| [1] |
Markets and Markets. Bioplastics & biopolymers market by type (non-biodegradable/bio-based, biodegradable), end-use industry (packaging, consumer goods, automotive & transportation, textiles, agriculture & horticulture), region–global forecast to 2025[R]. Report, 2020.
|
|
| [2] |
LEE JW, KIM HU, CHOI S, YI J, LEE SY. Microbial production of building block chemicals and polymers. Current Opinion in Biotechnology, 2011, 22(6): 758-767. DOI:10.1016/j.copbio.2011.02.011
|
|
| [3] |
DEGLI ESPOSTI M, MORSELLI D, FAVA F, BERTIN L, CAVANI F, VIAGGI D, FABBRI P. The role of biotechnology in the transition from plastics to bioplastics: an opportunity to reconnect global growth with sustainability. FEBS Open Bio, 2021, 11(4): 967-983. DOI:10.1002/2211-5463.13119
|
|
| [4] |
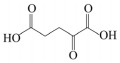
CHEN Y, NIELSEN J. Biobased organic acids production by metabolically engineered microorganisms. Current Opinion in Biotechnology, 2016, 37: 165-172. DOI:10.1016/j.copbio.2015.11.004
|
|
| [5] |
PORRO D, BRANDUARDI P. Production of organic acids by yeasts and filamentous fungi[M]//Biotechnology of Yeasts and Filamentous Fungi. Cham: Springer International Publishing, 2017: 205-223.
|
|
| [6] |
LIAUD N, GINIÉS C, NAVARRO D, FABRE N, CRAPART S, GIMBERT IH, LEVASSEUR A, RAOUCHE S, SIGOILLOT JC. Exploring fungal biodiversity: organic acid production by 66 strains of filamentous fungi. Fungal Biology and Biotechnology, 2014, 1(1): 1-10. DOI:10.1186/s40694-014-0001-z
|
|
| [7] |
SAUER M, PORRO D, MATTANOVICH D, BRANDUARDI P. Microbial production of organic acids: expanding the markets. Trends in Biotechnology, 2008, 26(2): 100-108. DOI:10.1016/j.tibtech.2007.11.006
|
|
| [8] | |
|
| [9] |
BECKER J, LANGE AN, FABARIUS J, WITTMANN C. Top value platform chemicals: bio-based production of organic acids. Current Opinion in Biotechnology, 2015, 36: 168-175. DOI:10.1016/j.copbio.2015.08.022
|
|
| [10] |
ALONSO S, RENDUELES M, DÍAZ M. Microbial production of specialty organic acids from renewable and waste materials. Critical Reviews in Biotechnology, 2015, 35(4): 497-513. DOI:10.3109/07388551.2014.904269
|
|
| [11] |
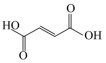
LIU JJ, LI JH, SHIN HD, LIU L, DU GC, CHEN J. Protein and metabolic engineering for the production of organic acids. Bioresource Technology, 2017, 239: 412-421. DOI:10.1016/j.biortech.2017.04.052
|
|
| [12] |
王颖珊, 郭峰, 严伟, 信丰学, 章文明, 姜岷. 四碳有机酸生物合成的代谢工程研究进展. 生物工程学报, 2021, 37(5): 1697-1720. WANG YS, GUO F, YAN W, XIN FX, ZHANG WM, JIANG M. Advances in the metabolic engineering for the production of tetracarbon organic acids. Chinese Journal of Biotechnology, 2021, 37(5): 1697-1720 (in Chinese). DOI:10.13345/j.cjb.200727
|
|
| [13] |
张勤, 张梁, 丁重阳, 王正祥, 石贵阳. 代谢工程改造野生耐酸酵母生产L-乳酸. 生物工程学报, 2011, 27(7): 1024-1031. ZHANG Q, ZHANG L, DING CY, WANG ZX, SHI GY. Metabolic engineering of wild acid-resistant yeast for -lactic acid production. Chinese Journal of Biotechnology, 2011, 27(7): 1024-1031 (in Chinese). DOI:10.13345/j.cjb.2011.07.004
|
|
| [14] |
荣兰新, 刘士琦, 朱坤, 孔婧, 苗琳, 王淑慧, 肖冬光, 于爱群. 代谢工程改造解脂耶氏酵母合成羧酸的研究进展. 生物工程学报, 2022, 38(4): 1360-1372. RONG LX, LIU SQ, ZHU K, KONG J, MIAO L, WANG SH, XIAO DG, YU AQ. Production of carboxylic acids by metabolically engineered Yarrowia lipolytica: a review. Chinese Journal of Biotechnology, 2022, 38(4): 1360-1372 (in Chinese). DOI:10.13345/j.cjb.210626
