途径靶向细胞器进行表达时,要充分分析细胞器的特点以及目标产物合成途径的特点,了解产物合成途径与细胞器之间的优、劣势,努力提高细胞器与产物之间的匹配性,提高合成效率;(2) 优化代谢途径,提高关键酶的定位效率和催化效率,尽可能减少细胞区室内的表达量以减轻可能出现的代谢负担;(3) 利用基因的动态调控策略,避免中间体代谢物的过度积累对细胞器造成压力或毒性,从而导致合成途径受阻;(4) 随着对酵母细胞内膜结构及其对特定底物的转运蛋白及相关分子转运机理研究的深入,针对不同代谢物的高效转运系统将被构建,届时代谢通量也将有大幅提升;(5) 对于长且复杂的产物合成途径,研究多区室的动态协作、交流以进一步提高化学品的生物合成。相信随着异源生物合成途径的长度和复杂性不断增加,需要将代谢途径的传统设计原则与代谢产物运输因素和非代谢信息相结合,以释放微生物生物制造平台的全部潜力[60]。同时,合成生物学和系统生物学的发展也将驱动酿酒酵母中各种天然产物生物合成的细胞器代谢工程的研究进程,充分挖掘细胞合成化学品的潜能,最大限度地提高微生物细胞工厂的合成能力,为化学品生物合成的工业化生产奠定基础。
| [1] |
DUSSÉAUX S, WAJN WT, LIU YX, IGNEA C, KAMPRANIS SC. Transforming yeast peroxisomes into microfactories for the efficient production of high-value isoprenoids. Proceedings of the National Academy of Sciences of the United States of America, 2020, 117(50): 31789-31799. DOI:10.1073/pnas.2013968117
|
| [2] |
JUNG SW, YEOM J, PARK JS, YOO SM. Recent advances in tuning the expression and regulation of genes for constructing microbial cell factories. Biotechnology Advances, 2021, 50: 107767. DOI:10.1016/j.biotechadv.2021.107767
|
| [3] |
张晓龙, 王晨芸, 刘延峰, 李江华, 刘龙, 堵国成. 基于合成生物技术构建高效生物制造系统的研究进展. 合成生物学, 2021, 2(6): 863-875. ZHANG XL, WANG CY, LIU YF, LI JH, LIU L, DU GC. Research progress of constructing efficient biomanufacturing system based on synthetic biotechnology. Synthetic Biology Journal, 2021, 2(6): 863-875 (in Chinese). |
| [4] |
王明, 栾韬, 赵建志, 李洪兴, 鲍晓明. 酿酒酵母转化木糖生产化学品的研究进展. 生物工程学报, 2021, 37(3): 1042-1057. WANG M, LUAN T, ZHAO JZ, LI HX, BAO XM. Progress in studies on production of chemicals from xylose by Saccharomyces cerevisiae. Chinese Journal of Biotechnology, 2021, 37(3): 1042-1057 (in Chinese). DOI:10.13345/j.cjb.200708 |
| [5] |
DAVID HAWKINS R, HON GC, REN B. Next-generation genomics: an integrative approach. Nature Reviews Genetics, 2010, 11(7): 476-486. DOI:10.1038/nrg2795
|
| [6] |
REAVES ML, RABINOWITZ JD. Metabolomics in systems microbiology. Current Opinion in Biotechnology, 2011, 22(1): 17-25. DOI:10.1016/j.copbio.2010.10.001
|
| [7] |
SNYDER M, GALLAGHER JEG. Systems biology from a yeast omics perspective. FEBS Letters, 2009, 583(24): 3895-3899. DOI:10.1016/j.febslet.2009.11.011
|
| [8] |
DURAN L, LÓPEZ JM, AVALOS JL. ¡Viva la mitochondria!: harnessing yeast mitochondria for chemical production. FEMS Yeast Research, 2020, 20(6): foaa037. DOI:10.1093/femsyr/foaa037
|
| [9] |
HU YT, ZHU ZW, NIELSEN J, SIEWERS V. Engineering Saccharomyces cerevisiae cells for production of fatty acid-derived biofuels and chemicals. Open Biology, 2019, 9(5): 190049. DOI:10.1098/rsob.190049
|
| [10] |
RO DK, PARADISE EM, OUELLET M, FISHER KJ, NEWMAN KL, NDUNGU JM, HO KA, EACHUS RA, HAM TS, KIRBY J, CHANG MCY, WITHERS ST, SHIBA Y, SARPONG R, KEASLING JD. Production of the antimalarial drug precursor artemisinic acid in engineered yeast. Nature, 2006, 440(7086): 940-943. DOI:10.1038/nature04640
|
| [11] |
WESTFALL PJ, PITERA DJ, LENIHAN JR, ENG DIANA, WOOLARD FX, REGENTIN R, HORNING T, TSURUTA H, MELIS DJ, OWENS A, FICKES S, DIOLA D, BENJAMIN KR, KEASLING JD, LEAVELL MD, McPHEE DJ, RENNINGER NS, NEWMAN JD, PADDON CJ. Production of amorphadiene in yeast, and its conversion to dihydroartemisinic acid, precursor to the antimalarial agent artemisinin. Proceedings of the National Academy of Sciences of the United States of America, 2012, 109(3): E111-E118.