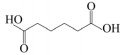
|
|
| [15] |
张金宏, 崔志勇, 祁庆生, 侯进. 解脂耶氏酵母表达调控工具的开发及天然产物合成的研究进展. 生物工程学报, 2022, 38(2): 478-505. ZHANG JH, CUI ZY, QI QS, HOU J. The recent advances in developing gene editing and expression tools and the synthesis of natural products in Yarrowia lipolytica. Chinese Journal of Biotechnology, 2022, 38(2): 478-505 (in Chinese). DOI:10.13345/j.cjb.210327
|
|
| [16] |
WERPY T, PETERSEN G. Top Value Added Chemicals from Biomass: Volume I–Results of Screening for Potential Candidates from Sugars and Synthesis Gas[M], US Department of Energy, Oak Ridge, TN, USA 2004.
|
|
| [17] |
LIU XT, ZHAO G, SUN SJ, FAN CL, FENG XJ, XIONG P. Biosynthetic pathway and metabolic engineering of succinic acid. Frontiers in Bioengineering and Biotechnology, 2022, 10: 843887. DOI:10.3389/fbioe.2022.843887
|
|
| [18] |
BABAEI M, RUEKSOMTAWIN KILDEGAARD K, NIAEI A, HOSSEINI M, EBRAHIMI S, SUDARSAN S, ANGELIDAKI I, BORODINA I. Engineering oleaginous yeast as the host for fermentative succinic acid production from glucose. Frontiers in Bioengineering and Biotechnology, 2019, 7: 361. DOI:10.3389/fbioe.2019.00361
|
|
| [19] |
CUI Z, GAO C, LI J, HOU J, LIN CSK, QI Q. Engineering of unconventional yeast Yarrowia lipolytica for efficient succinic acid production from glycerol at low pH. Metabolic Engineering, 2017, 42: 126-133. DOI:10.1016/j.ymben.2017.06.007
|
|
| [20] |
YANG XF, WANG HM, LI C, LIN CSK. Restoring of glucose metabolism of engineered Yarrowia lipolytica for succinic acid production via a simple and efficient adaptive evolution strategy. Journal of Agricultural and Food Chemistry, 2017, 65(20): 4133-4139. DOI:10.1021/acs.jafc.7b00519
|
|
| [21] |
BONDARENKO PY, FEDOROV AS, SINEOKY SP. Optimization of repeated-batch fermentation of a recombinant strain of the yeast Yarrowia lipolytica for succinic acid production at low pH. Applied Biochemistry and Microbiology, 2017, 53(9): 882-887. DOI:10.1134/S0003683817090022
|
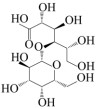
|
| [22] |
LI C, GAO S, YANG X, LIN CSK. Green and sustainable succinic acid production from crude glycerol by engineered Yarrowia lipolytica via agricultural residue based in situ fibrous bed bioreactor. Bioresource Technology, 2018, 249: 612-619. DOI:10.1016/j.biortech.2017.10.011
|
|
| [23] |
LI C, ONG KL, YANG X, Lin CSK. Bio-refinery of waste streams for green and efficient succinic acid production by engineered Yarrowia lipolytica without pH control. Chemical Engineering Journal, 2019, 371: 804-812. DOI:10.1016/j.cej.2019.04.092
|
|
| [24] |
LI C, YANG X, GAO S, CHUH AH, LIN CSK. Hydrolysis of fruit and vegetable waste for efficient succinic acid production with engineered Yarrowia lipolytica. Journal of Cleaner Production, 2018, 179: 151-159. DOI:10.1016/j.jclepro.2018.01.081
|
|
| [25] |
BILLERACH G, PREZIOSI-BELLOY L, LIN CSK, FULCRAND H, DUBREUCQ E, GROUSSEAU E. Impact of nitrogen deficiency on succinic acid production by engineered strains of Yarrowia lipolytica. Journal of Biotechnology, 2021, 336: 30-40. DOI:10.1016/j.jbiotec.2021.06.001
|
|
| [26] |