|
| [12] |
PADDON CJ, WESTFALL PJ, PITERA DJ, BENJAMIN K, FISHER K, MCPHEE D, LEAVELL MD, TAI A, MAIN A, ENG D, POLICHUK DR, TEOH KH, REED DW, TREYNOR T, LENIHAN J, FLECK M, BAJAD S, DANG G, DENGROVE D, DIOLA D, et al. High-level semi-synthetic production of the potent antimalarial artemisinin. Nature, 2013, 496(7446): 528-532. DOI:10.1038/nature12051
|
| [13] |
HOU J, TYO K, LIU ZH, PETRANOVIC D, NIELSEN J. Engineering of vesicle trafficking improves heterologous protein secretion in Saccharomyces cerevisiae. Metabolic Engineering, 2012, 14(2): 120-127. DOI:10.1016/j.ymben.2012.01.002
|
| [14] |
NIELSEN J. Production of biopharmaceutical proteins by yeast: advances through metabolic engineering. Bioengineered, 2013, 4(4): 207-211. DOI:10.4161/bioe.22856
|
| [15] |
MEADOWS AL, HAWKINS KM, TSEGAYE Y, ANTIPOV E, KIM Y, RAETZ L, DAHL RH, TAI AN, MAHATDEJKUL-MEADOWS T, XU L, ZHAO LS, DASIKA MS, MURARKA A, LENIHAN J, ENG DIANA, LENG JS, LIU CL, WENGER JW, JIANG HX, CHAO L, et al. Rewriting yeast central carbon metabolism for industrial isoprenoid production. Nature, 2016, 537(7622): 694-697. DOI:10.1038/nature19769
|
| [16] |
ZHANG W, SHAO W, ZHANG A. Isobutanol tolerance and production of Saccharomyces cerevisiae can be improved by engineering its TATA-binding protein Spt15. Letters in Applied Microbiology, 2021, 73(6): 694-707. DOI:10.1111/lam.13555
|
| [17] |
PURDUE PE, LAZAROW PB. Peroxisome biogenesis. Annual Review of Cell and Developmental Biology, 2001, 17: 701-752. DOI:10.1146/annurev.cellbio.17.1.701
|
| [18] |
GUIRIMAND G, KULAGINA N, PAPON N, HASUNUMA T, COURDAVAULT V. Innovative tools and strategies for optimizing yeast cell factories. Trends in Biotechnology, 2021, 39(5): 488-504. DOI:10.1016/j.tibtech.2020.08.010
|
| [19] |
DELOACHE WC, RUSS ZN, DUEBER JE. Towards repurposing the yeast peroxisome for compartmentalizing heterologous metabolic pathways. Nature Communications, 2016, 7: 11152. DOI:10.1038/ncomms11152
|
| [20] |
KANG W, MA T, LIU M, QU JL, LIU ZJ, ZHANG HW, SHI B, FU S, MA JC, LAI LTF, HE SC, QU JN, WING-NGOR AU S, HO KANG B, YU LAU WC, DENG ZX, XIA J, LIU TG. Modular enzyme assembly for enhanced cascade biocatalysis and metabolic flux. Nature Communications, 2019, 10: 4248. DOI:10.1038/s41467-019-12247-w
|
| [21] |
DU L, LI SY. Compartmentalized biosynthesis of fungal natural products. Current Opinion in Biotechnology, 2021, 69: 128-135. DOI:10.1016/j.copbio.2020.12.006
|
| [22] |
MA YS, LI JB, HUANG SW. Targeting pathway expression to subcellular organelles improves astaxanthin synthesis in Yarrowia lipolytica. Metabolic Engineering, 2021, 68: 152-161. DOI:10.1016/j.ymben.2021.10.004
|
| [23] |
HAMMER SK, AVALOS JL. Harnessing yeast organelles for metabolic engineering. Nature Chemical Biology, 2017, 13(8): 823-832. DOI:10.1038/nchembio.2429
|
| [24] |
LV XM, WANG F, ZHOU PP, YE LD, XIE WP, XU HM, YU HW. Dual regulation of cytoplasmic and mitochondrial acetyl-CoA utilization for improved isoprene production in Saccharomyces cerevisiae. Nature Communications, 2016, 7: 12851. DOI:10.1038/ncomms12851
|
| [25] |
THOMIK T, WITTIG I, CHOE JY, BOLES E, OREB M. An artificial transport metabolon facilitates improved substrate utilization in yeast. Nature Chemical Biology, 2017, 13(11): 1158-1163. DOI:10.1038/nchembio.2457
|
| [26] |
CAO X, YANG S, CAO CY, ZHOU YJ. Harnessing sub-organelle metabolism for biosynthesis of isoprenoids in yeast. Synthetic and Systems Biotechnology, 2020, 5(3): 179-186. DOI:10.1016/j.synbio.2020.06.005
|
| [27] |
ZHU ZT, DU MM, GAO B, TAO XY, ZHAO M, REN YH, WANG FQ, WEI DZ. Metabolic compartmentalization in yeast mitochondria: burden and solution for squalene overproduction. Metabolic Engineering, 2021, 68: 232-245. DOI:10.1016/j.ymben.2021.10.011
|
| [28] |
JIA HJ, CHEN TH, QU JZ, YAO MD, XIAO WH, WANG Y, LI C, YUAN YJ. Collaborative subcellular compartmentalization to improve GPP utilization and boost sabinene accumulation in Saccharomyces cerevisiae. Biochemical Engineering Journal, 2020, 164: 107768. DOI:10.1016/j.bej.2020.107768
|
| [29] |
SHI YS, WANG D, LI RS, HUANG LQ, DAI ZB, ZHANG XL. Engineering yeast subcellular compartments for increased production of the lipophilic natural products ginsenosides. Metabolic Engineering, 2021, 67: 104-111. DOI:10.1016/j.ymben.2021.06.002
|
| [30] |
MALINA C, LARSSON C, NIELSEN J. Yeast mitochondria: an overview of mitochondrial biology and the potential of mitochondrial systems biology. FEMS Yeast Research, 2018, 18(5): foy040.
|
| [31] |
ZHANG YY, WANG J, CAO XS, LIU W, YU HW, YE LD. High-level production of linalool by engineered Saccharomyces cerevisiae harboring dual mevalonate pathways in mitochondria and cytoplasm. Enzyme and Microbial Technology, 2020, 134: 109462. DOI:10.1016/j.enzmictec.2019.109462
|
| [32] |
ZHANG YM, SU M, WANG Z, NIELSEN J, LIU ZH. Rewiring regulation on respiro-fermentative metabolism relieved Crabtree effects in Saccharomyces cerevisiae. Synthetic and Systems Biotechnology, 2022, 7(4): 1034-1043. DOI:10.1016/j.synbio.2022.06.004
|
| [33] |
AVALOS JL, FINK GR, STEPHANOPOULOS G. Compartmentalization of metabolic pathways in yeast mitochondria improves the production of branched-chain alcohols. Nature Biotechnology, 2013, 31(4): 335-341. DOI:10.1038/nbt.2509
|
| [34] |
HAMMER SK, ZHANG YF, AVALOS JL. Mitochondrial compartmentalization confers specificity to the 2-ketoacid recursive pathway: increasing isopentanol production in Saccharomyces cerevisiae. ACS Synthetic Biology, 2020, 9(3): 546-555. DOI:10.1021/acssynbio.9b00420
|
| [35] |
LANE S, ZHANG YF, YUN EJ, ZIOLKOWSKI L, ZHANG GC, JIN YS, AVALOS JL. Xylose assimilation enhances the production of isobutanol in engineered Saccharomyces cerevisiae. Biotechnology and Bioengineering, 2020, 117(2): 372-381. DOI:10.1002/bit.27202
|
| [36] |
ZHANG YF, LANE S, CHEN JM, HAMMER SK, LUTTINGER J, YANG LF, JIN YS, AVALOS JL. Xylose utilization stimulates mitochondrial production of isobutanol and 2-methyl-1-butanol in Saccharomyces cerevisiae. Biotechnology for Biofuels, 2019, 12(1): 1-15. DOI:10.1186/s13068-018-1346-y
|
| [37] |
BURÉN S, PRATT K, JIANG X, GUO YS, JIMENEZ-VICENTE E, ECHAVARRI-ERASUN C, DEAN DR, SAAEM I, BENJAMIN GORDON D, VOIGT CA, RUBIO LM. Biosynthesis of the nitrogenase active-site cofactor precursor NifB-co in Saccharomyces cerevisiae. Proceedings of the National Academy of Sciences of the United States of America, 2019, 116(50): 25078-25086. DOI:10.1073/pnas.1904903116
|
| [38] |
GERKE J, FRAUENDORF H, SCHNEIDER D, WINTERGOLLER M, HOFMEISTER T, POEHLEIN A, ZEBEC Z, TAKANO E, SCRUTTON NS, BRAUS GH. Production of the fragrance geraniol in peroxisomes of a product-tolerant baker's yeast. Frontiers in Bioengineering and Biotechnology, 2020, 8: 582052. DOI:10.3389/fbioe.2020.582052
|
| [39] |
ZHANG CB, LI M, ZHAO GR, LU WY. Harnessing yeast peroxisomes and cytosol acetyl-CoA for sesquiterpene α-humulene production. Journal of Agricultural and Food Chemistry, 2020, 68(5): 1382-1389. DOI:10.1021/acs.jafc.9b07290
|
| [40] |
LIU GS, LI T, ZHOU W, JIANG M, TAO XY, LIU M, ZHAO M, REN YH, GAO B, WANG FQ, WEI DZ. The yeast peroxisome: a dynamic storage depot and subcellular factory for squalene overproduction. Metabolic Engineering, 2020, 57: 151-161. DOI:10.1016/j.ymben.2019.11.001
|
| [41] |
ZHANG Q, YU SQ, LYU YB, ZENG WZ, ZHOU JW. Systematically engineered fatty acid catabolite pathway for the production of (2S)-naringenin in Saccharomyces cerevisiae. ACS Synthetic Biology, 2021, 10(5): 1166-1175. DOI:10.1021/acssynbio.1c00002
|
| [42] |
SHENG JY, STEVENS J, FENG XY. Pathway compartmentalization in peroxisome of Saccharomyces cerevisiae to produce versatile medium chain fatty alcohols. Scientific Reports, 2016, 6: 26884. DOI:10.1038/srep26884
|
| [43] |
SIBIRNY AA. Yeast peroxisomes: structure, functions and biotechnological opportunities. FEMS Yeast Research, 2016, 16(4): fow038. DOI:10.1093/femsyr/fow038
|
| [44] |
DZANAEVA L, KRUK B, RUCHALA J, NIELSEN J, SIBIRNY A, DMYTRUK K. The role of peroxisomes in xylose alcoholic fermentation in the engineered Saccharomyces cerevisiae. Cell Biology International, 2020, 44(8): 1606-1615. DOI:10.1002/cbin.11353
|
| [45] |
THODEY K, GALANIE S, SMOLKE CD. A microbial biomanufacturing platform for natural and semisynthetic opioids. Nature Chemical Biology, 2014, 10(10): 837-844. DOI:10.1038/nchembio.1613