STYLIANOU E, PATERAKI C, LADAKIS D, DAMALA C, VLYSIDIS A, LATORRE-SÁNCHEZ M, COLL C, LIN CSK, KOUTINAS A. Bioprocess development using organic biowaste and sustainability assessment of succinic acid production with engineered Yarrowia lipolytica strain. Biochemical Engineering Journal, 2021, 174: 108099. DOI:10.1016/j.bej.2021.108099
|
|
| [27] |
JIANG ZN, CUI ZY, ZHU ZW, LIU YH, TANG YJ, HOU J, QI QS. Engineering of Yarrowia lipolytica transporters for high-efficient production of biobased succinic acid from glucose. Biotechnology for Biofuels, 2021, 14(1): 1-10. DOI:10.1186/s13068-020-01854-1
|
|
| [28] |
NARISETTY V, PRABHU AA, BOMMAREDDY RR, COX R, AGRAWAL D, MISRA A, ALI HAIDER M, BHATNAGAR A, PANDEY A, KUMAR V. Development of hypertolerant strain of Yarrowia lipolytica accumulating succinic acid using high levels of acetate. ACS Sustainable Chemistry & Engineering, 2022, 10(33): 10858-10869.
|
|
| [29] |
XIBERRAS J, KLEIN M, de HULSTER E, MANS R, NEVOIGT E. Engineering Saccharomyces cerevisiae for succinic acid production from glycerol and carbon dioxide. Frontiers in Bioengineering and Biotechnology, 2020, 8: 566. DOI:10.3389/fbioe.2020.00566
|
|
| [30] |
PRABHU AA, LEDESMA-AMARO R, LIN CSK, COULON F, THAKUR VK, KUMAR V. Bioproduction of succinic acid from xylose by engineered Yarrowia lipolytica without pH control. Biotechnology for Biofuels, 2020, 13(1): 1-15. DOI:10.1186/s13068-019-1642-1
|
|
| [31] |
于青林, 孟令莉, 霍海亮, 高翠娟. 重组解脂酵母发酵生产琥珀酸的条件优化. 食品与发酵工业, 2018, 44(4): 119-123. YU QL, MENG LL, HUO HL, GAO CJ. Fermentation optimization of recombinant Yarrowia lipolytica for its efficient succinic acid production. Food and Fermentation Industries, 2018, 44(4): 119-123 (in Chinese).
|
|
| [32] |
XI YY, ZHAN T, XU HT, CHEN J, BI CH, FAN FY, ZHANG XL. Characterization of JEN family carboxylate transporters from the acid-tolerant yeast Pichia kudriavzevii and their applications in succinic acid production. Microbial Biotechnology, 2021, 14(3): 1130-1147. DOI:10.1111/1751-7915.13781
|
|
| [33] |
XU GQ, LIU LM, CHEN J. Reconstruction of cytosolic fumaric acid biosynthetic pathways in Saccharomyces cerevisiae. Microbial Cell Factories, 2012, 11(1): 1-10. DOI:10.1186/1475-2859-11-1
|
|
| [34] |
WEI L, LIU J, QI H, WEN J. Engineering Scheffersomyces stipitis for fumaric acid production from xylose. Bioresource Technology, 2015, 187: 246-254. DOI:10.1016/j.biortech.2015.03.122
|
|
| [35] |
CAO N, DU J, GONG CS, TSAO GT. Simultaneous production and recovery of fumaric acid from immobilized Rhizopus oryzae with a rotary biofilm contactor and an adsorption column. Applied and Environmental Microbiology, 1996, 62(8): 2926-2931. DOI:10.1128/aem.62.8.2926-2931.1996
|
|
| [36] |
WANG GY, BAI TT, MIAO ZG, NING WG, LIANG WX. Simultaneous production of single cell oil and fumaric acid by a newly isolated yeast Aureobasidium pullulans var.aubasidani DH177. Bioprocess and Biosystems Engineering, 2018, 41(11): 1707-1716. DOI:10.1007/s00449-018-1994-0
|
|
| [37] |
WEI X, ZHANG M, WANG GY, LIU GL, CHI ZM, CHI Z. The ornithine-urea cycle involves fumaric acid biosynthesis in Aureobasidium pullulans var.aubasidani, a green and eco-friendly process for fumaric acid production. Synthetic and Systems Biotechnology, 2022, 8(1): 33-45.
|
|
| [38] |
TAING O, TAING K. Production of malic and succinic acids by sugar-tolerant yeast Zygosaccharomyces rouxii. European Food Research and Technology, 2007, 224: 343-347.
|
|
| [39] |
CHEN XL, WANG YC, DONG XX, HU GP, LIU LM. Engineering rTCA pathway and C4-dicarboxylate transporter for l-malic acid production. Applied Microbiology and Biotechnology, 2017, 101(10): 4041-4052. DOI:10.1007/s00253-017-8141-8
|
|
| [40] |
ZHANG T, GE CY, DENG L, TAN TW, WANG F. C4-dicarboxylic acid production by overexpressing the reductive TCA pathway. FEMS Microbiology Letters, 2015, 362(9): fnv052.
|
|
| [41] |
PANDURIC N, SALIC A, ZELIC B. Fully integrated biotransformation of fumaric acid by permeabilized baker's yeast cells with in situ separation of L-malic acid using ultrafiltration, acidification and electrodialysis. Biochemical Engineering Journal, 2017, 125: 221-229. DOI:10.1016/j.bej.2017.06.005
|
|
| [42] |
MENEGATTI T, ŽNIDARŠIC-PLAZL P. Copolymeric hydrogel-based immobilization of yeast cells for continuous biotransformation of fumaric acid in a microreactor. Micromachines, 2019, 10(12): 867. DOI:10.3390/mi10120867
|
|
| [43] |
梁欣泉, 李宁, 任勤, 刘继栋. 代谢工程改造酿酒酵母生产L-乳酸的研究进展. 中国生物工程杂志, 2016, 36(2): 109-114. LIANG XQ, LI N, REN Q, LIU JD. Progress in the metabolic engineering of Saccharomyces cerevisiae for l-lactic acid production. China Biotechnology, 2016, 36(2): 109-114 (in Chinese). DOI:10.13523/j.cb.20160216
|
|
| [44] |
BAEK SH, KWON EY, KIM YH, HAHN JS. Metabolic engineering and adaptive evolution for efficient production of d-lactic acid in Saccharomyces cerevisiae. Applied Microbiology and Biotechnology, 2016, 100(6): 2737-2748. DOI:10.1007/s00253-015-7174-0
|
|
| [45] |
JANG BK, JU YB, JEONG D, JUNG SK, KIM CK, CHUNG YS, KIM SR. L-lactic acid production using engineered Saccharomyces cerevisiae with improved organic acid tolerance. Journal of Fungi, 2021, 7(11): 928. DOI:10.3390/jof7110928
|
|
| [46] |
WATCHARAWIPAS A, SAE-TANG K, SANSATCHANON K, SUDYING P, BOONCHOO K, TANAPONGPIPAT S, KOCHARIN K, RUNGUPHAN W. Systematic engineering of Saccharomyces cerevisiae for D-lactic acid production with near theoretical yield. FEMS Yeast Research, 2021, 21(4): foab024. DOI:10.1093/femsyr/foab024
|
|
| [47] |
KUANYSHEV N, RAO CV, DIEN B, JIN YS. Domesticating a food spoilage yeast into an organic acid-tolerant metabolic engineering host: lactic acid production by engineered Zygosaccharomyces bailii. Biotechnology and Bioengineering, 2021, 118(1): 372-382. DOI:10.1002/bit.27576
|
|
| [48] |
KOIVURANTA KT, ILMÉN M, WIEBE MG, RUOHONEN L, SUOMINEN P, PENTTILÄ M. L-lactic acid production from D-xylose with Candida sonorensis expressing a heterologous lactate dehydrogenase encoding gene. Microbial Cell Factories, 2014, 13(1): 1-14. DOI:10.1186/1475-2859-13-1
|
|
| [49] |
MELO N, MULDER K, NICOLA A, CARVALHO L, MENINO G, MULINARI E, PARACHIN N. Effect of pyruvate decarboxylase knockout on product distribution using Pichia pastoris ( Komagataella phaffii) engineered for lactic acid production. Bioengineering, 2018, 5(1): 17. DOI:10.3390/bioengineering5010017
|
|
| [50] |