|
| [46] |
ARENDT P, MIETTINEN K, POLLIER J, de RYCKE R, CALLEWAERT N, GOOSSENS A. An endoplasmic reticulum-engineered yeast platform for overproduction of triterpenoids. Metabolic Engineering, 2017, 40: 165-175. DOI:10.1016/j.ymben.2017.02.007
|
| [47] |
KIM JE, JANG IS, SON SH, KO YJ, CHO BK, KIM SC, LEE JY. Tailoring the Saccharomyces cerevisiae endoplasmic reticulum for functional assembly of terpene synthesis pathway. Metabolic Engineering, 2019, 56: 50-59. DOI:10.1016/j.ymben.2019.08.013
|
| [48] |
WEI PP, ZHANG CB, BIAN XK, LU WY. Metabolic engineering of Saccharomyces cerevisiae for heterologous carnosic acid production. Frontiers in Bioengineering and Biotechnology, 2022, 10: 916605. DOI:10.3389/fbioe.2022.916605
|
| [49] |
BAO JC, HUANG MT, PETRANOVIC D, NIELSEN J. Balanced trafficking between the ER and the golgi apparatus increases protein secretion in yeast. AMB Express, 2018, 8(1): 1-10. DOI:10.1186/s13568-017-0531-x
|
| [50] |
HUANG MT, WANG GK, QIN JF, PETRANOVIC D, NIELSEN J. Engineering the protein secretory pathway of Saccharomyces cerevisiae enables improved protein production. Proceedings of the National Academy of Sciences of the United States of America, 2018, 115(47): E11025-E11032.
|
| [51] |
YU Y, RASOOL A, LIU HR, LV B, CHANG PC, SONG H, WANG Y, LI C. Engineering Saccharomyces cerevisiae for high yield production of α-amyrin via synergistic remodeling of α-amyrin synthase and expanding the storage pool. Metabolic Engineering, 2020, 62: 72-83. DOI:10.1016/j.ymben.2020.08.010
|
| [52] |
MANZOOR R, AHMED M, RIAZ N, KIANI BH, KALEEM U, RASHID Y, NAWAZ A, AWAN MUF, KHAN H, IMTIAZ U, RASHEED Y, KALEEM I, RASOOL A. Self-redirection of metabolic flux toward squalene and ethanol pathways by engineered yeast. Metabolites, 2020, 10(2): 56. DOI:10.3390/metabo10020056
|
| [53] |
MA T, SHI B, YE ZL, LI XW, LIU M, CHEN Y, XIA J, NIELSEN J, DENG ZX, LIU TG. Lipid engineering combined with systematic metabolic engineering of Saccharomyces cerevisiae for high-yield production of lycopene. Metabolic Engineering, 2019, 52: 134-142. DOI:10.1016/j.ymben.2018.11.009
|
| [54] |
SUN ZJ, LIAN JZ, ZHU L, JIANG YQ, LI GS, XUE HL, WU MB, YANG LR, LIN JP. Combined biosynthetic pathway engineering and storage pool expansion for high-level production of ergosterol in industrial Saccharomyces cerevisiae. Frontiers in Bioengineering and Biotechnology, 2021, 9: 681666. DOI:10.3389/fbioe.2021.681666
|
| [55] |
ZHAO YJ, ZHANG YP, NIELSEN J, LIU ZH. Production of β-carotene in Saccharomyces cerevisiae through altering yeast lipid metabolism. Biotechnology and Bioengineering, 2021, 118(5): 2043-2052. DOI:10.1002/bit.27717
|
| [56] |
BU X, LIN JY, DUAN CQ, KOFFAS MAG, YAN GL. Dual regulation of lipid droplet-triacylglycerol metabolism and ERG9 expression for improved β-carotene production in Saccharomyces cerevisiae. Microbial Cell Factories, 2022, 21(1): 1-13. DOI:10.1186/s12934-021-01718-9
|
| [57] |
GUO XJ, YAO MD, XIAO WH, WANG Y, ZHAO GR, YUAN YJ. Compartmentalized reconstitution of post-squalene pathway for 7-dehydrocholesterol overproduction in Saccharomyces cerevisiae. Frontiers in Microbiology, 2021, 12: 663973. DOI:10.3389/fmicb.2021.663973
|
| [58] |
张思琪, 周景文, 张国强, 陈坚. 产对香豆酸酿酒酵母工程菌株的构建与优化. 生物工程学报, 2020, 36(9): 1838-1848. ZHANG SQ, ZHOU JW, ZHANG GQ, CHEN J. Construction and optimization of p-coumaric acid-producing Saccharomyces cerevisiae. Chinese Journal of Biotechnology, 2020, 36(9): 1838-1848 (in Chinese). DOI:10.13345/j.cjb.200003 |
| [59] |
SRINIVASAN P, SMOLKE CD. Biosynthesis of medicinal tropane alkaloids in yeast. Nature, 2020, 585(7826): 614-619. DOI:10.1038/s41586-020-2650-9
|
| [60] |