PARK HJ, BAE JH, KO HJ, LEE SH, SUNG BH, HAN JI, SOHN JH. Low-pH production of d-lactic acid using newly isolated acid tolerant yeast Pichia kudriavzevii NG7. Biotechnology and Bioengineering, 2018, 115(9): 2232-2242. DOI:10.1002/bit.26745
|
|
| [51] |
BIANCHI MM, BRAMBILLA L, PROTANI F, LIU CL, LIEVENSE J, PORRO D. Efficient homolactic fermentation by Kluyveromyces lactis strains defective in pyruvate utilization and transformed with the heterologous LDH gene. Applied and Environmental Microbiology, 2001, 67(12): 5621-5625. DOI:10.1128/AEM.67.12.5621-5625.2001
|
|
| [52] |
YU JL, XIA XX, ZHONG JJ, QIAN ZG. Direct biosynthesis of adipic acid from a synthetic pathway in recombinant Escherichia coli. Biotechnology and Bioengineering, 2014, 111(12): 2580-2586. DOI:10.1002/bit.25293
|
|
| [53] |
JU JH, OH BR, HEO SY, LEE YU, SHON JH, KIM CH, KIM YM, SEO JW, HONG WK. Production of adipic acid by short- and long-chain fatty acid acyl-CoA oxidase engineered in yeast Candida tropicalis. Bioprocess and Biosystems Engineering, 2020, 43(1): 33-43. DOI:10.1007/s00449-019-02202-w
|
|
| [54] |
ZHANG X, LIU Y, WANG J, ZHAO Y, DENG Y. Biosynthesis of adipic acid in metabolically engineered Saccharomyces cerevisiae. The Journal of Microbiology, 2020, 58(12): 1065-1075. DOI:10.1007/s12275-020-0261-7
|
|
| [55] |
张熙, 李国辉, 周胜虎, 毛银, 赵运英, 邓禹. 酿酒酵母异源合成己二酸. 食品与发酵工业, 2020, 46(7): 1-9. ZHANG X, LI GH, ZHOU SH, MAO Y, ZHAO YY, DENG Y. Production of adipic acid in recombinant Saccharomyces cerevisiae. Food and Fermentation Industries, 2020, 46(7): 1-9 (in Chinese).
|
|
| [56] |
RAJ K, PARTOW S, CORREIA K, KHUSNUTDINOVA AN, YAKUNIN AF, MAHADEVAN R. Biocatalytic production of adipic acid from glucose using engineered Saccharomyces cerevisiae. Metabolic Engineering Communications, 2018, 6: 28-32. DOI:10.1016/j.meteno.2018.02.001
|
|
| [57] |
KARLSSON E, MAPELLI V, OLSSON L. Adipic acid tolerance screening for potential adipic acid production hosts. Microbial Cell Factories, 2017, 16(1): 1-17. DOI:10.1186/s12934-016-0616-2
|
|
| [58] |
FLETCHER E, MERCURIO K, WALDEN EA, BAETZ K. A yeast chemogenomic screen identifies pathways that modulate adipic acid toxicity. iScience, 2021, 24(4): 102327. DOI:10.1016/j.isci.2021.102327
|
|
| [59] |
SALUSJÄRVI L, HAVUKAINEN S, KOIVISTOINEN O, TOIVARI M. Biotechnological production of glycolic acid and ethylene glycol: current state and perspectives. Applied Microbiology and Biotechnology, 2019, 103(6): 2525-2535. DOI:10.1007/s00253-019-09640-2
|
|
| [60] |
KOIVISTOINEN OM, KUIVANEN J, BARTH D, TURKIA H, PITKÄNEN JP, PENTTILÄ M, RICHARD P. Glycolic acid production in the engineered yeasts Saccharomyces cerevisiae and Kluyveromyces lactis. Microbial Cell Factories, 2013, 12(1): 1-16. DOI:10.1186/1475-2859-12-1
|
|
| [61] |
SALUSJÄRVI L, TOIVARI M, VEHKOMÄKI ML, KOIVISTOINEN O, MOJZITA D, NIEMELÄ K, PENTTILÄ M, RUOHONEN L. Production of ethylene glycol or glycolic acid from D-xylose in Saccharomyces cerevisiae. Applied Microbiology and Biotechnology, 2017, 101(22): 8151-8163. DOI:10.1007/s00253-017-8547-3
|
|
| [62] |
于新磊, 毛雨丰, 张晓霞, 陆凌雪, 王智文, 陈涛. 生物法生产3-羟基丙酸研究进展. 化工进展, 2018, 37(11): 4427-4436. YU XL, MAO YF, ZHANG XX, LU LX, WANG ZW, CHEN T. Recent progress in microbial production of 3-hydroxypropionic acid. Chemical Industry and Engineering Progress, 2018, 37(11): 4427-4436 (in Chinese).