SRINIVASAN P, SMOLKE CD. Engineering cellular metabolite transport for biosynthesis of computationally predicted tropane alkaloid derivatives in yeast. Proceedings of the National Academy of Sciences of the United States of America, 2021, 118(25): e2104460118. DOI:10.1073/pnas.2104460118
|
| [61] |
KAHAR P, ITOMI A, TSUBOI H, ISHIZAKI M, YASUDA M, KIHIRA C, OTSUKA H, BINTI AZMI N, MATSUMOTO H, OGINO C, KONDO A. The flocculant Saccharomyces cerevisiae strain gains robustness via alteration of the cell wall hydrophobicity. Metabolic Engineering, 2022, 72: 82-96. DOI:10.1016/j.ymben.2022.03.001
|
| [62] |
SON SH, KIM JE, OH SS, LEE JY. Engineering cell wall integrity enables enhanced squalene production in yeast. Journal of Agricultural and Food Chemistry, 2020, 68(17): 4922-4929. DOI:10.1021/acs.jafc.0c00967
|
| [63] |
LI J, ZHANG MM, WAN C, den HAAN R, BAI FW, ZHAO XQ. Improved cellulase production in recombinant Saccharomyces cerevisiae by disrupting the cell wall protein-encoding gene CWP2. Journal of Bioscience and Bioengineering, 2020, 129(2): 165-171. DOI:10.1016/j.jbiosc.2019.08.012
|
| [64] |
KONG ML, LI XW, LI TT, ZHAO XB, JIN MJ, ZHOU X, GU HQ, MRŠA V, XIAO W, CAO LM. Overexpressing CCW12 in Saccharomyces cerevisiae enables highly efficient ethanol production from lignocellulose hydrolysates. Bioresource Technology, 2021, 337: 125487. DOI:10.1016/j.biortech.2021.125487
|
| [65] |
INOKUMA K, KURONO H, den HAAN R, van ZYL WH, HASUNUMA T, KONDO A. Novel strategy for anchorage position control of GPI-attached proteins in the yeast cell wall using different GPI-anchoring domains. Metabolic Engineering, 2020, 57: 110-117. DOI:10.1016/j.ymben.2019.11.004
|
| [66] |
INOKUMA K, KITADA Y, BAMBA T, KOBAYASHI Y, YUKAWA T, HAAN R, ZYL WH, KONDO A, HASUNUMA T. Improving the functionality of surface-engineered yeast cells by altering the cell wall morphology of the host strain. Applied Microbiology and Biotechnology, 2021, 105(14/15): 5895-5904.
|
| [67] |
CHEAH LC, STARK T, ADAMSON LSR, ABIDIN RS, LAU YH, SAINSBURY F, VICKERS CE. Artificial self-assembling nanocompartment for organizing metabolic pathways in yeast. ACS Synthetic Biology, 2021, 10(12): 3251-3263. DOI:10.1021/acssynbio.1c00045
|
| [68] |
ZHANG Q, ZENG WZ, XU S, ZHOU JW. Metabolism and strategies for enhanced supply of acetyl-CoA in Saccharomyces cerevisiae. Bioresource Technology, 2021, 342: 125978. DOI:10.1016/j.biortech.2021.125978
|
| [69] |
ENGLISH AM, SCHULER MH, XIAO TY, KORNMANN B, SHAW JM, HUGHES AL. ER-mitochondria contacts promote mitochondrial-derived compartment biogenesis. Journal of Cell Biology, 2020, 219(12): e202002144. DOI:10.1083/jcb.202002144
|
| [70] |
YUAN JF, CHING CB. Mitochondrial acetyl-CoA utilization pathway for terpenoid productions. Metabolic Engineering, 2016, 38: 303-309. DOI:10.1016/j.ymben.2016.07.008
|
| [71] |
BUU LM, CHEN YC, LEE FJ S. Functional characterization and localization of acetyl-CoA hydrolase, Ach1p, in Saccharomyces cerevisiae. Journal of Biological Chemistry, 2003, 278(19): 17203-17209. DOI:10.1074/jbc.M213268200
|
| [72] |
WEINERT BT, IESMANTAVICIUS V, MOUSTAFA T, SCHÖLZ C, WAGNER SA, MAGNES C, ZECHNER R, CHOUDHARY C. Acetylation dynamics and stoichiometry in Saccharomyces cerevisiae. Molecular Systems Biology, 2015, 11(10): 833. DOI:10.15252/msb.156513
|
| [73] |
KRIVORUCHKO A, ZHANG YM, SIEWERS V, CHEN Y, NIELSEN J. Microbial acetyl-CoA metabolism and metabolic engineering. Metabolic Engineering, 2015, 28: 28-42. DOI:10.1016/j.ymben.2014.11.009
|
| [74] |
SIKKEMA J, de BONT JA, POOLMAN B. Mechanisms of membrane toxicity of hydrocarbons. Microbiological Reviews, 1995, 59(2): 201-222. DOI:10.1128/mr.59.2.201-222.1995
|
| [75] |
KWAK S, JIN YS. Production of fuels and chemicals from xylose by engineered Saccharomyces cerevisiae: a review and perspective. Microbial Cell Factories, 2017, 16(1): 1-15. DOI:10.1186/s12934-016-0616-2
|
| [76] |
SALUSJÄRVI L, KANKAINEN M, SOLIYMANI R, PITKÄNEN JP, PENTTILÄ M, RUOHONEN L. Regulation of xylose metabolism in recombinant Saccharomyces cerevisiae. Microbial Cell Factories, 2008, 7(1): 1-16. DOI:10.1186/1475-2859-7-1
|
| [77] |
ZAMPAR GG, KÜMMEL A, EWALD J, JOL S, NIEBEL B, PICOTTI P, AEBERSOLD R, SAUER U, ZAMBONI N, HEINEMANN M. Temporal system-level organization of the switch from glycolytic to gluconeogenic operation in yeast. Molecular Systems Biology, 2013, 9(1): 651. DOI:10.1038/msb.2013.11
|
| [78] |
HEYLAND J, FU JN, BLANK LM. Correlation between TCA cycle flux and glucose uptake rate during respiro-fermentative growth of Saccharomyces cerevisiae. Microbiology, 2009, 155(12): 3827-3837. DOI:10.1099/mic.0.030213-0
|
| [79] |
KWAK S, JO JH, YUN EJ, JIN YS, SEO JH. Production of biofuels and chemicals from xylose using native and engineered yeast strains. Biotechnology Advances, 2019, 37(2): 271-283. DOI:10.1016/j.biotechadv.2018.12.003
|
| [80] |
KILDEGAARD KR, WANG Z, CHEN Y, NIELSEN J, BORODINA I. Production of 3-hydroxypropionic acid from glucose and xylose by metabolically engineered Saccharomyces cerevisiae. Metabolic Engineering Communications, 2015, 2: 132-136. DOI:10.1016/j.meteno.2015.10.001
|
| [81] |
WANG PP, LI CJ, LI XD, HUANG WJ, WANG Y, WANG JL, ZHANG YJ, YANG XM, YAN X, WANG Y, ZHOU ZH. Complete biosynthesis of the potential medicine icaritin by engineered Saccharomyces cerevisiae and Escherichia coli. Science Bulletin, 2021, 66(18): 1906-1916. DOI:10.1016/j.scib.2021.03.002
|
| [82] |
BEACH A, LEONOV A, ARLIA-CIOMMO A, SVISTKOVA V, LUTCHMAN V, TITORENKO V. Mechanisms by which different functional states of mitochondria define yeast longevity. International Journal of Molecular Sciences, 2015, 16(12): 5528-5554. DOI:10.3390/ijms16035528
|
| [83] |
GREWAL PS, SAMSON JA, BAKER JJ, CHOI B, DUEBER JE. Peroxisome compartmentalization of a toxic enzyme improves alkaloid production. Nature Chemical Biology, 2021, 17(1): 96-103. DOI:10.1038/s41589-020-00668-4
|
| [84] |
van ROERMUND CW, HETTEMA EH, KAL AJ, van den BERG M, TABAK HF, WANDERS RJ. Peroxisomal beta-oxidation of polyunsaturated fatty acids in Saccharomyces cerevisiae: isocitrate dehydrogenase provides NADPH for reduction of double bonds at even positions. The EMBO Journal, 1998, 17(3): 677-687. DOI:10.1093/emboj/17.3.677
|
| [85] |
SCHUELLER N, HOLTON SJ, FODOR K, MILEWSKI M, KONAREV P, STANLEY WA, WOLF J, ERDMANN R, SCHLIEBS W, SONG YH, WILMANNS M. The peroxisomal receptor Pex19p forms a helical mPTS recognition domain. The EMBO Journal, 2010, 29(15): 2491-2500. DOI:10.1038/emboj.2010.115
|
| [86] |
PHILLIPS MJ, VOELTZ GK. Structure and function of ER membrane contact sites with other organelles. Nature Reviews Molecular Cell Biology, 2016, 17(2): 69-82. DOI:10.1038/nrm.2015.8
|
| [87] |
赵妍, 喻其林. 内质网靶向纳米药物的研究进展. 生物工程学报, 2021, 37(2): 418-428. ZHAO Y, YU QL. Advance in endoplasmic reticulum-targeting nanodrugs. Chinese Journal of Biotechnology, 2021, 37(2): 418-428 (in Chinese). DOI:10.13345/j.cjb.200292 |
| [88] |
HAUSJELL J, HALBWIRTH H, SPADIUT O. Recombinant production of eukaryotic cytochrome P450s in microbial cell factories. Bioscience Reports, 2018, 38(2): BSR20171290. DOI:10.1042/BSR20171290
|
| [89] |
NOWROUZI B, RIOS-SOLIS L. Redox metabolism for improving whole-cell P450-catalysed terpenoid biosynthesis. Critical Reviews in Biotechnology, 2022, 42(8): 1213-1237. DOI:10.1080/07388551.2021.1990210
|
| [90] |
JIANG LH, HUANG L, CAI J, XU ZN, LIAN JZ. Functional expression of eukaryotic cytochrome P450s in yeast. Biotechnology and Bioengineering, 2021, 118(3): 1050-1065. DOI:10.1002/bit.27630
|
| [91] |
ZHENG XY, LI P, LU X. Research advances in cytochrome P450-catalysed pharmaceutical terpenoid biosynthesis in plants. Journal of Experimental Botany, 2019, 70(18): 4619-4630. DOI:10.1093/jxb/erz203