|
|
| [63] |
KILDEGAARD KR, HALLSTRÖM BM, BLICHER TH, SONNENSCHEIN N, JENSEN NB, SHERSTYK S, HARRISON SJ, MAURY J, HERRGÅRD MJ, JUNCKER AS, FORSTER J, NIELSEN J, BORODINA I. Evolution reveals a glutathione-dependent mechanism of 3-hydroxypropionic acid tolerance. Metabolic Engineering, 2014, 26: 57-66. DOI:10.1016/j.ymben.2014.09.004
|
|
| [64] |
QIN N, LI LY, JI X, LI XW, ZHANG YM, LARSSON C, CHEN Y, NIELSEN J, LIU ZH. Rewiring central carbon metabolism ensures increased provision of acetyl-CoA and NADPH required for 3-OH-propionic acid production. ACS Synthetic Biology, 2020, 9(12): 3236-3244. DOI:10.1021/acssynbio.0c00264
|
|
| [65] |
YU W, CAO X, GAO J, ZHOU YJ. Overproduction of 3-hydroxypropionate in a super yeast chassis. Bioresource Technology, 2022, 361: 127690. DOI:10.1016/j.biortech.2022.127690
|
|
| [66] |
KILDEGAARD KR, JENSEN NB, SCHNEIDER K, CZARNOTTA E, ÖZDEMIR E, KLEIN T, MAURY J, EBERT BE, CHRISTENSEN HB, CHEN Y, KIM IK, HERRGÅRD MJ, BLANK LM, FORSTER J, NIELSEN J, BORODINA I. Engineering and systems-level analysis of Saccharomyces cerevisiae for production of 3-hydroxypropionic acid via malonyl-CoA reductase-dependent pathway. Microbial Cell Factories, 2016, 15(1): 1-13.
|
|
| [67] |
FINA A, HEUX S, ALBIOL J, FERRER P. Combining metabolic engineering and multiplexed screening methods for 3-hydroxypropionic acid production in Pichia pastoris. Frontiers in Bioengineering and Biotechnology, 2022, 10: 942304.
|
|
| [68] |
ZHAO ML, LU XY, ZONG H, LI JY, ZHUGE B. Itaconic acid production in microorganisms. Biotechnology Letters, 2018, 40(3): 455-464.
|
|
| [69] |
高寅岭, 张凤娇, 赵贵众, 张宏森, 王风芹, 宋安东. 衣康酸发酵研究进展. 中国生物工程杂志, 2021, 41(5): 105-113. GAO YL, ZHANG FJ, ZHAO GZ, ZHANG HS, WANG FQ, SONG AD. Research progress of itaconic acid fermentation. China Biotechnology, 2021, 41(5): 105-113 (in Chinese).
|
|
| [70] |
KRULL S, HEVEKERL A, KUENZ A, PRÜßE U. Process development of itaconic acid production by a natural wild type strain of Aspergillus terreus to reach industrially relevant final titers. Applied Microbiology and Biotechnology, 2017, 101(10): 4063-4072.