|
| [92] |
RENAULT H, BASSARD JE, HAMBERGER B, WERCK-REICHHART D. Cytochrome P450-mediated metabolic engineering: current progress and future challenges. Current Opinion in Plant Biology, 2014, 19: 27-34. DOI:10.1016/j.pbi.2014.03.004
|
| [93] |
WANG X, PEREIRA JH, TSUTAKAWA S, FANG XY, ADAMS PD, MUKHOPADHYAY A, LEE TS. Efficient production of oxidized terpenoids via engineering fusion proteins of terpene synthase and cytochrome P450. Metabolic Engineering, 2021, 64: 41-51. DOI:10.1016/j.ymben.2021.01.004
|
| [94] |
AJIKUMAR PK, XIAO WH, TYO KEJ, WANG Y, SIMEON F, LEONARD E, MUCHA O, PHON TH, PFEIFER B, STEPHANOPOULOS G. Isoprenoid pathway optimization for taxol precursor overproduction in Escherichia coli. Science, 2010, 330(6000): 70-74. DOI:10.1126/science.1191652
|
| [95] |
BIGGS BW, LIM CG, SAGLIANI K, SHANKAR S, STEPHANOPOULOS G, de MEY M, AJIKUMAR PK. Overcoming heterologous protein interdependency to optimize P450-mediated taxol precursor synthesis in Escherichia coli. Proceedings of the National Academy of Sciences of the United States of America, 2016, 113(12): 3209-3214. DOI:10.1073/pnas.1515826113
|
| [96] |
KIM JE, JANG IS, SUNG BH, KIM SC, LEE JY. Rerouting of NADPH synthetic pathways for increased protopanaxadiol production in Saccharomyces cerevisiae. Scientific Reports, 2018, 8: 15820. DOI:10.1038/s41598-018-34210-3
|
| [97] |
ZHUANG X, CHAPPELL J. Building terpene production platforms in yeast. Biotechnology and Bioengineering, 2015, 112(9): 1854-1864. DOI:10.1002/bit.25588
|
| [98] |
MALHOTRA JD, MIAO HZ, ZHANG KZ, WOLFSON A, PENNATHUR S, PIPE SW, KAUFMAN RJ. Antioxidants reduce endoplasmic reticulum stress and improve protein secretion. Proceedings of the National Academy of Sciences of the United States of America, 2008, 105(47): 18525-18530. DOI:10.1073/pnas.0809677105
|
| [99] |
SUDA Y, NAKANO A. The yeast golgi apparatus. Traffic, 2012, 13(4): 505-510. DOI:10.1111/j.1600-0854.2011.01316.x
|
| [100] |
IYER P, BHAVE M, JAIN BK, RoyCHOWDHURY S, BHATTACHARYYA D. Vps74p controls golgi size in an Arf1-dependent manner. FEBS Letters, 2018, 592(22): 3720-3735. DOI:10.1002/1873-3468.13266
|
| [101] |
NODA Y, YODA K. Molecular mechanisms of the localization of membrane proteins in the yeast golgi compartments. Bioscience, Biotechnology, and Biochemistry, 2013, 77(3): 435-445. DOI:10.1271/bbb.120982
|
| [102] |
SUDA Y, KUROKAWA K, NAKANO A. Regulation of ER-golgi transport dynamics by GTPases in budding yeast. Frontiers in Cell and Developmental Biology, 2018, 5: 122. DOI:10.3389/fcell.2017.00122
|
| [103] |
MUNRO S. What is the golgi apparatus, and why are we asking?. BMC Biology, 2011, 9(1): 1-5. DOI:10.1186/1741-7007-9-1
|
| [104] |
BAO JC, HUANG MT, PETRANOVIC D, NIELSEN J. Moderate expression of SEC16 increases protein secretion by Saccharomyces cerevisiae. Applied and Environmental Microbiology, 2017, 83(14): e03400-16.
|
| [105] |
OLZMANN JA, CARVALHO P. Dynamics and functions of lipid droplets. Nature Reviews Molecular Cell Biology, 2019, 20(3): 137-155. DOI:10.1038/s41580-018-0085-z
|
| [106] |
TEIXEIRA PG, DAVID F, SIEWERS V, NIELSEN J. Engineering lipid droplet assembly mechanisms for improved triacylglycerol accumulation in Saccharomyces cerevisiae. FEMS Yeast Research, 2018, 18(6): foy060.
|
| [107] |
柯霞, 沈逸, 曹丽莎, 张博, 柳志强. 酵母中甾醇定向存储转运及理性强化技术研究进展. 生物工程学报, 2021, 37(11): 3975-3987. KE X, SHEN Y, CAO LS, ZHANG B, LIU ZQ. Reinforcement of sterols production through directed storage and transportation in yeast: a review. Chinese Journal of Biotechnology, 2021, 37(11): 3975-3987 (in Chinese). DOI:10.13345/j.cjb.200772 |
| [108] |
HENNE M, GOODMAN JM, HARIRI H. Spatial compartmentalization of lipid droplet biogenesis. Biochimica et Biophysica Acta (BBA)-Molecular and Cell Biology of Lipids, 2020, 1865(1): 158499.
|
| [109] |
ZONI V, KHADDAJ R, CAMPOMANES P, THIAM AR, SCHNEITER R, VANNI S. Pre-existing bilayer stresses modulate triglyceride accumulation in the ER versus lipid droplets. eLife, 2021, 10: 62886. DOI:10.7554/eLife.62886
|
| [110] |
NETTEBROCK NT, BOHNERT M. Born this way-biogenesis of lipid droplets from specialized ER subdomains. Biochimica et Biophysica Acta (BBA)-Molecular and Cell Biology of Lipids, 2020, 1865(1): 158448.
|
| [111] |
SCHEPERS J, BEHL C. Lipid droplets and autophagy—links and regulations from yeast to humans. Journal of Cellular Biochemistry, 2021, 122(6): 602-611. DOI:10.1002/jcb.29889
|
| [112] |
PALAGE AM, WARD VC. Strategies for production of hydrophobic compounds. Current Opinion in Biotechnology, 2022, 75: 102681. DOI:10.1016/j.copbio.2022.102681
|
| [113] |
THIAM AR, BELLER M. The why, when and how of lipid droplet diversity. Journal of Cell Science, 2017, 130(2): 315-324.
|
| [114] |
YOKOTA S, GOTOH T. Effects of rubber elongation factor and small rubber particle protein from rubber-producing plants on lipid metabolism in Saccharomyces cerevisiae. Journal of Bioscience and Bioengineering, 2019, 128(5): 585-592. DOI:10.1016/j.jbiosc.2019.04.018
|
| [115] |
WINKLER MBL, NEL L, FRAIN KM, DEDIC E, OLESEN E, PEDERSEN BP. Sterol uptake by the NPC system in eukaryotes: a Saccharomyces cerevisiae perspective. FEBS Letters, 2022, 596(2): 160-179. DOI:10.1002/1873-3468.14253
|
| [116] |
LI SC, KANE PM. The yeast lysosome-like vacuole: endpoint and crossroads. Biochimica et Biophysica Acta (BBA)-Molecular Cell Research, 2009, 1793(4): 650-663. DOI:10.1016/j.bbamcr.2008.08.003
|
| [117] |
BANTA LM, ROBINSON JS, KLIONSKY DJ, EMR SD. Organelle assembly in yeast: characterization of yeast mutants defective in vacuolar biogenesis and protein sorting. Journal of Cell Biology, 1988, 107(4): 1369-1383. DOI:10.1083/jcb.107.4.1369
|
| [118] |
KLIONSKY DJ, HERMAN PK, EMR SD. The fungal vacuole: composition, function, and biogenesis. Microbiological Reviews, 1990, 54(3): 266-292. DOI:10.1128/mr.54.3.266-292.1990
|
| [119] |
KAWANO-KAWADA M, KAKINUMA Y, SEKITO T. Transport of amino acids across the vacuolar membrane of yeast: its mechanism and physiological role. Biological and Pharmaceutical Bulletin, 2018, 41(10): 1496-1501. DOI:10.1248/bpb.b18-00165
|
| [120] |
ZHANG WP, DU GC, ZHOU JW, CHEN J. Regulation of sensing, transportation, and catabolism of nitrogen sources in Saccharomyces cerevisiae. Microbiology and Molecular Biology Reviews, 2018, 82(1): e00040-17.
|
| [121] |
ORLEAN P. Architecture and biosynthesis of the Saccharomyces cerevisiae cell wall. Genetics, 2012, 192(3): 775-818. DOI:10.1534/genetics.112.144485
|
| [122] |
CABIB E, ARROYO J. How carbohydrates sculpt cells: chemical control of morphogenesis in the yeast cell wall. Nature Reviews Microbiology, 2013, 11(9): 648-655. DOI:10.1038/nrmicro3090
|
| [123] |
AUESUKAREE C. Molecular mechanisms of the yeast adaptive response and tolerance to stresses encountered during ethanol fermentation. Journal of Bioscience and Bioengineering, 2017, 124(2): 133-142. DOI:10.1016/j.jbiosc.2017.03.009
|
| [124] |
BOROVIKOVA D, TEPARIĆ R, MRŠA V, RAPOPORT A. Anhydrobiosis in yeast: cell wall mannoproteins are important for yeast Saccharomyces cerevisiae resistance to dehydration. Yeast, 2016, 33(8): 347-353. DOI:10.1002/yea.3164
|
| [125] |
THAK EJ, YOO SJ, MOON HY, KANG HA. Yeast synthetic biology for designed cell factories producing secretory recombinant proteins. FEMS Yeast Research, 2020, 20(2): foaa009. DOI:10.1093/femsyr/foaa009
|
| [126] |
WILLIAMS EM, JUNG SM, COFFMAN JL, LUTZ S. Pore engineering for enhanced mass transport in encapsulin nanocompartments. ACS Synthetic Biology, 2018, 7(11): 2514-2517. DOI:10.1021/acssynbio.8b00295
|
| [127] |
ZHU K, KONG J, ZHAO BX, RONG LX, LIU SQ, LU ZH, ZHANG CY, XIAO DG, PUSHPANATHAN K, FOO JL, WONG A, YU AQ. Metabolic engineering of microbes for monoterpenoid production. Biotechnology Advances, 2021, 53: 107837. DOI:10.1016/j.biotechadv.2021.107837
|
| [128] |
JIN K, XIA HZ, LIU YF, LI JH, DU GC, LV XQ, LIU L. Compartmentalization and transporter engineering strategies for terpenoid synthesis. Microbial Cell Factories, 2022, 21(1): 1-12. DOI:10.1186/s12934-021-01718-9
|