|
|
| [71] |
HOSSEINPOUR TEHRANI H, BECKER J, BATOR I, SAUR K, MEYER S, RODRIGUES LÓIA AC, BLANK LM, WIERCKX N. Integrated strain- and process design enable production of 220 gL−1 itaconic acid with Ustilago maydis. Biotechnology for Biofuels, 2019, 12(1): 1-11.
|
|
| [72] |
BLAZECK J, HILL A, JAMOUSSI M, PAN A, MILLER J, ALPER HS. Metabolic engineering of Yarrowia lipolytica for itaconic acid production. Metabolic Engineering, 2015, 32: 66-73.
|
|
| [73] |
SUN W, VILA-SANTA A, LIU N, PROZOROV T, XIE DM, FARIA NT, FERREIRA FC, MIRA NP, SHAO ZY. Metabolic engineering of an acid-tolerant yeast strain Pichia kudriavzevii for itaconic acid production. Metabolic Engineering Communications, 2020, 10: e00124.
|
|
| [74] |
YOUNG EM, ZHAO Z, GIELESEN BEM, WU L, BENJAMIN GORDON D, ROUBOS JA, VOIGT CA. Iterative algorithm-guided design of massive strain libraries, applied to itaconic acid production in yeast. Metabolic Engineering, 2018, 48: 33-43.
|
|
| [75] |
RONG LX, MIAO L, WANG SH, WANG YP, LIU SQ, LU ZH, ZHAO BX, ZHANG CY, XIAO DG, PUSHPANATHAN K, WONG A, YU AQ. Engineering Yarrowia lipolytica to produce itaconic acid from waste cooking oil. Frontiers in Bioengineering and Biotechnology, 2022, 10: 888869.
|
|
| [76] |
XU YY, LI ZM. Utilization of ethanol for itaconic acid biosynthesis by engineered Saccharomyces cerevisiae. FEMS Yeast Research, 2021, 21(6): foab043.
|
|
| [77] |
LI S, FU W, SU R, ZHAO Y, DENG Y. Metabolic engineering of the malonyl-CoA pathway to efficiently produce malonate in Saccharomyces cerevisiae. Metabolic Engineering, 2022, 73: 1-10.
|
|
| [78] |
TOIVARI M, VEHKOMÄKI ML, NYGÅRD Y, PENTTILÄ M, RUOHONEN L, WIEBE MG. Low pH D-xylonate production with Pichia kudriavzevii. Bioresource Technology, 2013, 133: 555-562.
|
|
| [79] |
KOVACEVIC G, ELGAHWASH R, BLAZIC M, PANTIĆ N. Production of fructose and gluconic acid from sucrose with cross-linked yeast cell walls expressing glucose oxidase on the surface. Molecular Catalysis, 2022, 522: 112215.
|
|
| [80] |
信丰学, 章文明, 钱秀娟, 周大伟, 董维亮, 周杰, 姜岷. 一株联产油脂与葡萄糖酸的产油酵母菌及其应用: CN112760242A[P]. 2021-05-07.
XIN FX, ZHANG WM, QIAN XJ, ZHOU DW, DONG WL, ZHOU J, JIANG M. Oleaginous saccharomycetes for co-production of grease and gluconic acid and application thereof: CN112760242A[P]. 2021-05-07 (in Chinese).
|
|
| [81] |
GUPTA A, HICKS MA, MANCHESTER SP, PRATHER KLJ. Porting the synthetic D-glucaric acid pathway from Escherichia coli to Saccharomyces cerevisiae. Biotechnology Journal, 2016, 11(9): 1201-1208.
|
|
| [82] |
ZHANG X, XU C, LIU YL, WANG J, ZHAO YY, DENG Y. Enhancement of glucaric acid production in Saccharomyces cerevisiae by expressing Vitreoscilla hemoglobin. Biotechnology Letters, 2020, 42(11): 2169-2178.
|
|
| [83] |
ZHANG M, ZHANG K, MEHMOOD MA, ZHAO ZK, BAI F, ZHAO X. Deletion of acetate transporter gene ADY2 improved tolerance of Saccharomyces cerevisiae against multiple stresses and enhanced ethanol production in the presence of acetic acid. Bioresource Technology, 2017, 245: 1461-1468.
|
|
 2023, Vol. 39
2023, Vol. 39